Quantum mechanics is the theory that we use to describe the
... light packets, or photons, also come in integer multiples of h, with here being the frequency of the light. A paradigm shift was beginning. In 1913 Neils Bohr was able to explain the observed spectral lines of a hydrogen atom by quantising the atom. He postulated that atoms could only exists in s ...
... light packets, or photons, also come in integer multiples of h, with here being the frequency of the light. A paradigm shift was beginning. In 1913 Neils Bohr was able to explain the observed spectral lines of a hydrogen atom by quantising the atom. He postulated that atoms could only exists in s ...
Quantum physics
... velocities) the Newtonian description of mechanics breaks down and the relativistic treatment discovered by Einstein must be used. Now, we will see that the description of light entirely in terms of waves breaks down at very small scales. In addition, we will see that objects that have mass, whi ...
... velocities) the Newtonian description of mechanics breaks down and the relativistic treatment discovered by Einstein must be used. Now, we will see that the description of light entirely in terms of waves breaks down at very small scales. In addition, we will see that objects that have mass, whi ...
Quantum Mechanics
... arbitrary accuracy momentum (p) and position (x) of a particle cannot be known exactly at the same time ...
... arbitrary accuracy momentum (p) and position (x) of a particle cannot be known exactly at the same time ...
1. INTRODUCTION (increasing the number of accessible PCs that could be
... for numerically solving the time-dependent Schrödinger equation for an atom in a laser field [1]. These calculations make use of the single-active-electron approximation (SAE) [2], which means that the atom is modeled as a single electron moving in a potential well describing the nuclear attraction ...
... for numerically solving the time-dependent Schrödinger equation for an atom in a laser field [1]. These calculations make use of the single-active-electron approximation (SAE) [2], which means that the atom is modeled as a single electron moving in a potential well describing the nuclear attraction ...
The stability of an atom depends on the ratio and number of protons
... conversion. During this process, the radionuclide is said to undergo radioactive decay. Radioactive decay results in the emission of gamma rays and/or subatomic particles such as alpha or beta particles, as shown in . These emissions constituteionizing radiation. Radionuclides occur naturally but ca ...
... conversion. During this process, the radionuclide is said to undergo radioactive decay. Radioactive decay results in the emission of gamma rays and/or subatomic particles such as alpha or beta particles, as shown in . These emissions constituteionizing radiation. Radionuclides occur naturally but ca ...
Chapter 8
... *The effective nuclear charge also increases down a group. The increase in the n quantum number is more important. ...
... *The effective nuclear charge also increases down a group. The increase in the n quantum number is more important. ...
Quantum Mechanics-Atomic, molecular, and optical physics
... result, he believed that electrons revolved around the proton.Niels Bohr, in 1913, combined the Rutherford model of the atom with the quantisation ideas of Planck. Only specific and well-defined orbits of the electron could exist, which also do not radiate light. In jumping orbit the electron would ...
... result, he believed that electrons revolved around the proton.Niels Bohr, in 1913, combined the Rutherford model of the atom with the quantisation ideas of Planck. Only specific and well-defined orbits of the electron could exist, which also do not radiate light. In jumping orbit the electron would ...
Atomic 1
... number l should be 1/2, but we know that l can only be an integer. Clearly a problem here. What is going on? in the early 1920’sW. Pauli was the first to suggest that a fourth quantum number, ( in addition to n,l,mL) assigned to the electron was needed to solve this problem. In 1925 S. Goudsmit and ...
... number l should be 1/2, but we know that l can only be an integer. Clearly a problem here. What is going on? in the early 1920’sW. Pauli was the first to suggest that a fourth quantum number, ( in addition to n,l,mL) assigned to the electron was needed to solve this problem. In 1925 S. Goudsmit and ...
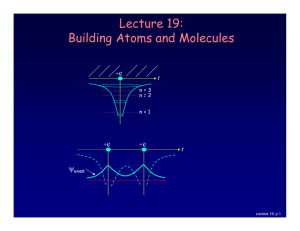
Lecture 19: Building Atoms and Molecules
... No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: • Electrons do not pile up in the lowest energy state. It’ ...
... No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that each atomic orbital (n,l,ml) can hold 2 electrons: ms = ±½. Important consequence: • Electrons do not pile up in the lowest energy state. It’ ...
Free electron theory of Metals Introduction The electrons in
... 3/2Ru while the actual values are about 3Ru and 0.015Ru. 3. Electrical conductivity of semiconductor or insulators couldn’t be explained using this model. ...
... 3/2Ru while the actual values are about 3Ru and 0.015Ru. 3. Electrical conductivity of semiconductor or insulators couldn’t be explained using this model. ...
Chemistry 106: General Chemistry
... (25) In molecular orbital theory, the σ1s orbital is _________ and the σ*1s orbital is__________ in the H2 molecule. (a) filled, filled (b) filled, empty (c) filled, half-filled (d) half-filled, filled (e) empty, filled (26) The ground state electronic configuration of fluorine is (a) (b) (c) (d) (e ...
... (25) In molecular orbital theory, the σ1s orbital is _________ and the σ*1s orbital is__________ in the H2 molecule. (a) filled, filled (b) filled, empty (c) filled, half-filled (d) half-filled, filled (e) empty, filled (26) The ground state electronic configuration of fluorine is (a) (b) (c) (d) (e ...
Introduction to Atomic Physics Lab Report
... interact with each other. The inner electrons (with low n and l values) are ’screening’ the positive nucleus from the outer electrons, which are thereby looser bound to the atom. The higher n and l the electrons have, the farther away the electron is from the nucleus and the larger will this screeni ...
... interact with each other. The inner electrons (with low n and l values) are ’screening’ the positive nucleus from the outer electrons, which are thereby looser bound to the atom. The higher n and l the electrons have, the farther away the electron is from the nucleus and the larger will this screeni ...
Chemistry of Living Things Outline
... Negatively charged particles, called ______________ (-1) revolve around the nucleus at different distances from the nucleus. The electrons move in paths called shells or ____________________. Atoms have the ________ number of electrons and protons. Therefore, they are electrically ____________ ...
... Negatively charged particles, called ______________ (-1) revolve around the nucleus at different distances from the nucleus. The electrons move in paths called shells or ____________________. Atoms have the ________ number of electrons and protons. Therefore, they are electrically ____________ ...
2. Chemistry of Living Things Outline
... Negatively charged particles, called ______________ (-1) revolve around the nucleus at different distances from the nucleus. The electrons move in paths called shells or ____________________. Atoms have the ________ number of electrons and protons. Therefore, they are electrically ____________ (have ...
... Negatively charged particles, called ______________ (-1) revolve around the nucleus at different distances from the nucleus. The electrons move in paths called shells or ____________________. Atoms have the ________ number of electrons and protons. Therefore, they are electrically ____________ (have ...
atomic number
... Rutherford proposed that the nucleus had a particle that had the same amount of charge as an electron but opposite sign - based on measurements of the nuclear charge of the elements These particles are called protons - protons have a charge of +1 and a mass of 1 amu Since protons and electrons have ...
... Rutherford proposed that the nucleus had a particle that had the same amount of charge as an electron but opposite sign - based on measurements of the nuclear charge of the elements These particles are called protons - protons have a charge of +1 and a mass of 1 amu Since protons and electrons have ...
The concepts of an atom and chemical bond in physics and chemistry
... and it is worth to take a closer look at the picture emerging from this physical theory. In quantum mechanics, the atom is treated as a system of interacting particles – positively charged nucleus surrounded by negatively charged electrons. As a result, isolated atoms are electrically neutral. The s ...
... and it is worth to take a closer look at the picture emerging from this physical theory. In quantum mechanics, the atom is treated as a system of interacting particles – positively charged nucleus surrounded by negatively charged electrons. As a result, isolated atoms are electrically neutral. The s ...
Relativistic theory of one– and two electron systems: valley of
... by Sommerfeld (in the framework of the elliptical orbits model) and after in the year 1926 by use of the relativistic wave equation established by Dirac after the discovery of the spin electron (1925) by Uhlenberg and Goudsmith. However, it would be very interesting to make relativistic correction o ...
... by Sommerfeld (in the framework of the elliptical orbits model) and after in the year 1926 by use of the relativistic wave equation established by Dirac after the discovery of the spin electron (1925) by Uhlenberg and Goudsmith. However, it would be very interesting to make relativistic correction o ...
Exam Review - hrsbstaff.ednet.ns.ca
... Which of the following statements is not a part of Dalton's atomic theory? a) Atoms of the same element may differ in mass. b) All atoms of one element differ from the atoms of every other element. c) Chemical change is the union or separation of atoms. d) Atoms combine in small whole number ratios. ...
... Which of the following statements is not a part of Dalton's atomic theory? a) Atoms of the same element may differ in mass. b) All atoms of one element differ from the atoms of every other element. c) Chemical change is the union or separation of atoms. d) Atoms combine in small whole number ratios. ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.