Miss Pang`s 2012 Review
... 16. The atomic number of magnesium is 12. How many protons and valence electrons respectively does magnesium have? A) 12 and 12 ...
... 16. The atomic number of magnesium is 12. How many protons and valence electrons respectively does magnesium have? A) 12 and 12 ...
electron configuration
... A few terms to define to understand this more fully… • Valence shell: outermost EL that is occupied by ein the electron cloud • Valence shell electrons: an e- that is available to be lost, gained, or shared in the outer EL – These electrons are of primary concern because they are the electrons most ...
... A few terms to define to understand this more fully… • Valence shell: outermost EL that is occupied by ein the electron cloud • Valence shell electrons: an e- that is available to be lost, gained, or shared in the outer EL – These electrons are of primary concern because they are the electrons most ...
... the nature of cathode rays was the same irrespective of the metal used for the cathode or the gas filled in the cathode ray tube. This led Thomson to conclude that all atoms must contain electrons. This meant that the atom is not indivisible as was believed by Dalton and others. In other words, we c ...
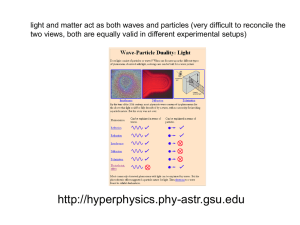
lecture 5 radiation and matter
... Assume for now that we are talking only about how our probe (laser) and sample interact. In CSLM, all of the above occur and can be important. Think about how each would effect a focused laser as it probes the sample. In CSLM, we normally image in fluorescence mode The following are important consid ...
... Assume for now that we are talking only about how our probe (laser) and sample interact. In CSLM, all of the above occur and can be important. Think about how each would effect a focused laser as it probes the sample. In CSLM, we normally image in fluorescence mode The following are important consid ...
AP Biology chap 2 HW - yhs
... may change shape when covalent bonds are formed. d. never contain more than 1 electron each. e. more than one of the preceding is correct. 8. Which of the following bonds and properties are correctly matched? a. ionic bonds--are strong only if the participating ions are hydrated b. hydrogen bonds--a ...
... may change shape when covalent bonds are formed. d. never contain more than 1 electron each. e. more than one of the preceding is correct. 8. Which of the following bonds and properties are correctly matched? a. ionic bonds--are strong only if the participating ions are hydrated b. hydrogen bonds--a ...
Review for Exam 1
... So far only considered S one-electron atomic orbitals p orbitals are different p has nonzero angular momentum L = l(l+1)½ h/ l is equal to______ for electron occupying orbitals in p subshell. All orbitals with l > 0 have zero amplitude at the nucleus so no probability of finding electron there. Fact ...
... So far only considered S one-electron atomic orbitals p orbitals are different p has nonzero angular momentum L = l(l+1)½ h/ l is equal to______ for electron occupying orbitals in p subshell. All orbitals with l > 0 have zero amplitude at the nucleus so no probability of finding electron there. Fact ...
Sample Exam 3
... 17. You may have noticed that a bound electron (q = −e, m = me ) orbiting a proton (q = +e, m = mp ) in the Bohr model atom obeys the following relation: 2 KEn = –PEn . (a) If an excited electron orbits a proton at a distance of 1.9044 nm, what is the potential energy of this electron in eV? (b) Wha ...
... 17. You may have noticed that a bound electron (q = −e, m = me ) orbiting a proton (q = +e, m = mp ) in the Bohr model atom obeys the following relation: 2 KEn = –PEn . (a) If an excited electron orbits a proton at a distance of 1.9044 nm, what is the potential energy of this electron in eV? (b) Wha ...
Unit 1 – Physical Science and Chemical Reactions
... This work led one of Rutherford’s students (H. G. Mosely) to discover that the positive charge inside the nucleus increased by one from element to element in Mendeleev’s periodic table. From this he coined the phrase atomic number (the number of protons inside the nucleus of an atom). This disco ...
... This work led one of Rutherford’s students (H. G. Mosely) to discover that the positive charge inside the nucleus increased by one from element to element in Mendeleev’s periodic table. From this he coined the phrase atomic number (the number of protons inside the nucleus of an atom). This disco ...
atomicspectra1-2
... • The multiplicity of the L term is equal to 2S + 1 = 2s + 1 = 2.: doublet : two levels, with J = L ± 1/2, respectively • The Coulomb interaction between the nucleus and the single electron is dominant, so that the largest energy separations are associated with levels having different n • hyperfine ...
... • The multiplicity of the L term is equal to 2S + 1 = 2s + 1 = 2.: doublet : two levels, with J = L ± 1/2, respectively • The Coulomb interaction between the nucleus and the single electron is dominant, so that the largest energy separations are associated with levels having different n • hyperfine ...
Document
... This is like the classical rule but using the quantum numbers rather than the angular momentum vector. The total angular momentum quantum number j takes values between the sum and difference of the corresponding quantum numbers for l and s in integer steps. For each j, there are 2j+1 possible values ...
... This is like the classical rule but using the quantum numbers rather than the angular momentum vector. The total angular momentum quantum number j takes values between the sum and difference of the corresponding quantum numbers for l and s in integer steps. For each j, there are 2j+1 possible values ...
atoms-chemical
... • While all atoms of a given element have the same number of protons (atomic number), they may differ in the number of neutrons and atomic mass. • Two atoms of the same element that differ in the number of neutrons are called isotopes. • For example, 99% of carbon atoms have 6 neutrons (12C). 1% of ...
... • While all atoms of a given element have the same number of protons (atomic number), they may differ in the number of neutrons and atomic mass. • Two atoms of the same element that differ in the number of neutrons are called isotopes. • For example, 99% of carbon atoms have 6 neutrons (12C). 1% of ...
CHAPTER 1 Practice Exercises 1.1 12.3 g Cd 1.3 26.9814 u 1.5
... A reactant is a chemical species which is transformed in a chemical reaction A chemical reaction is a process whereby one or more chemical species is/are transformed into different chemical species. This generally involves the making and/or breaking of chemical bonds. A product is the species formed ...
... A reactant is a chemical species which is transformed in a chemical reaction A chemical reaction is a process whereby one or more chemical species is/are transformed into different chemical species. This generally involves the making and/or breaking of chemical bonds. A product is the species formed ...
UNIT 1 WORKSHEET 1. Name three methods for the separation of
... 7.69 g Z. The second sample was 35.9% A and 64.1% Z. It was observed that 0.718 g A reacted with Z to form 2.00 g of the third sample. Show that these data illustrate the law of definite composition. ...
... 7.69 g Z. The second sample was 35.9% A and 64.1% Z. It was observed that 0.718 g A reacted with Z to form 2.00 g of the third sample. Show that these data illustrate the law of definite composition. ...
Chemistry Comes Alive: Part A
... • Can be separated physically, such as by straining or filtering • Heterogeneous or homogeneous ...
... • Can be separated physically, such as by straining or filtering • Heterogeneous or homogeneous ...
Name - cloudfront.net
... What were the two major conclusions drawn from Rutherford’s Gold Foil experiment? 1. The nucleus is small, dense and positively charged. 2. Most of an atom’s volume is empty space. ...
... What were the two major conclusions drawn from Rutherford’s Gold Foil experiment? 1. The nucleus is small, dense and positively charged. 2. Most of an atom’s volume is empty space. ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.