* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Peptide Bonds
Implicit solvation wikipedia , lookup
Structural alignment wikipedia , lookup
Homology modeling wikipedia , lookup
Protein purification wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein folding wikipedia , lookup
Protein domain wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Circular dichroism wikipedia , lookup
List of types of proteins wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Alpha helix wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
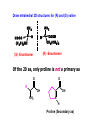
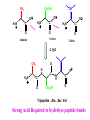
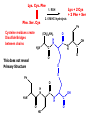
Biological Chemistry First Year Organic Chemistry Lecture Eleven : Amino Acids, Peptides and Proteins Convener : Dr. Fawaz Aldabbagh R H2 N CH CO2H All DNA encoded aa are CHO All are chiral, except Glycine R=H H OH CHO HO CH2OH CH2OH D- All DNA encoded aa are usually L- H LR CHO = HO CH2OH H (S) - Glyceraldehyde (-) - = H2N C CO2H H (L) - Amino Acids (-) - Draw tetrahedral 3D structures for (R) and (S) valine NH2 NH2 C HOOC (H 3C) 2HC H H C COOH CH9(CH 3 )2 (R) -Enantiomer (S) -Enantiomer Of the 20 aa, only proline is not a primary aa O O R OH NH2 OH N H Proline (Secondary aa) aa are high melting point solids! Why? Answer = aa are ionic compounds under normal conditions LOW pH NEUTRAL C O O O R HIGH pH R C OH NH3 ammonium Form R C O NH3 Zwitterion O NH2 Carboxylate Form Isoelectric Point = concentration of zwitterion is at a maximum and the concentration of cations and anions is equal r aa with basic R-groups, we require higher pHs, and for aa with acidic R-groups, we require lower pHs to reach the Isoelectric Point aa are covalently linked by amide bonds (Peptide Bonds) The resulting molecules are called Peptides & Proteins R' R' N C O R N C R O Features of a Peptide Bond; 1. Usually inert 2. Planar to allow delocalisation 3. Restricted Rotation about the amide bond 4. Rotation of Groups (R and R’) attached to the amide bond is relatively free aa that are part of a peptide or protein are referred to as residues. Peptides are made up of about 50 residues, and do not possess a well-defined 3D-structure Proteins are larger molecules that usually contain at least 50 residues, and sometimes 1000. The most important feature of proteins is that they possess well-defined 3D-structure. Primary Structure is the order (or sequence) of amino acid residues Peptides are always written and named with the amino terminus on the left and the carboxy terminus on the right CH2OH CH3 O O H3 N CH H3 N C O C H3 N C O O O Serine Alanine Valine - 2 H2 O CH3 O H N H3 N C C O CH2OH O N H C O Tripeptide : Ala . Ser. Val Strong Acid Required to hydrolyse peptide bonds Lys. Cys. Phe 1. RSH Phe. Ser. Cys 2. 6 M HCl hydrolysis Lys + 2 Cys + 2 Phe + Ser Ph Cysteine residues create Disulfide Bridges between chains (CH2)4NH2 O H N H2 N C C OH N H O O This does not reveal Primary Structure S S Ph O H N H2 N C OH N H O C O HO C REVERSIBLE DENATURING Oxidation RS SR RS H Reduction Prof. Linus Pauling Dr. Frederick Sanger, Prof. R. B. Merrifield Nobel Prize for Chemistry Nobel Prize for Chemistry 1984 1958 and 1980 Automated Peptide Synthesis Peptide sequencing Secondary Structure The Development of Regular patterns of Hydrogen Bonding, which result in distinct folding patterns -helix -pleated sheets Tertiary Structure This is the 3D structure resulting from further regular folding of the polypeptide chains using H-bonding, Van der Waals, disulfide bonds and electrostatic forces – Often detected by X-ray crystallographic methods Globular Proteins – “Spherical Shape” , include Insulin, Hemoglobin, Enzymes, Antibodies ---polar hydrophilic groups are aimed outwards towards water, whereas non-polar “greasy” hydrophobic hydrocarbon portions cluster inside the molecule, so protecting them from the hostile aqueous environment ----- Soluble Proteins Fibrous Proteins – “Long thin fibres” , include Hair, wool, skin, nails – less folded ----- e.g. keratin - the -helix strands are wound into a “superhelix”. The superhelix makes one complete turn for each 35 turns of the -helix. In globular proteins this tertiary structure or macromolecular shape determines biological properties Bays or pockets in proteins are called Active Sites Enzymes are Stereospecific and possess Geometric Specificity The range of compounds that an enzyme excepts varies from a particular functional group to a specific compound Emil Fischer formulated the lock-and-key mechanism for enzymes All reactions which occur in living cells are mediated by enzymes and are catalysed by 106-108 Some enzymes may require the presence of a Cofactor. This may be a metal atom, which is essential for its redox activity. Others may require the presence of an organic molecule, such as NAD+, called a Coenzyme. If the Cofactor is permanently bound to the enzyme, it is called a Prosthetic Group. For a protein composed of a single polypeptide molecule, tertiary structure is the highest level of structure that is attained Myoglobin and hemoglobin were the first proteins to be successfully subjected to completely successful X-rays analysis by J. C. Kendrew and Max Perutz (Nobel Prize for Chemistry 1962) Quaternary Structure When multiple sub-units are held together in aggregates by Van der Waals and electrostatic forces (not covalent bonds) Hemoglobin is tetrameric myglobin For example, Hemoglobin has four heme units, the protein globin surrounds the heme – Takes the shape of a giant tetrahedron – Two identical and globins. The and chains are very similar but distinguishable in both primary structure and folding