* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introduction to Macromolecular Structures
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Gene expression wikipedia , lookup
Bottromycin wikipedia , lookup
Expanded genetic code wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Protein moonlighting wikipedia , lookup
Genetic code wikipedia , lookup
Biosynthesis wikipedia , lookup
Western blot wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein domain wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein folding wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein adsorption wikipedia , lookup
X-ray crystallography wikipedia , lookup
Homology modeling wikipedia , lookup
Biochemistry wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Introduction to
Macromolecular Structures
Zhi-Jie Liu
Institute of Biophysics
Chinese Academy of Sciences
Outline
1. Varieties of macromolecules
2. Macromolecular structures
3. Structure determination by X-ray crystallography
4. Structure validation and deposition.
Varieties of macromolecules
1. Proteins
2. DNA
3. RNA
4. Complexes: protein-protein, protein-DNA/RNA
Lipids, peptides, sugars, etc are categorized as
non macromolecules
Our discussion is more focused on protein
molecules
DNA/RNA
Deoxyribonucleic acid, DNA:
consists of two long polymers of simple
units called nucleotides, Cytosine,
Guanine, Adenine and Thymine.
The sequence of these four bases
along the backbone encodes
information, or the genetic code.
RNA has the same nucleotides except
that Thymine is replaced by Uracil.
Genetic code
A series of codons in part of a
mRNA molecule. Each codon
consists of three nucleotides,
usually representing a single
amino acid.
Macromolecular structures
Proteins
Composed of one or more polypeptides which is a single
linear polymer chain of amino acids. The sequence of amino
acids in a protein is defined by the sequence of a gene, which
is encoded in the genetic code.
Proteins are the molecular
building block of life. Protein
molecules are three-dimensional,
so is life.
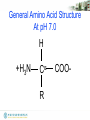
General Amino Acid Structure
At pH 7.0
H
+H3N
α
C
R
COO-
General Amino Acid Structure
Chirality of amino acids
The "CORN" rule for determining the D/L isomeric form of an
amino acid :
COOH, R, NH2 and H (where R is a variant carbon chain)
are arranged around the chiral center C atom. Starting with
the hydrogen atom away from the viewer, if these groups are
arranged clockwise around the carbon atom, then it is the Dform. If counter-clockwise, it is the L-form.
L
D
Varieties of amino acids
tending to avoid water, nonpolar and uncharged, relatively
insoluble in water. Side chains tend to associate with each
other to minimize their contact with water or polar side
chains.
Protein Structure & Function, ©2004 New Science Press Ltd
Varieties of amino acids
Interact with water, polar or charged,very soluble in water.
side chains tend to associate with other hydrophilic side
chains, or with water molecules, usually by means of
hydrogen bonds.
Protein Structure & Function, ©2004 New Science Press Ltd
Varieties of amino acids
having both polar and nonpolar character
and therefore a tendency to form interfaces between
hydrophobic and hydrophilic molecules.
Protein Structure & Function, ©2004 New Science Press Ltd
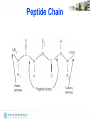
Peptide Chain
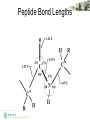
Peptide Bond Lengths
Protein Conformation
Framework
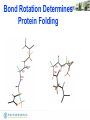
• Bond rotation determines protein
folding, 3D structure
• Double bond disallows rotation
Bond Rotation Determines
Protein Folding
Protein Conformation
Framework
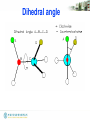
• Torsion angle (dihedral angle)
– Measures orientation of four linked
atoms in a molecule: A, B, C, D
Dihedral angle
Protein Conformation
Framework
• Torsion angle (dihedral angle)
– Measures orientation of four linked
atoms in a molecule: A, B, C, D
– ԎABCD defined as the angle between
the normal to the plane of atoms A-BC and normal to the plane of atoms BC-D
– Three repeating torsion angles along
protein backbone: ω, φ, ψ
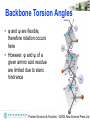
Backbone Torsion Angles
Backbone Torsion Angles
• Dihedral angle ω : rotation about the peptide
bond, namely Cα1-{C-N}- Cα2
Backbone Torsion Angles
• Dihedral angle φ : rotation about the bond
between N and Cα
Backbone Torsion Angles
• Dihedral angle ψ : rotation about the bond
between Cα and the carbonyl carbon
Backbone Torsion Angles
• ω angle tends to be planar (0º - cis, or 180 º trans) due to delocalization of carbonyl π
electrons and nitrogen lone pair
Backbone Torsion Angles
• φ and ψ are flexible,
therefore rotation occurs
here
• However, φ and ψ of a
given amino acid residue
are limited due to steric
hindrance
Protein Structure & Function, ©2004 New Science Press Ltd
Steric Hindrance
• Interference to rotation caused by spatial
arrangement of atoms within molecule
• Atoms cannot overlap
• Atom size defined by van der Waals radii
• Electron clouds repel each other
G.N. Ramachandran
• Used computer models of small polypeptides to
systematically vary φ and ψ with the objective of finding
stable conformations
• For each conformation, the structure was examined for
close contacts between atoms
• Atoms were treated as hard spheres with dimensions
corresponding to their van der Waals radii
• Therefore, φ and ψ angles which cause spheres to
collide correspond to sterically disallowed conformations
of the polypeptide backbone
• Only 10% of the {φ, ψ} combinations are generally
observed for proteins
• First noticed by G.N. Ramachandran
Ramachandran Plot
• Plot of φ vs. ψ
• The computed angles which are
sterically allowed fall on certain
regions of plot
Computed Ramachandran Plot
White = sterically disallowed
conformations (atoms come
closer than sum of van der
Waals radii)
Blue = sterically allowed
conformations
Experimental
Ramachandran Plot
φ, ψ distribution in 42 high-resolution protein
structures (x-ray crystallography)
Ramachandran Plot
And Secondary Structure
• Repeating values of φ and ψ along the chain
result in regular structure
• For example, repeating values of φ ~ -57°
and ψ ~ -47° give a right-handed helical fold
(the alpha-helix)
The structure of cytochrome C shows many segments of
helix and the Ramachandran plot shows a tight grouping of
φ, ψ angles near -50,-50
alpha-helix
cytochrome C
Ramachandran plot
Similarly, repetitive values in the region of φ = -110 to
–140 and ψ = +110 to +135 give beta sheets. The
structure of plastocyanin is composed mostly of beta
sheets; the Ramachandran plot shows values in the
–110, +130 region:
beta-sheet
plastocyanin
Ramachandran plot
φ, ψ and Secondary Structure
Name
φ
ψ
Structure
------------------- ------- ------- --------------------------------alpha-L
57
47
left-handed alpha helix
3-10 Helix
-49 -26
right-handed.
π helix
-57 -80
right-handed.
Type II helices -79 150
left-handed helices
formed by polyglycine
and polyproline.
Collagen
-51 153 right-handed coil formed
of three left handed
helicies.
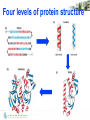
Four levels of protein structure
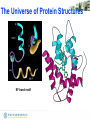
The Universe of Protein Structures
How many proteins in the universe?
The smallest archaea genome encodes above 600 ORFs
Pyrococcus furiosus encodes 2200 ORFs
Homo sapiens encodes around 30,000 ORFS
The facts:
The number of protein folds is large but limited. the number of different
protein folds in nature is limited. They are used
repeatedly in different combinations to create the diversity of proteins
found in living organisms.
The Universe of Protein Structures
Protein structures are
modular and proteins can
be grouped into
families on the basis of the
domains they contain
There are around 1000 different protein folds
The Universe of Protein Structures
Protein motifs may be defined by their primary sequence or by the
arrangement of secondary structure elements
Zinc finger motif
The Universe of Protein Structures
EF-hand motif
Protein Function in Cell
1. Enzymes
•
Catalyze biological reactions
2. Structural role
•
•
•
Cell wall
Cell membrane
Cytoplasm
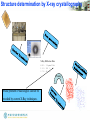
Structure determination by X-ray crystallography
X-Ray Diffraction Data
H K L
I SgimaI Phi
2 5 9
101
5
3 7 8
49
4
…
Phase problem: Phase angles can not be
recoded by current X-Ray techniques.
Phasing
Crystal mounting and
Cryo-Crystallography
X-ray sources: Rotation anode X-rays
Crystal mounting and
Cryo-Crystallography
X-ray sources: synchrotron X-rays, 106 times stronger.
Shanghai Synchrotron Radiation Facility
Crystal mounting and
Cryo-Crystallography
Data Collection:
Crystal mounting and
Cryo-Crystallography
Data Collection:
Crystal mounting and
Cryo-Crystallography
Advantages:
1. Lack of radiation damage thus increased crystal lifetime
2. Lower X-ray background and increased resolution
3. Fewer crystals required
4. Transport and ship in LN2
5. Mount when crystals are ready.
Crystal mounting and
Cryo-Crystallography
Crystal mounting and
Cryo-Crystallography
Mounting:
Crystal mounting and
Cryo-Crystallography
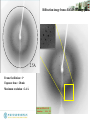
Robotic crystal diffraction quality screen
Crystal mounting robot
Data collection strategy
and data processing
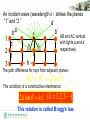
Bragg’s law
Lawrence,
Henry
In 1913, William Henry Bragg (1862–1942) and his son,
William Lawrence Bragg (1890–1971), derived a formula to
explain the diffraction of
X-ray by crystals.
They won the Nobel Prize
in physics for their
seminal roles in X-ray
Crystallography.
An incident wave (wavelength λ)strikes the planes
“1” and “2 ”
a’
1
2
3
b
a
b’
A
d
B
d
C
D
AB and AC vertical
with lights a and a’
respectively.
h
The path difference for rays from adjacent planes:
BD DC 2d sin
The condition of a constructive interference:
2d sin k (k 1.2.3)
This relation is called Bragg’s law.
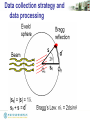
Data collection strategy and
data processing
Data collection strategy and
data processing
Diffraction image from a RAXIS-IV image plate
2.5A
Frame Oscillation = 1o
Exposure time = 20 min
Maximum resolution = 2.4 Å
Data collection strategy and
data processing
Data collection strategy and
data processing
Data collection strategy and
data processing
Data processing:
Indexing (finding the unit cell, orientation &
space group)
Integrating (determining the intensities of
each spot)
Merging (scaling data, averaging data &
determining data quality)
Calculating structure factor amplitudes from
merged intensities
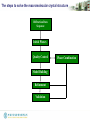
The steps to solve the macromolecular crystal structure
Diffraction Data
Sequence
Initial Phases
Quality Control
Model Building
Refinement
Validation
Phase Combination
Phasing Methods in Macromolecular
Crystallography
Molecular Replacement Method (MR)
Isomorphs Replacement Method (MIR, SIR)
Anomalous Dispersion Method (MAD, SAD, SIRAS)
Direct Method
Other Methods
Phasing Methods in Macromolecular
Crystallography
The phasing problem
The phase ambiguity in SIR
|FP(h)|
|Fp(h)|
|FPH(h)|
FH(h)
Phasing Methods in Macromolecular
Crystallography
How to break the phase ambiguity?
MIR
Fourier Transformation and Electron Density Maps
Fourier Transformation
r (x, y, z) 1 F (h, k, l ) exp[- 2pi (hx ky lz) i a (h, k, l )]
V
h
k
l
X-Ray diffraction
Experiment
Phasing method
0.5
sigmma
0.33
sigmma
A
B
1.0
sigmma
C
Fig. 1 Effect of chainging countor level on the electron density map. In (A) a section of aldehyde
dehydrogenase[2] density at 3.0Å resolution is shown using the 0.33 sigmma for the
minimium countor level. The solvent is very noisy and the difference between protein and
solvent is not obvious. In (B) the minimium countor level is increased to 0.5 sigmma. The
solvent is less noisy and the protein and solvent is distinguishable. In (C) the minimium
countor level is increased to 1.0 sigmma. The solvent is very clean and it is very easy to
identify the protein boundry.
FIG. 2 Effect of increasing phase
error on the electron density
map.
A: Density map at 2.0 Å resolution
is shown using the final refined
phases
B: An average of 22˚ of random error
has been added to each phase.
C: An average of 45˚ of random error
has been added to each phase.
D: An average of 67˚ of random
error has been added to each
phase.
(“Practical protein crystallography”
by D E Mcree, Page 190)
3. A good map should show clear secondary
structures ( helixes or b-sheet).
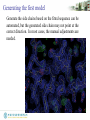
Model Building:
Steps in making the first trace in electron
density map
(1). Generating Ca chain trace.
The only rule one has to observe is that the distance between Ca
atoms of adjacent residues is always approximately 3.8 Å. Try to
look for large pieces of secondary structure, such as helices and
sheets, to start the Ca trace.
(2). Identifying chain direction
The side chains on a
helix point to the
nitrogen-terminal
end. Another way to
put it: the a-helix
resembles a
Christmas tree, when
viewed with the Nterminal end down,
and the C-terminal
end up.
(3). Generating main chain trace
Main chain can be automatically generated
from a well traced Ca chain by many
computer programs. In helices, the side
chain positions are so highly constrained
that you can accurately predict the main
chain and Cb atom positions with a refined
a-helix from another protein.
Example of generated α-helix and β-sheet
in electron density map
(4). Fitting the chemical sequence.
Finding the first match of sequence to the
map is a milestone in structure
determination. Some tips are listed below:
Heavy atoms bind to some specific residues.
Hg-Cys, Pt-Met
Start the fitting from a well defined main
chain trace where the density should be
clear and rich in side chain information.
These regions are often located inside the
molecule.
The sulfur or Se-methionines are the perfect
starting point for the sequence fitting if the
map is from sulfur SAS or Se-MAD phases.
Tryptophan is so much larger than all the
other amino acids it can often be recognized.
Hydrophilic side chains are often disordered.
A correct fitting should be easily extended in
both directions.
Representative electron density for amino acid
side chains arranged in order of increasing size.
From an experimental electron density map calculated at 1.5 Angstrom resolution.
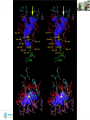
Generating the first model
Generate the side chains based on the fitted sequence can be
automated, but the generated side chain may not point at the
correct direction. In most cases, the manual adjustments are
needed.
Structure Validation and Deposition
Generate symmetry related molecules. The
atoms at the contacts cannot come any closer than
Van der Waals packing distance.
Structure Validation and Deposition
The side chains should fit the electron density map
all over the whole molecule. If the fitting suddenly
becomes bad in some region, it may indicate that
something wrong with the fitting.
Missing density is much better than extra density.
It’s rarely seen that there is a blob of extra density
for Gly, Ala or Pro residue.
The model should make chemical sense and satisfy
all that is known about the macromolecule.
Structure Validation and Deposition
It may be useful to evaluate the overall
distribution of some residues, such as
hydrophobic residues, glycine, and proline.
If certain residues have been identified as
being in the active site, are they close together
in the model?
Structure Validation and Deposition
The stereochemical parameters such as bond
length, bond angle etc, should within the standard
deviation from their ideal values.
The Ramachandran Plot should be normal.
http://molprobity.biochem.duke.edu/
Structure Validation and Deposition
Atomic coordinates should be deposited to
Protein Data Bank
http://www.pdb.org
谢谢!