SOME ELEMENTS OF ATOMIC STRUCTURE THEORY
... As everyone knows, an atom is the smallest unit of a chemical element, and atoms combine to form molecules and solids. Each element is uniquely specified by its atomic number Z. However a given element may occur in several different versions, called isotopes; these have the same Z but different atomic ...
... As everyone knows, an atom is the smallest unit of a chemical element, and atoms combine to form molecules and solids. Each element is uniquely specified by its atomic number Z. However a given element may occur in several different versions, called isotopes; these have the same Z but different atomic ...
Word document - FacStaff Home Page for CBU
... “…determination of the stable motion of electrons in the atom introduces integers, and up to this point the only phenomena involving integers in physics were those of interference and of normal modes of vibration. This fact suggested to me the idea that electrons too could not be considered simply a ...
... “…determination of the stable motion of electrons in the atom introduces integers, and up to this point the only phenomena involving integers in physics were those of interference and of normal modes of vibration. This fact suggested to me the idea that electrons too could not be considered simply a ...
Chapter Five: Many electron atom
... any direction. The component oriented along the magnetic field gradient (say the Z direction) would determine the force on the electron, and hence how much it would be deflected. • If electrons were like ordinary magnets with random orientations, they would show a continuous distribution of paths. T ...
... any direction. The component oriented along the magnetic field gradient (say the Z direction) would determine the force on the electron, and hence how much it would be deflected. • If electrons were like ordinary magnets with random orientations, they would show a continuous distribution of paths. T ...
Intro to Quantum Mechanics
... bulb. A 40/75/115 watt bulb can only shine light at those three wattage's, and when you switch from one setting to the next, the power immediately jumps to the new setting instead of just gradually increasing. It is the fact that electrons can only exist at discrete energy levels which prevents them ...
... bulb. A 40/75/115 watt bulb can only shine light at those three wattage's, and when you switch from one setting to the next, the power immediately jumps to the new setting instead of just gradually increasing. It is the fact that electrons can only exist at discrete energy levels which prevents them ...
Chap. 7 - Quantum Chemistry
... 1. Yellow light exhibits a wavelength of approximately 570 nm. Determine the frequency of this light and the total energy of the photon being emitted in units of J and kJ/mol. 2. When an electron beam strikes a block of copper, x-rays with a frequency of 2.0 x 1018 Hz are emitted. How much energy is ...
... 1. Yellow light exhibits a wavelength of approximately 570 nm. Determine the frequency of this light and the total energy of the photon being emitted in units of J and kJ/mol. 2. When an electron beam strikes a block of copper, x-rays with a frequency of 2.0 x 1018 Hz are emitted. How much energy is ...
0321813545_07_final
... very little energy. Gamma rays and X‐rays are much more likely to damage cells and tissue. Radiation may have a negative connotation, but radiation keeps the Earth warm enough to inhabit, and ionizing radiation from gamma rays and X‐rays can be useful. The analogy with water waves can go too ...
... very little energy. Gamma rays and X‐rays are much more likely to damage cells and tissue. Radiation may have a negative connotation, but radiation keeps the Earth warm enough to inhabit, and ionizing radiation from gamma rays and X‐rays can be useful. The analogy with water waves can go too ...
Study Guide For Final Exam
... Shows mass is a property of energy Energy – Momentum relationship: ...
... Shows mass is a property of energy Energy – Momentum relationship: ...
O Strong-Arming Electron Spin Dynamics
... spin, let alone demonstrate control of quantum coherence. In this talk I will describe recent progress in the field, focusing on two new methods for single spin control that have been developed by my group at Princeton. The first method is based on quantum interference and implements spin-interferom ...
... spin, let alone demonstrate control of quantum coherence. In this talk I will describe recent progress in the field, focusing on two new methods for single spin control that have been developed by my group at Princeton. The first method is based on quantum interference and implements spin-interferom ...
CH107 Special Topics
... Aftermath – the Successes and Failures of Bohr’s Model • For the first time, Bohr was able to give a theoretical explanation of the stability of the Rutherford H atom, and of the line spectra of hydrogen and other single electron species (e.g. He+, Li2+, etc). • However, Bohr’s theory failed totall ...
... Aftermath – the Successes and Failures of Bohr’s Model • For the first time, Bohr was able to give a theoretical explanation of the stability of the Rutherford H atom, and of the line spectra of hydrogen and other single electron species (e.g. He+, Li2+, etc). • However, Bohr’s theory failed totall ...
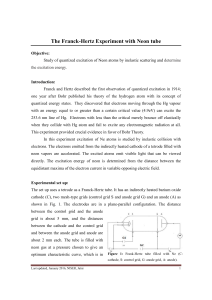
The Franck-Hertz Experiment with Neon tube
... one year after Bohr published his theory of the hydrogen atom with its concept of ...
... one year after Bohr published his theory of the hydrogen atom with its concept of ...
Unit B review - mvhs
... In general, as one moves across a row of the periodic table from the alkali metals to the halogens: (A) A, B, and C will decrease. (B) A, B, and C will increase. (C) A will increase, B and C will decrease. (D) A and B will increase, C will decrease. (E) A will decrease, B and C will increase. 15. In ...
... In general, as one moves across a row of the periodic table from the alkali metals to the halogens: (A) A, B, and C will decrease. (B) A, B, and C will increase. (C) A will increase, B and C will decrease. (D) A and B will increase, C will decrease. (E) A will decrease, B and C will increase. 15. In ...
Name: Date: Period: Who is the Father of Atomic Theory? What
... 13. Circle the correct answers. “The mass of the products (always / sometimes / never) equals the mass of the reactants. This statement summarizes the Law of Conservation of (mass / energy).” 14. How are the following terms related: mole, formula mass, and atomic mass units? ...
... 13. Circle the correct answers. “The mass of the products (always / sometimes / never) equals the mass of the reactants. This statement summarizes the Law of Conservation of (mass / energy).” 14. How are the following terms related: mole, formula mass, and atomic mass units? ...
Units 1-6
... I can calculate conversions of all types (metric prefix, energy, temperature, density, etc.) including set up, sig figs and units I understand that energy takes different forms, and I can classify energy as potential (chemical, positional, gravitational), kinetic (including temperature) or radiant. ...
... I can calculate conversions of all types (metric prefix, energy, temperature, density, etc.) including set up, sig figs and units I understand that energy takes different forms, and I can classify energy as potential (chemical, positional, gravitational), kinetic (including temperature) or radiant. ...
Name - Net Start Class
... 29. If one variable increases while the other variable decreases, what type of relationship is it? Sketch a graph of this relationship. An inversely proportional relationship ...
... 29. If one variable increases while the other variable decreases, what type of relationship is it? Sketch a graph of this relationship. An inversely proportional relationship ...
Lecture Notes V: Spin, Pauli Exclusion Principle, Symmetric
... other source of magnetic dipole: With field on, atoms will deflect in “all” directions. (The “funny” shape is due to the magnet geometry.) ...
... other source of magnetic dipole: With field on, atoms will deflect in “all” directions. (The “funny” shape is due to the magnet geometry.) ...
ψ 2
... The nucleus is the very dense region consisting of nucleons (protons and neutrons) at the center of an atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in t ...
... The nucleus is the very dense region consisting of nucleons (protons and neutrons) at the center of an atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in t ...
prereq reading
... The Schrödinger equation can only be completely solved for the hydrogen atom, or isoelectronic ions, with just one electron. Approximation methods must be used for multielectron atoms and polyatomic molecules. ...
... The Schrödinger equation can only be completely solved for the hydrogen atom, or isoelectronic ions, with just one electron. Approximation methods must be used for multielectron atoms and polyatomic molecules. ...
Solutions - Dynamic Science
... 7) An atom has 10 electrons, 10 neutrons and 12 protons. Which comment is true? a) The atom has a charge of +2. b) The atom is neutral. c) The atom has a charge of -2. d) The atom has an atomic number 10. ...
... 7) An atom has 10 electrons, 10 neutrons and 12 protons. Which comment is true? a) The atom has a charge of +2. b) The atom is neutral. c) The atom has a charge of -2. d) The atom has an atomic number 10. ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.