collective states of 2d electron-hole system under the influence of
... Institute of Applied Physics of the Academy of Sciences of Moldova Key words: magnetoexciton, Bose-Einstein condensation, electron-hole liquid This study is concerned with a two-dimensional (2D) electron–hole system in an ideal symmetric 2D layer in a strong perpendicular magnetic field with special ...
... Institute of Applied Physics of the Academy of Sciences of Moldova Key words: magnetoexciton, Bose-Einstein condensation, electron-hole liquid This study is concerned with a two-dimensional (2D) electron–hole system in an ideal symmetric 2D layer in a strong perpendicular magnetic field with special ...
Measuring Planck`s Constant Using Light Emitting Diodes - IFSC-USP
... The results yielded should give an accurate value for Planck’s constant. This method, depending on the results, can then be used in an entry level physics lab, such as that of a high school physics lab. ...
... The results yielded should give an accurate value for Planck’s constant. This method, depending on the results, can then be used in an entry level physics lab, such as that of a high school physics lab. ...
Examination 3 Multiple Choice Questions
... iii) Why were the results so "shocking?" -Particles were fired at a thin sheet of Gold foil. The deflection of the particles was then measured. The deflection results, which were unexcepted because they thought the particles would simply plow right through the low density material, indicated the at ...
... iii) Why were the results so "shocking?" -Particles were fired at a thin sheet of Gold foil. The deflection of the particles was then measured. The deflection results, which were unexcepted because they thought the particles would simply plow right through the low density material, indicated the at ...
Part 3 - MGNet
... Using a diffusion scaling in the full quantum hydrodynamic equations, similar as in the full hydrodynamic model, the so-called quantum energy-transport model: ...
... Using a diffusion scaling in the full quantum hydrodynamic equations, similar as in the full hydrodynamic model, the so-called quantum energy-transport model: ...
Problems
... the conduction electrons in the metal. Here is our mode of attack: when the light wave experiences a mismatch in the refractive index, part of it is transmitted, and part reflected. So we will ask Maxwell’s equations to give us the reflection coefficient when a light beam is incident from air on a m ...
... the conduction electrons in the metal. Here is our mode of attack: when the light wave experiences a mismatch in the refractive index, part of it is transmitted, and part reflected. So we will ask Maxwell’s equations to give us the reflection coefficient when a light beam is incident from air on a m ...
Covalent Bonds - WordPress.com
... subatomic particles An element’s atomic number is the number of protons It is written as a subscript before the symbol for elements For example: 2He means that an atom of helium has 2 protons in nucleus An element’s mass number is the sum of protons plus neutrons in the nucleus (it is written as a s ...
... subatomic particles An element’s atomic number is the number of protons It is written as a subscript before the symbol for elements For example: 2He means that an atom of helium has 2 protons in nucleus An element’s mass number is the sum of protons plus neutrons in the nucleus (it is written as a s ...
hydrogen
... HYDROGEN ATOM AND HYDROGEN-LIKE IONS Hydrogen is the simplest of all the atoms with only one electron surrounding the nucleus. Ions such as He+ and Li2+ are hydrogen-like since they also have only a single electron. In each case the mass of the electron is much less the nuclear mass, therefore, we w ...
... HYDROGEN ATOM AND HYDROGEN-LIKE IONS Hydrogen is the simplest of all the atoms with only one electron surrounding the nucleus. Ions such as He+ and Li2+ are hydrogen-like since they also have only a single electron. In each case the mass of the electron is much less the nuclear mass, therefore, we w ...
Electronic Structure
... If an atom absorb energy, its outermost electrons are promoted form the ground state into higher energy level. If sufficient energy is supplied, an excited electron may reach the energy level of infinity. This electron is completely detached from the nucleus and the atom becomes an ion on losing thi ...
... If an atom absorb energy, its outermost electrons are promoted form the ground state into higher energy level. If sufficient energy is supplied, an excited electron may reach the energy level of infinity. This electron is completely detached from the nucleus and the atom becomes an ion on losing thi ...
Quantum (wave) mechanics
... Hydrogen atom The Rutherford-Bohr model of the atom described the electron orbiting around the nucleus in circular orbits. This is a planetary model like the planets orbiting the Sun. However, in terms of Quantum Mechanics the electron has to be regarded as a wave so that experimental observations a ...
... Hydrogen atom The Rutherford-Bohr model of the atom described the electron orbiting around the nucleus in circular orbits. This is a planetary model like the planets orbiting the Sun. However, in terms of Quantum Mechanics the electron has to be regarded as a wave so that experimental observations a ...
chapter 2 - Scranton Prep Biology
... Energy: Ability to do work potential energ/ = Energy that matter storesbecauseof its position or location. ...
... Energy: Ability to do work potential energ/ = Energy that matter storesbecauseof its position or location. ...
Franck-Hertz Experiment – Quantized Energy Levels in Atoms
... reduction in the expected current means that some of the electron’s kinetic energy was used to “pump” neon atoms into excited states. The observation of discrete current peaks is the signature for verifying discrete energy states within the neon atom. I. ...
... reduction in the expected current means that some of the electron’s kinetic energy was used to “pump” neon atoms into excited states. The observation of discrete current peaks is the signature for verifying discrete energy states within the neon atom. I. ...
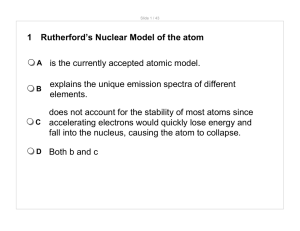
1 Rutherford`s Nuclear Model of the atom A is the currently accepted
... In the quantum-mechanical model of the atom, which of the following is NOT one of the four quantum numbers needed to specify the probable location of an electron? ...
... In the quantum-mechanical model of the atom, which of the following is NOT one of the four quantum numbers needed to specify the probable location of an electron? ...
The Schrödinger equation in 3-D
... emission is proportional to Z – 1, where Z is the atomic number of the atom (see Figure 41.24 below). Larger Z means a higher frequency and more energetic emitted x-ray photons. This is consistent with our model of multielectron atoms. Bombarding an atom with a high-energy electron can knock an atom ...
... emission is proportional to Z – 1, where Z is the atomic number of the atom (see Figure 41.24 below). Larger Z means a higher frequency and more energetic emitted x-ray photons. This is consistent with our model of multielectron atoms. Bombarding an atom with a high-energy electron can knock an atom ...
Bonding in Atoms
... • Modeled by the Lewis Dot Diagram • Gain of electrons = anion • Loss of electrons = cations ...
... • Modeled by the Lewis Dot Diagram • Gain of electrons = anion • Loss of electrons = cations ...
Atomic Structure and Periodicity
... lines correspond to discrete wavelengths of specific (quantized) energy. Only certain energies are allowed for the electron in the hydrogen atom. The hydrogen emission spectrum is called a line spectrum. ...
... lines correspond to discrete wavelengths of specific (quantized) energy. Only certain energies are allowed for the electron in the hydrogen atom. The hydrogen emission spectrum is called a line spectrum. ...
Chap8_theatom
... An Atomic Electron Has 4 in All In the quantum theory of the atom, an electron has NO FIXED ORBIT but is free to move about in three dimensions. Think of the electron as circulating in a probability cloud that forms a certain pattern in space. ...
... An Atomic Electron Has 4 in All In the quantum theory of the atom, an electron has NO FIXED ORBIT but is free to move about in three dimensions. Think of the electron as circulating in a probability cloud that forms a certain pattern in space. ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.