Honors Chemistry Exam Review Questions
... C For the results of an experiment to be accepted, the experiment must produce the same results no matter how many times it is repeated. D The scientific process is repeated until a hypothesis either fits all the observed experimental facts or is discarded. 8. What is the volume of the diagram to th ...
... C For the results of an experiment to be accepted, the experiment must produce the same results no matter how many times it is repeated. D The scientific process is repeated until a hypothesis either fits all the observed experimental facts or is discarded. 8. What is the volume of the diagram to th ...
Assignment 10 - Duke Physics
... (of the form (2S+1) LJ ). In the final column of your table give the degeneracy of each term. Here, L ~l1 + ~l2 + ~l3 , with an analogous definition for S. ~ J~ = L ~ + S. ~ This table represents all the possibilities were the particles distinguishable. Some of the entries will be repeated and it is ...
... (of the form (2S+1) LJ ). In the final column of your table give the degeneracy of each term. Here, L ~l1 + ~l2 + ~l3 , with an analogous definition for S. ~ J~ = L ~ + S. ~ This table represents all the possibilities were the particles distinguishable. Some of the entries will be repeated and it is ...
Quantum physics I
... •von Neumann Chain- the wave function collapse can be located anywhere on the chain from quon gun to observer’s consciousness, but not eliminated ...
... •von Neumann Chain- the wave function collapse can be located anywhere on the chain from quon gun to observer’s consciousness, but not eliminated ...
Worksheet on Ionic and Atomic Size Trends
... 12. The sodium ion is smaller than the atom, because when sodium loses its valence electron to form an ion, it also loses its 3rd energy level. 13. The chlorine ion is larger than the chlorine atom, because adding an additional electron to the 3 rd energy level causes the energy level to expand beca ...
... 12. The sodium ion is smaller than the atom, because when sodium loses its valence electron to form an ion, it also loses its 3rd energy level. 13. The chlorine ion is larger than the chlorine atom, because adding an additional electron to the 3 rd energy level causes the energy level to expand beca ...
Test - Chemical Bonding- Practice Test
... Match each item with the correct statement below. NOTE: Each item may be used once, more than once, or not at all. A. B. C. D. E. F. ...
... Match each item with the correct statement below. NOTE: Each item may be used once, more than once, or not at all. A. B. C. D. E. F. ...
N 2
... For a normal population of atoms, there will always be more atoms in the lower energy levels than in the upper ones. Since the probability for an individual atom to absorb a photon is the same as the probability for an excited atom to emit a photon via stimulated emission, the collection of real ato ...
... For a normal population of atoms, there will always be more atoms in the lower energy levels than in the upper ones. Since the probability for an individual atom to absorb a photon is the same as the probability for an excited atom to emit a photon via stimulated emission, the collection of real ato ...
Biochemistry I (CHE 418 / 5418)
... • Second Messenger in cellular signaling. – Nitric oxide is a powerful vasodilator. • Regulates blood pressure, controls muscles that dilate arteries and blood vessels. • Nitric oxide is produced when nitroglycerin is placed under the ...
... • Second Messenger in cellular signaling. – Nitric oxide is a powerful vasodilator. • Regulates blood pressure, controls muscles that dilate arteries and blood vessels. • Nitric oxide is produced when nitroglycerin is placed under the ...
Lecture 2
... atoms are composed of positively charged nuclei surrounded by a cloud of “orbiting” electrons. But, – Orbiting (or accelerating charge radiates energy electrons should spiral into nucleus all of matter should be unstable!) – Spectroscopy results of H (Rydberg states) indicated that energy of an ...
... atoms are composed of positively charged nuclei surrounded by a cloud of “orbiting” electrons. But, – Orbiting (or accelerating charge radiates energy electrons should spiral into nucleus all of matter should be unstable!) – Spectroscopy results of H (Rydberg states) indicated that energy of an ...
Lectures in Physics, summer 2008/09 3
... those treated previously (e.g. rectangular box). The potential energy U(r) results from Coulomb interaction between a single electron and proton in nucleus. U(r) ...
... those treated previously (e.g. rectangular box). The potential energy U(r) results from Coulomb interaction between a single electron and proton in nucleus. U(r) ...
Practice Bypass Answers
... a) Is aluminum chloride an ionic or covalent compound? How does the [ionic or covalent, depending on what you selected] bond form? It is an ionic compound. Aluminum is a metal that has three valence electrons and low attraction for electrons. In order for it to achieve stable octet it needs to lose ...
... a) Is aluminum chloride an ionic or covalent compound? How does the [ionic or covalent, depending on what you selected] bond form? It is an ionic compound. Aluminum is a metal that has three valence electrons and low attraction for electrons. In order for it to achieve stable octet it needs to lose ...
The format of this test is MULTIPLE CHOICE
... content in each unit covered and allow you practice with the content from this semester. It is not intended to address any specific test question on the final exam. Completion of this packet does not guarantee success on the Final Exam, but practicing with the content is a great idea. Unit 1: Scient ...
... content in each unit covered and allow you practice with the content from this semester. It is not intended to address any specific test question on the final exam. Completion of this packet does not guarantee success on the Final Exam, but practicing with the content is a great idea. Unit 1: Scient ...
Chapter 1 - Atoms: The Quantum World
... diffraction, constructive and destructive interference, duality of electromagnetic radiation and matter, linear momentum, de Broglie relation, Heisenberg uncertainty principle (complementarity of location and momentum), trajectories, wavefunction, Born interpretation, node of the wavefunction, proba ...
... diffraction, constructive and destructive interference, duality of electromagnetic radiation and matter, linear momentum, de Broglie relation, Heisenberg uncertainty principle (complementarity of location and momentum), trajectories, wavefunction, Born interpretation, node of the wavefunction, proba ...
Using the Franck-Hertz Experiment To Illustrate Quantization
... figure 3displays a partial encrgy-level diagram for neon ...
... figure 3displays a partial encrgy-level diagram for neon ...
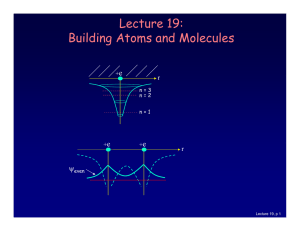
Lecture 19: Building Atoms and Molecules
... No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: • Electrons do not pile up in the lowest energy state. It’ ...
... No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: • Electrons do not pile up in the lowest energy state. It’ ...
The format of this test is MULTIPLE CHOICE
... content in each unit covered and allow you practice with the content from this semester. It is not intended to address any specific test question on the final exam. Completion of this packet does not guarantee success on the Final Exam, but practicing with the content is a great idea. Unit 1: Scient ...
... content in each unit covered and allow you practice with the content from this semester. It is not intended to address any specific test question on the final exam. Completion of this packet does not guarantee success on the Final Exam, but practicing with the content is a great idea. Unit 1: Scient ...
ELECTRONIC STRUCTURE OF THE ATOM
... The spin quantum number led to the PAULI'S EXCLUSION PRINCIPLE. In a given atom, no two electrons can have the same set of four quantum numbers. This implies that an orbital can accommodate a maximum of two electrons. The electron configuration of an atom describes the arrangements of electrons in t ...
... The spin quantum number led to the PAULI'S EXCLUSION PRINCIPLE. In a given atom, no two electrons can have the same set of four quantum numbers. This implies that an orbital can accommodate a maximum of two electrons. The electron configuration of an atom describes the arrangements of electrons in t ...
Chapter 9. Electrons in magnetic fields
... cyclotron frequency ωc. Indeed, there should be a “zero point energy” correspond to the smallest possible orbital with n=0. i,e, More correctly, ...
... cyclotron frequency ωc. Indeed, there should be a “zero point energy” correspond to the smallest possible orbital with n=0. i,e, More correctly, ...
Elements, mixtures and compounds lecture
... • Matter is made of atoms which can be arranged in almost infinite ways to make the world as we know it (brick houses?) • Atoms are on the order of a trillionth of a meter in size: 10-10 meters • Atoms are constantly moving in irregular-jerky like motion called Brownian motion (model?) • So, though ...
... • Matter is made of atoms which can be arranged in almost infinite ways to make the world as we know it (brick houses?) • Atoms are on the order of a trillionth of a meter in size: 10-10 meters • Atoms are constantly moving in irregular-jerky like motion called Brownian motion (model?) • So, though ...
Scanning Electron Microscopy / Electron Probe X
... incident electron that transfers energy to an electron of the sample. This excited electron then leaves the sample with a very small kinetic energy. Due to this low energy, only SE’s that are created near the surface can exit the sample and can be detected. Any variation in topography of the surface ...
... incident electron that transfers energy to an electron of the sample. This excited electron then leaves the sample with a very small kinetic energy. Due to this low energy, only SE’s that are created near the surface can exit the sample and can be detected. Any variation in topography of the surface ...
PHY492: Nuclear & Particle Physics Lecture 4 Nature of the nuclear force
... Nuclear mass and binding energy The mass of a bound system is always less than the mass of its component parts. For example, the mass of the hydrogen atom is 13.5 eV/c2 less than proton mass plus electron mass. When the hydrogen atom is formed, 13.5 eV is released in photons. mH c 2 − m p + me c 2 ...
... Nuclear mass and binding energy The mass of a bound system is always less than the mass of its component parts. For example, the mass of the hydrogen atom is 13.5 eV/c2 less than proton mass plus electron mass. When the hydrogen atom is formed, 13.5 eV is released in photons. mH c 2 − m p + me c 2 ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.