chapter 7 part 2
... this is the equation for the wave function of the hydrogen atom, it entirely describes how the electron moves in the electrostatic potential of the proton, (just as Newton’s second law described how classical objects move) ...
... this is the equation for the wave function of the hydrogen atom, it entirely describes how the electron moves in the electrostatic potential of the proton, (just as Newton’s second law described how classical objects move) ...
II. Units of Measurement
... to just how precisely we can know both the position and velocity of a particle at a given time. ...
... to just how precisely we can know both the position and velocity of a particle at a given time. ...
Quantum Theory of Hydrogen
... There are “zillions” of web sites about the hydrogen atom. Every physics teacher who does anything relating to modern physics and puts class material on the web seems to do the hydrogen atom. A search using Google for “hydrogen atom schrodinger equation” (but don’t put the words in quotes when you ...
... There are “zillions” of web sites about the hydrogen atom. Every physics teacher who does anything relating to modern physics and puts class material on the web seems to do the hydrogen atom. A search using Google for “hydrogen atom schrodinger equation” (but don’t put the words in quotes when you ...
Hydrogen Atom Simulator – Exercises
... fraction of atoms in the Hydrogen exist at a certain energy level. This next section will explore what conditions one might expect to have if a “red” cloud of Hydrogen gas is observed in space. ...
... fraction of atoms in the Hydrogen exist at a certain energy level. This next section will explore what conditions one might expect to have if a “red” cloud of Hydrogen gas is observed in space. ...
Copyright The McGraw-Hill Companies, Inc
... The uncertainty ("x) is given as ±1% (0.01) of 6x106 m/s. Once we calculate this, plug it into the uncertainty equation. ...
... The uncertainty ("x) is given as ±1% (0.01) of 6x106 m/s. Once we calculate this, plug it into the uncertainty equation. ...
Chapter7 - FSU Chemistry
... The atom then emits two photons: one of wavelength 1281 nm to reach an intermediate level, and a second to return to the ground state. (a) What level did the electron reach? (b) What intermediate level did the electron reach? (c) What was the wavelength of the second photon emitted? Bohr and Schrödi ...
... The atom then emits two photons: one of wavelength 1281 nm to reach an intermediate level, and a second to return to the ground state. (a) What level did the electron reach? (b) What intermediate level did the electron reach? (c) What was the wavelength of the second photon emitted? Bohr and Schrödi ...
Handout
... made of more than one particle is entangled if the state is not a trivial product state: | + +i is not entangled, as each particle is spin up regardless of the other. The state | + −i + | − +i is entangled, as if we measure the first electron to be spin up the second one must then be spin down, and ...
... made of more than one particle is entangled if the state is not a trivial product state: | + +i is not entangled, as each particle is spin up regardless of the other. The state | + −i + | − +i is entangled, as if we measure the first electron to be spin up the second one must then be spin down, and ...
Atomic structure ls on a periodic table.
... Atomic structure ls on a periodic table. -metals, and noble gases are found on a periodic table. Specify how to predict the charge expected for the most stable ion of an atom. the line spectra of atomic species relate to the idea of quantized states of electrons in atoms. A Jablonksi diagram may be ...
... Atomic structure ls on a periodic table. -metals, and noble gases are found on a periodic table. Specify how to predict the charge expected for the most stable ion of an atom. the line spectra of atomic species relate to the idea of quantized states of electrons in atoms. A Jablonksi diagram may be ...
Chem 150 Problem Set Introductory Quantum Chemistry 1
... a) According to the Bohr model an electron in the ground state of a hydrogen atom orbits the nucleus at a specific radius of 0.53 x 10-10 m. In the quantum mechanical description of the hydrogen atom, the most probable distance of the electron from the nucleus is 0.53 x 10 -10 m. Why are these two s ...
... a) According to the Bohr model an electron in the ground state of a hydrogen atom orbits the nucleus at a specific radius of 0.53 x 10-10 m. In the quantum mechanical description of the hydrogen atom, the most probable distance of the electron from the nucleus is 0.53 x 10 -10 m. Why are these two s ...
do physics online from quanta to quarks the bohr model of the atom
... electron in “allowed” circular stable orbit such that the electron’s angular momentum was quantised. The electron in a stable orbit did not lose energy by the emission of electromagnetic radiation. Bohr assumed that classical electromagnetic theory was not completely valid for atomic systems. ...
... electron in “allowed” circular stable orbit such that the electron’s angular momentum was quantised. The electron in a stable orbit did not lose energy by the emission of electromagnetic radiation. Bohr assumed that classical electromagnetic theory was not completely valid for atomic systems. ...
4.1 Schr¨ odinger Equation in Spherical Coordinates ~
... rigid body rotation: ‘spin’ for rotation about its center of mass; ‘orbital’ for rotation of its center of mass about another axis. The same two words are used in quantum mechanical systems, but they do not refer to similar types of motion. Experiments have shown that the behavior of electrons in ma ...
... rigid body rotation: ‘spin’ for rotation about its center of mass; ‘orbital’ for rotation of its center of mass about another axis. The same two words are used in quantum mechanical systems, but they do not refer to similar types of motion. Experiments have shown that the behavior of electrons in ma ...
+l.
... •This quantum number distinguishes orbitals of a given n and l , that is, of a given energy and shape but having different orientations. •The magnetic quantum number depends on the value of l and can have any integer value from –l to 0 to +l. Each different value represents a different orbital. For ...
... •This quantum number distinguishes orbitals of a given n and l , that is, of a given energy and shape but having different orientations. •The magnetic quantum number depends on the value of l and can have any integer value from –l to 0 to +l. Each different value represents a different orbital. For ...
Electronic Structure of Atoms (i.e., Quantum Mechanics)
... possible wavelengths of EM; below 700 K, very little visible EM is produced; above 700 K visible E is produced starting at red, orange, yellow, and white before ending up at blue as the temperature increases ◦ discovery that light intensity (energy emitted per unit of time) is proportional to T4; ho ...
... possible wavelengths of EM; below 700 K, very little visible EM is produced; above 700 K visible E is produced starting at red, orange, yellow, and white before ending up at blue as the temperature increases ◦ discovery that light intensity (energy emitted per unit of time) is proportional to T4; ho ...
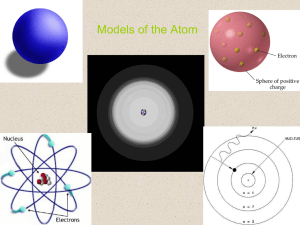
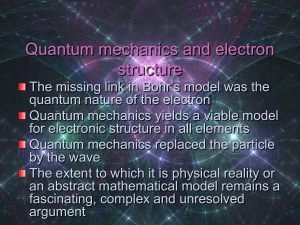
Quantum mechanics and electron structure
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstract mathematical model remains a fascinat ...
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for electronic structure in all elements Quantum mechanics replaced the particle by the wave The extent to which it is physical reality or an abstract mathematical model remains a fascinat ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).