Atomic Structure Practice Test
... 30) An atom with atomic number 9 is in its ground state. How many electrons are in its outermost shell? ...
... 30) An atom with atomic number 9 is in its ground state. How many electrons are in its outermost shell? ...
Pauli Exclusion Principle
... i off order d α2mc2 : ~100 eV Fine structure: of order α4mc2 : ~10-4 eV Lamb shift: of order α5mc2 : ~10-6 eV Hyperfine splitting: of order (m/mp)α4mc2 : ~10-6 eV ...
... i off order d α2mc2 : ~100 eV Fine structure: of order α4mc2 : ~10-4 eV Lamb shift: of order α5mc2 : ~10-6 eV Hyperfine splitting: of order (m/mp)α4mc2 : ~10-6 eV ...
The Hydrogen Atom 24.1 Radial Wavefunction
... As with the infinite square well, it makes sense to let κ = −2~m E (negative inside the square root, now – bound states will have E < 0 and we want to make κ real). We want to define a new “coordinate” ρ ≡ κ r. The advantage is to render the coordinate variable itself unitless. Whenever we want to c ...
... As with the infinite square well, it makes sense to let κ = −2~m E (negative inside the square root, now – bound states will have E < 0 and we want to make κ real). We want to define a new “coordinate” ρ ≡ κ r. The advantage is to render the coordinate variable itself unitless. Whenever we want to c ...
Chapter 2 Notes
... C. States of Matter- on Earth matter occurs in 4 physical states 1. solids- molecules are in a fixed position relative to each other; atoms or molecules may vibrate, but do not switch positions; a solid resists changes in shape and volume 2. liquids- atoms or molecules remain close to one another bu ...
... C. States of Matter- on Earth matter occurs in 4 physical states 1. solids- molecules are in a fixed position relative to each other; atoms or molecules may vibrate, but do not switch positions; a solid resists changes in shape and volume 2. liquids- atoms or molecules remain close to one another bu ...
HW / Unit 2
... 1. Explain what each of the following scientists contributed to atomic theory. a. Niels Bohr b. Erwin Schrodinger 2. Give two uses of the phenomena whereby atoms give off colorful light when supplied with energy. 3. Explain what led Bohr to believe that electrons could only be found at certain energ ...
... 1. Explain what each of the following scientists contributed to atomic theory. a. Niels Bohr b. Erwin Schrodinger 2. Give two uses of the phenomena whereby atoms give off colorful light when supplied with energy. 3. Explain what led Bohr to believe that electrons could only be found at certain energ ...
Honors Unit 5 Practice Test
... ____ 65. The strength of London dispersion forces between molecules depends on a. only the number of electrons in the molecule. b. only the number of protons in the molecule. c. both the number of electrons in the molecule and the mass of the molecule. d. both the number of electrons and the number ...
... ____ 65. The strength of London dispersion forces between molecules depends on a. only the number of electrons in the molecule. b. only the number of protons in the molecule. c. both the number of electrons in the molecule and the mass of the molecule. d. both the number of electrons and the number ...
Intro to Chapter 5 Development of the Periodic Table
... example of how scientific theory comes into being. Through random observations followed by organization of data into trends resulted in a consistent hypothesis which could explain known facts and makes correct predictions of the elements. B. Mendeleev s organized chemical information by: 1) listing ...
... example of how scientific theory comes into being. Through random observations followed by organization of data into trends resulted in a consistent hypothesis which could explain known facts and makes correct predictions of the elements. B. Mendeleev s organized chemical information by: 1) listing ...
2. NH3 - Huffman Chemistry Website!
... Directions: Answer the following questions below. Show all work for full credit, label all units, put all calculation answers in correct scientific notation (when needed) and significant figures and BOX all answers. ...
... Directions: Answer the following questions below. Show all work for full credit, label all units, put all calculation answers in correct scientific notation (when needed) and significant figures and BOX all answers. ...
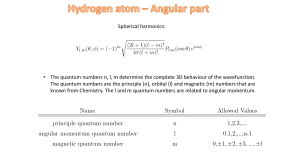
Spherical harmonics: • The quantum numbers n, l, m determine the
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
Molecular Statistics
... The electrons come out when a light is shining onto a metal. The frequency of light controls whether or not electrons are emitted, but not light intensity. The number of electrons emitted is proportional to the intensity. ...
... The electrons come out when a light is shining onto a metal. The frequency of light controls whether or not electrons are emitted, but not light intensity. The number of electrons emitted is proportional to the intensity. ...
atomic structure sm
... In 1913, Niels Bohr proposed a model for electron distributions that was a combination of classical and the new emerging quantum theories. ...
... In 1913, Niels Bohr proposed a model for electron distributions that was a combination of classical and the new emerging quantum theories. ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.