PHY4605–Introduction to Quantum Mechanics II Spring 1997 Problem Set 4 Jan. 31, 2005
... point-proton model to be δV (r) = V1 (r) − V0 (r). Use 1st-order perturbation theory to find the correction δE0 to the ground state energy due to the finite size of the proton. Obtain a numerical value for the fractional correction δE0 /E0 . (Hint: you can simplify the calculation by noting that r0 ...
... point-proton model to be δV (r) = V1 (r) − V0 (r). Use 1st-order perturbation theory to find the correction δE0 to the ground state energy due to the finite size of the proton. Obtain a numerical value for the fractional correction δE0 /E0 . (Hint: you can simplify the calculation by noting that r0 ...
Document
... 2. (a) What is the wavelength of light for the east energetic photon emitted in the Lyman series of hydrogen atom spectrum lines? (b) What is the wavelength of the series limit for the Lyman series? ANSWER: (a) 122 nm; (b) 91.1 nm 3. What are the (a) energy, (b) magnitude of the momentum, and (c) wa ...
... 2. (a) What is the wavelength of light for the east energetic photon emitted in the Lyman series of hydrogen atom spectrum lines? (b) What is the wavelength of the series limit for the Lyman series? ANSWER: (a) 122 nm; (b) 91.1 nm 3. What are the (a) energy, (b) magnitude of the momentum, and (c) wa ...
electron orbits atomic spectra the Bohr atom
... Did you spot the example of the misleading Physicsspeak* on the previous slide? Evidently, from the Balmer formula and its extension to general integers m, n, these allowed non-radiating orbits, the stationary states, could be labeled 1, 2, 3, ... , n, ... and had energies -1, -1/4, -1/9, ..., -1/n ...
... Did you spot the example of the misleading Physicsspeak* on the previous slide? Evidently, from the Balmer formula and its extension to general integers m, n, these allowed non-radiating orbits, the stationary states, could be labeled 1, 2, 3, ... , n, ... and had energies -1, -1/4, -1/9, ..., -1/n ...
The Atom
... atomic mass unit (amu) atomic mass electromagnetic radiation wavelength frequency amplitude ...
... atomic mass unit (amu) atomic mass electromagnetic radiation wavelength frequency amplitude ...
Models of an atom and old quantum theory
... • These postulates de ne Bohr's model of an atom, which turns out to be applicable for Hydrogen atoms (Z = 1) and ions containing only one electron such as singly ionized Helium (Z = 2), doubly ionized Lithium (Z = 3), etc. • The postulated quantization of angular momentum leads to electron energy q ...
... • These postulates de ne Bohr's model of an atom, which turns out to be applicable for Hydrogen atoms (Z = 1) and ions containing only one electron such as singly ionized Helium (Z = 2), doubly ionized Lithium (Z = 3), etc. • The postulated quantization of angular momentum leads to electron energy q ...
The Quantum Theory of Atoms and Molecules
... Same as the Rydberg formula Bohr’s theory is in agreement with experimentally observed spectra. Combining de Broglie’s relation with Bohr condition shows that the circumference of the orbit of radius r must be an integer number of wavelengths, . ...
... Same as the Rydberg formula Bohr’s theory is in agreement with experimentally observed spectra. Combining de Broglie’s relation with Bohr condition shows that the circumference of the orbit of radius r must be an integer number of wavelengths, . ...
PowerPoint
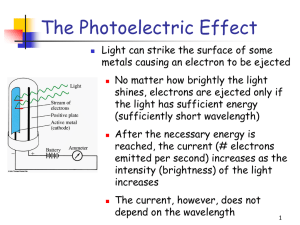
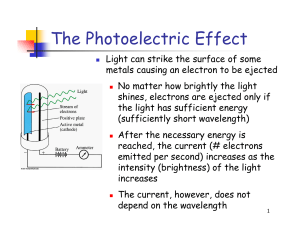
... Light consists of photons, each with a particular amount of energy, called a quantum of energy Upon collision, each photon can transfer its energy to a single electron The more photons strike the surface of the metal, the more electrons are liberated and the higher is the current ...
... Light consists of photons, each with a particular amount of energy, called a quantum of energy Upon collision, each photon can transfer its energy to a single electron The more photons strike the surface of the metal, the more electrons are liberated and the higher is the current ...
Electron Configuration Notes
... • electrons move around nucleus in orbits similar to how planets orbit the sun • energy levels for electrons are quantized Major developments that put Bohr’s Model into question: Einstein: Light energy exhibits properties of matter. Matter and energy are different forms of the same thing. De Broglie ...
... • electrons move around nucleus in orbits similar to how planets orbit the sun • energy levels for electrons are quantized Major developments that put Bohr’s Model into question: Einstein: Light energy exhibits properties of matter. Matter and energy are different forms of the same thing. De Broglie ...
Rutherford Model 1911 - University of St Andrews
... them from responding to the Coulomb force of attraction and being sucked into the nucleus. But the atomic diameter would then be ~ nuclear diameter. ...
... them from responding to the Coulomb force of attraction and being sucked into the nucleus. But the atomic diameter would then be ~ nuclear diameter. ...
Modern Model of the Atom
... Do not confuse with orbits! The electrons are NOT “orbiting” the nucleus. We cannot predict the actual location of an electron like we can predict planets orbiting the sun! Different orbital shapes: s, p, d, f (lowest to highest energy) ...
... Do not confuse with orbits! The electrons are NOT “orbiting” the nucleus. We cannot predict the actual location of an electron like we can predict planets orbiting the sun! Different orbital shapes: s, p, d, f (lowest to highest energy) ...
The study of biology can help you better understand
... 10. Write electron configuration notation for He _____________ Ne ___________________ Ar ___________________ 11. Write electron configuration notation for N _____________ F ___________________ S ___________________ 12. Write noble gas electron configuration notation for N ______________ F __________ ...
... 10. Write electron configuration notation for He _____________ Ne ___________________ Ar ___________________ 11. Write electron configuration notation for N _____________ F ___________________ S ___________________ 12. Write noble gas electron configuration notation for N ______________ F __________ ...
Chapter2. Elements of quantum mechanics
... An observation by Plank : radiation from a heated sample is emitted in discrete units of energy, called quanta ; the energy units were described by h, where is the frequency of the radiation, and h is a quantity called Plank’s constant En=nhν=nћω, h = 6.63 × 10-34 J·s, ћ=h/2π Quantization of ligh ...
... An observation by Plank : radiation from a heated sample is emitted in discrete units of energy, called quanta ; the energy units were described by h, where is the frequency of the radiation, and h is a quantity called Plank’s constant En=nhν=nћω, h = 6.63 × 10-34 J·s, ћ=h/2π Quantization of ligh ...
Modern Atomic Theory Notes Sheet
... Said Hydrogen’s single electron could only be in certain allowed orbits around the nucleus higher orbit = theorized that electrons existed in distinct orbitals or energy levels around the nucleus, and it took an exact amount of energy or quanta to move an electron from one orbital to another, ...
... Said Hydrogen’s single electron could only be in certain allowed orbits around the nucleus higher orbit = theorized that electrons existed in distinct orbitals or energy levels around the nucleus, and it took an exact amount of energy or quanta to move an electron from one orbital to another, ...
Problem Set 4
... hydrogen like atom in the presence of an electric field upto second order in Hel given in problem 23 in terms of an infinte sum involving all the eigenstates of the unperturbed hydrogen atom. Estimate the shift using only the n = 2 term ( n is the principal quantum number)in electron volts for the s ...
... hydrogen like atom in the presence of an electric field upto second order in Hel given in problem 23 in terms of an infinte sum involving all the eigenstates of the unperturbed hydrogen atom. Estimate the shift using only the n = 2 term ( n is the principal quantum number)in electron volts for the s ...
Document
... Bohr’s quantum model of the Atom (1913) Four postulates: 1. An electron in an atom moves in a circular orbit about the nucleus under the influence of the Coulomb attraction between the electron and the nucleus 2. The allowed orbit is a stationary orbit with a constant energy E 3. Electron radiates ...
... Bohr’s quantum model of the Atom (1913) Four postulates: 1. An electron in an atom moves in a circular orbit about the nucleus under the influence of the Coulomb attraction between the electron and the nucleus 2. The allowed orbit is a stationary orbit with a constant energy E 3. Electron radiates ...
Atomic Emission Spectra and Quantum mechanical Model
... • http://www.youtube.com/watch?v=8M72pX5wlyE ...
... • http://www.youtube.com/watch?v=8M72pX5wlyE ...
Lecture 24: Quantum mechanics
... Question then arose how do we prove the wave nature of electrons. In a brilliant experiment, Stern and Gerlach proved this. They exploited Huygen’s principle that was used to prove light is wave. The primary effect of wave nature is the observation of diffraction or interference phenomena. Using sin ...
... Question then arose how do we prove the wave nature of electrons. In a brilliant experiment, Stern and Gerlach proved this. They exploited Huygen’s principle that was used to prove light is wave. The primary effect of wave nature is the observation of diffraction or interference phenomena. Using sin ...
Bohr Model, Quantum Mechanical Model
... b. energy is involved in moving an electron from one level to another. 4. Heisenberg Uncertainty Principle- It is impossible to know the momentum (mass of electron times velocity) of an electron and its position in space at the same time. One or the other. 5. Quantum Mechanical Model- a mathematical ...
... b. energy is involved in moving an electron from one level to another. 4. Heisenberg Uncertainty Principle- It is impossible to know the momentum (mass of electron times velocity) of an electron and its position in space at the same time. One or the other. 5. Quantum Mechanical Model- a mathematical ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.