Chemistry Test Review - Greenslime Home Page
... a. Proton – located in the nucleus – mass of 1 amu – charge is positive (+) b. Neutron – located in the nucleus – mass of 1 amu – charge is neutral (0) c. Electron – located in electron cloud (shells, orbitals) – mass of approx. 0 amu – charge is negative (-) 9. Name 3 elements that have the most si ...
... a. Proton – located in the nucleus – mass of 1 amu – charge is positive (+) b. Neutron – located in the nucleus – mass of 1 amu – charge is neutral (0) c. Electron – located in electron cloud (shells, orbitals) – mass of approx. 0 amu – charge is negative (-) 9. Name 3 elements that have the most si ...
Artificial atoms
... In a natural atom one has little control over the spectrum of energies for adding or removing electrons. There the electrons interact with the fixed potential of the nucleus and with each other, and these two kinds of interaction determine the spectrum. In an artificial atom, however, one can change ...
... In a natural atom one has little control over the spectrum of energies for adding or removing electrons. There the electrons interact with the fixed potential of the nucleus and with each other, and these two kinds of interaction determine the spectrum. In an artificial atom, however, one can change ...
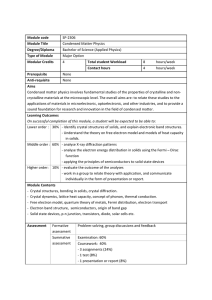
Module code SP-2306 Module Title Condensed Matter Physics
... Condensed matter physics involves fundamental studies of the properties of crystalline and noncrystalline materials at the microscopic level. The overall aims are: to relate these studies to the applications of materials in microelectronic, optoelectronic, and other industries, and to provide a so ...
... Condensed matter physics involves fundamental studies of the properties of crystalline and noncrystalline materials at the microscopic level. The overall aims are: to relate these studies to the applications of materials in microelectronic, optoelectronic, and other industries, and to provide a so ...
Name: Date: Chemistry 1 – Midterm Review Sheet Unit 1 – Scientific
... 3. The energy levels of the hydrogen atom (and all atoms) are ______________, meaning that only certain discrete energy levels are allowed. a. varied b. quantized c. ramp-like d. continuous e. two of these 4. The form of EMR that has less energy than microwaves is a. microwaves b. radio waves c. ga ...
... 3. The energy levels of the hydrogen atom (and all atoms) are ______________, meaning that only certain discrete energy levels are allowed. a. varied b. quantized c. ramp-like d. continuous e. two of these 4. The form of EMR that has less energy than microwaves is a. microwaves b. radio waves c. ga ...
Term paper
... It agrees qualitativelt, i.e predicts the increase of wavelength, but quantitative result is poor as, they have 110% average absolute error. It fails for conjugated ployenes, but, works well for polymethine ions (CH3 )2 N+ =CH(-CH=CH)k -N̈(CH3 )2 ...
... It agrees qualitativelt, i.e predicts the increase of wavelength, but quantitative result is poor as, they have 110% average absolute error. It fails for conjugated ployenes, but, works well for polymethine ions (CH3 )2 N+ =CH(-CH=CH)k -N̈(CH3 )2 ...
Name - Quia
... Explain the relationship between nucleon number and stability of nuclei. Explain why nuclear reactions occur and know how to balance a nuclear equation. Define and relate the terms radioactive decay and nuclear radiation. Describe the different types of radioactive decay and their effects on the nuc ...
... Explain the relationship between nucleon number and stability of nuclei. Explain why nuclear reactions occur and know how to balance a nuclear equation. Define and relate the terms radioactive decay and nuclear radiation. Describe the different types of radioactive decay and their effects on the nuc ...
quantum number
... spatial configuration of positive ion cores and outer electrons has less total energy than any other configuration (including infinite separation of the respective atoms). The energy deficience of the configuration compared with isolated atoms is known as a cohesive energy, and ranges in value from ...
... spatial configuration of positive ion cores and outer electrons has less total energy than any other configuration (including infinite separation of the respective atoms). The energy deficience of the configuration compared with isolated atoms is known as a cohesive energy, and ranges in value from ...
Chapter 5 Mendeleev`s Periodic Table
... The Bohr Model of the Atom • In 1913, Niels Bohr suggested a new model of the atom that explained why hydrogen had a discrete line spectrum rather than a continuous spectrum. • Bohr's basic theory: electrons in atoms can only be at certain energy levels, and they can give off or absorb radiation onl ...
... The Bohr Model of the Atom • In 1913, Niels Bohr suggested a new model of the atom that explained why hydrogen had a discrete line spectrum rather than a continuous spectrum. • Bohr's basic theory: electrons in atoms can only be at certain energy levels, and they can give off or absorb radiation onl ...
File
... Quantum Mechanical Model • Each orbital can contain two electrons. • Since negative-negative repel, these electrons occupy the orbital with opposite spins. ...
... Quantum Mechanical Model • Each orbital can contain two electrons. • Since negative-negative repel, these electrons occupy the orbital with opposite spins. ...
Document
... Lithium has Z=3. Two electrons are in a 1s state and one electron is excited into the 3d state. How does the energy of this excited electron compare to the energy of the electron in a hydrogen atom which is also in the 3d state? A. The lithium electron energy is significantly higher (less negative) ...
... Lithium has Z=3. Two electrons are in a 1s state and one electron is excited into the 3d state. How does the energy of this excited electron compare to the energy of the electron in a hydrogen atom which is also in the 3d state? A. The lithium electron energy is significantly higher (less negative) ...
Chapter 7 Quantum Theory of the Atom
... by absorbing or emitting a photon Energy of a photon is the difference in energy between the energy levels Emission of light during a transition gives the line spectrum of the element results from an e– moving from a higher energy level to a lower energy level Energy of an emitted photon ...
... by absorbing or emitting a photon Energy of a photon is the difference in energy between the energy levels Emission of light during a transition gives the line spectrum of the element results from an e– moving from a higher energy level to a lower energy level Energy of an emitted photon ...
Quantum Mechanics as dissolver of the sensate universe: this is
... sources produce interference patterns; yet in 1839, it was first shown that light waves falling on metal caused the emission of electrons, which suggests that light has particle properties. Louis de Brogli generalized wave particle duality by associating a wave length not only with mass-less photons ...
... sources produce interference patterns; yet in 1839, it was first shown that light waves falling on metal caused the emission of electrons, which suggests that light has particle properties. Louis de Brogli generalized wave particle duality by associating a wave length not only with mass-less photons ...
Name: Date: Period: _____ Unit 2 Notes, Part 1 – The Basics of
... number of protons is considered the atom’s atomic number and determines the atom’s position on the periodic table of elements. Since protons and neutrons are the only particles in the atom with a significant mass, the sum of the masses of protons and neutrons is considered an atom’s atomic mass. 6. ...
... number of protons is considered the atom’s atomic number and determines the atom’s position on the periodic table of elements. Since protons and neutrons are the only particles in the atom with a significant mass, the sum of the masses of protons and neutrons is considered an atom’s atomic mass. 6. ...
Quantum-Electrodynamics and the Magnetic Moment of the
... interaction between matter and radiation, which requires that the electromagnetic mass be a small correction (∼ (e2 /hc)m0 ) to the mechanical mass m0 . The new Hamiltonian is superior to the original one in essentially three ways: it involves the experimental electron mass, rather than the unobser ...
... interaction between matter and radiation, which requires that the electromagnetic mass be a small correction (∼ (e2 /hc)m0 ) to the mechanical mass m0 . The new Hamiltonian is superior to the original one in essentially three ways: it involves the experimental electron mass, rather than the unobser ...
Document
... quantity L2x L2y . Write an expression for this quantity in terms of l and ml . (b) What is the meaning of L2x L2y ? (c) For a state of nonzero orbital angular momentum, find the maximum and minimum values of L2x L2y . Explain your results. ...
... quantity L2x L2y . Write an expression for this quantity in terms of l and ml . (b) What is the meaning of L2x L2y ? (c) For a state of nonzero orbital angular momentum, find the maximum and minimum values of L2x L2y . Explain your results. ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.