ERC-focus (English)
... plays a role in the so-called weak decay of particles through the exchange of yet another set of force particles, which have large masses coming from the Higgs field. The recent likely discovery of the excitation of this field is an important step in our quest what is beyond the so-called Standard M ...
... plays a role in the so-called weak decay of particles through the exchange of yet another set of force particles, which have large masses coming from the Higgs field. The recent likely discovery of the excitation of this field is an important step in our quest what is beyond the so-called Standard M ...
Chemistry
... All atoms consist of even smaller particles—protons, neutrons, and electrons. The center of an atom is called the nucleus, which is made up of protons and neutrons. A proton is a tiny particle that has mass and a positive electric charge. A neutron is a tiny particle with approximately the same mas ...
... All atoms consist of even smaller particles—protons, neutrons, and electrons. The center of an atom is called the nucleus, which is made up of protons and neutrons. A proton is a tiny particle that has mass and a positive electric charge. A neutron is a tiny particle with approximately the same mas ...
Time Evolution in Quantum Mechanics
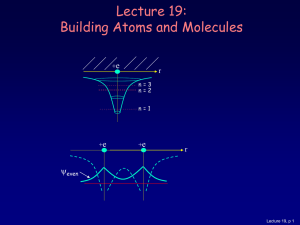
... Figure 15.2: Potential experienced by electron in O−2 ion. For increasing V0 (the height of the barrier), the off-diagonal element of the Hamiltonian decreases, becoming zero when the barrier is infinitely high. So, from a classical physics perspective, the electron would reside in the vicinity of t ...
... Figure 15.2: Potential experienced by electron in O−2 ion. For increasing V0 (the height of the barrier), the off-diagonal element of the Hamiltonian decreases, becoming zero when the barrier is infinitely high. So, from a classical physics perspective, the electron would reside in the vicinity of t ...
3/27 Lecture Slides
... – After CO32precipitates to near completion, pHg drops to the point where CrO42- starts to precipitate Which anion was initially present at higher concentration? ...
... – After CO32precipitates to near completion, pHg drops to the point where CrO42- starts to precipitate Which anion was initially present at higher concentration? ...
PPT
... For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: Electrons do not pile up in the lowest energy state. It’s more like filling a bucket with water. They are ...
... For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: Electrons do not pile up in the lowest energy state. It’s more like filling a bucket with water. They are ...
Strong Interactions I
... nucleons. This is the dominant term in the formula. Other terms show the variation of the binding energy as a function of N and Z. The second term is called the surface term with as = 18.56 MeV, representing that the binding energy is lost somehow proportional to the surface area. These two terms c ...
... nucleons. This is the dominant term in the formula. Other terms show the variation of the binding energy as a function of N and Z. The second term is called the surface term with as = 18.56 MeV, representing that the binding energy is lost somehow proportional to the surface area. These two terms c ...
Atomic Structure | Topic Notes
... • fire α-particles at thin gold foil • large no. not deflected - essentially empty space • many deflected at small angles • some deflected at large angles - passed close to positive charge • few rebounded - collided directly with a small, dense nucleus of positive charge • model: mostly empty space, ...
... • fire α-particles at thin gold foil • large no. not deflected - essentially empty space • many deflected at small angles • some deflected at large angles - passed close to positive charge • few rebounded - collided directly with a small, dense nucleus of positive charge • model: mostly empty space, ...
Nuclear Binding Energy -
... discovered earlier by J.J. Thomson himself. Knowing that atoms are electrically neutral, Thomson postulated that there must be a positive charge as well. In his plum pudding model, Thomson suggested that an atom consisted of negative electrons randomly scattered within a sphere of positive charge. E ...
... discovered earlier by J.J. Thomson himself. Knowing that atoms are electrically neutral, Thomson postulated that there must be a positive charge as well. In his plum pudding model, Thomson suggested that an atom consisted of negative electrons randomly scattered within a sphere of positive charge. E ...
Chapter 6. Electronic Structure of Atoms
... Be able to distinguish between an emission or absorption spectrum Be able to calculation various Energy transitions between two energy levels. Be able to write electronic structure both long & short-cut forms, Energy level diagram, and identifications by structures including atoms and ions Be able t ...
... Be able to distinguish between an emission or absorption spectrum Be able to calculation various Energy transitions between two energy levels. Be able to write electronic structure both long & short-cut forms, Energy level diagram, and identifications by structures including atoms and ions Be able t ...
Effective mass theorem, dynamics of electrons and
... is therefore h̄kF /m∗ . Lorentz force = h̄∂k/∂t = −ev × B. Plug the velocity in, we have: ǫf B/m∗ = ∂k/∂t = 2πkF /τ . (The time it takes to go around one cycle, τ , will vary with k, but the dominant comes from orbits whose periods are stationary with respect to change in k.) In the above equation, ...
... is therefore h̄kF /m∗ . Lorentz force = h̄∂k/∂t = −ev × B. Plug the velocity in, we have: ǫf B/m∗ = ∂k/∂t = 2πkF /τ . (The time it takes to go around one cycle, τ , will vary with k, but the dominant comes from orbits whose periods are stationary with respect to change in k.) In the above equation, ...
Using the Franck-Hertz Experiment To Illustrate Quantization
... of couisions may occur, inelastic collisions where the bombarding electrons lose energy to the electrons of the neon atoms, and elastic collisions where translational energy may be exchanged hetween bombarding electrons and neon atoms. Elastic collisions do not change the energy of accelerated elect ...
... of couisions may occur, inelastic collisions where the bombarding electrons lose energy to the electrons of the neon atoms, and elastic collisions where translational energy may be exchanged hetween bombarding electrons and neon atoms. Elastic collisions do not change the energy of accelerated elect ...
Chem Ch. 4.4
... beta particle is emitted by the nucleus of an atom. • How, you may ask, is it possible for an electron to come from a nucleus?? • Scientists believe that neutrons are actually composed of 2 particles - an electron and a proton. • In beta decay, the electron is emitted and the proton is left, increas ...
... beta particle is emitted by the nucleus of an atom. • How, you may ask, is it possible for an electron to come from a nucleus?? • Scientists believe that neutrons are actually composed of 2 particles - an electron and a proton. • In beta decay, the electron is emitted and the proton is left, increas ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.