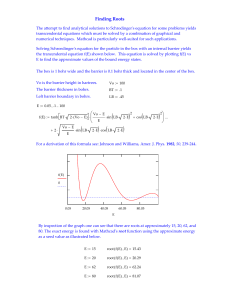
key concepts of matter
... electrons. Key Concept 3: Protons and neutrons are located in the nucleus of an atom and determine the mass of an atom. Key Concept 4: Protons have a positive electrical charge and electrons have a negative electrical charge. Atoms that have the same number of protons and electrons do not have an el ...
... electrons. Key Concept 3: Protons and neutrons are located in the nucleus of an atom and determine the mass of an atom. Key Concept 4: Protons have a positive electrical charge and electrons have a negative electrical charge. Atoms that have the same number of protons and electrons do not have an el ...
Lecture IV : Feb 8, 2016 Learning from Two Hole Experiment (A
... It turns out that it is IMPOSSIBLE to design any experiment where we can observe which hole the electron goes and not to destroy the interference ...
... It turns out that it is IMPOSSIBLE to design any experiment where we can observe which hole the electron goes and not to destroy the interference ...
Chapter 2 Molecular Mechanics
... which are due to the behavior of the electrons and nuclei • Electrons are too small and too light to be described by classical mechanics and need to be described by quantum mechanics • Accurate potential energy surfaces for molecules can be calculated using modern electronic structure methods ...
... which are due to the behavior of the electrons and nuclei • Electrons are too small and too light to be described by classical mechanics and need to be described by quantum mechanics • Accurate potential energy surfaces for molecules can be calculated using modern electronic structure methods ...
First of all, do you know any methods to check
... (1) Required: The same instrumental settings, e.g. resolution, e-beam energy, for both the determination sensitivity factors and sample analysis. (2) Needed: The same peak shapes for all peaks; Reduce effect of the peak shape using high energy peaks. Alternatively, different sensitivity factor for d ...
... (1) Required: The same instrumental settings, e.g. resolution, e-beam energy, for both the determination sensitivity factors and sample analysis. (2) Needed: The same peak shapes for all peaks; Reduce effect of the peak shape using high energy peaks. Alternatively, different sensitivity factor for d ...
File first semester final study guide key
... Their presence is what allows us to calculate the ___average atomic mass____ which is our final number on the periodic table. Short Answer: Using a brief statement, answer each of the following questions about atomic structure. 1. John Dalton’s atomic theory states that, “atoms of a given element ar ...
... Their presence is what allows us to calculate the ___average atomic mass____ which is our final number on the periodic table. Short Answer: Using a brief statement, answer each of the following questions about atomic structure. 1. John Dalton’s atomic theory states that, “atoms of a given element ar ...
Chapter 2: Matter
... nucleus of an atoms is made of _________ &___________. The _______________ of an atom are in constant motion around the nucleus. ...
... nucleus of an atoms is made of _________ &___________. The _______________ of an atom are in constant motion around the nucleus. ...
1.5.16(Chem) - mrcarlsonschemistryclass
... Cations and Anions • Cations are ions with a POSITIVE charge. • Anions are ions with a NEGATIVE charge. • Draw the funny way to remember cations and anions: ...
... Cations and Anions • Cations are ions with a POSITIVE charge. • Anions are ions with a NEGATIVE charge. • Draw the funny way to remember cations and anions: ...
Atoms and Molecules in Mirce Mechanics Approach to Reliability
... energy to remove an outer electron from one of these elements than from any other element in the periodic table. The strong reducing ability of these elements is readily accounted for. The variation in the relative reducing power of the elements across a given period or within a given group is deter ...
... energy to remove an outer electron from one of these elements than from any other element in the periodic table. The strong reducing ability of these elements is readily accounted for. The variation in the relative reducing power of the elements across a given period or within a given group is deter ...
Lecture 4
... approach and obtained Curie’s Law for a paramagnet. • We saw that quantization of angular momentum and spin will be important components of a quantum theory of paramagnetism. ...
... approach and obtained Curie’s Law for a paramagnet. • We saw that quantization of angular momentum and spin will be important components of a quantum theory of paramagnetism. ...
Wave-particle_duality
... (ii) State and explain what would happen to this pressure if the light is reflected rather than absorbed by the surface. ...
... (ii) State and explain what would happen to this pressure if the light is reflected rather than absorbed by the surface. ...
General Chemistry
... There is a mix of attractive and repulsive dipole-dipole forces as the molecules tumble If two molecules have about the same mass and size, then dipole-dipole forces increase with increasing polarity. 3- London Dispersion Forces • Weakest of all intermolecular forces. • It is possible for two adjace ...
... There is a mix of attractive and repulsive dipole-dipole forces as the molecules tumble If two molecules have about the same mass and size, then dipole-dipole forces increase with increasing polarity. 3- London Dispersion Forces • Weakest of all intermolecular forces. • It is possible for two adjace ...
Document
... No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: • Electrons do not pile up in the lowest energy state. It’ ...
... No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: • Electrons do not pile up in the lowest energy state. It’ ...
(TEQ) Model of the Electron - Superluminal quantum models of the
... • is a helically moving point-like quantum object having a frequency and a wavelength, and carrying energy and momentum. • can easily pass through the speed of light (being massless). • can generate a photon or an electron depending on whether the energy quantum’s helical trajectory is open or close ...
... • is a helically moving point-like quantum object having a frequency and a wavelength, and carrying energy and momentum. • can easily pass through the speed of light (being massless). • can generate a photon or an electron depending on whether the energy quantum’s helical trajectory is open or close ...
The Weird World of Quantum Information
... Example: spin and measurements In 1922, O. Stern and W. Gerlach conducted experiment to measure the magnetic dipole moments of atoms. The results of these experiments could not be explained by classical mechanics. First, let's discuss why would atom poses a magnetic moment. Even in Bohr's model of t ...
... Example: spin and measurements In 1922, O. Stern and W. Gerlach conducted experiment to measure the magnetic dipole moments of atoms. The results of these experiments could not be explained by classical mechanics. First, let's discuss why would atom poses a magnetic moment. Even in Bohr's model of t ...
che-20028 QC lecture 2 - Rob Jackson`s Website
... The electron diffraction experiment - 1 • What happens if you ‘fire’ a beam of electrons at a crystal surface? • This experiment was first performed in 1925 by Davisson and Germer, who used a nickel metal surface, and observed that the electrons were diffracted by the surface like light is when it ...
... The electron diffraction experiment - 1 • What happens if you ‘fire’ a beam of electrons at a crystal surface? • This experiment was first performed in 1925 by Davisson and Germer, who used a nickel metal surface, and observed that the electrons were diffracted by the surface like light is when it ...
Notes on Atomic Structure 1. Introduction 2. Hydrogen Atoms and
... The sum of the energies of the two emitted photons is E2s−E1s = 10.2 eV. The photons have a continuous spectrum since there is no other constraint on their energies. This is a major contri ...
... The sum of the energies of the two emitted photons is E2s−E1s = 10.2 eV. The photons have a continuous spectrum since there is no other constraint on their energies. This is a major contri ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.