* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download functional group
Survey
Document related concepts
Vectors in gene therapy wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Peptide synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Signal transduction wikipedia , lookup
Proteolysis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
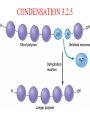
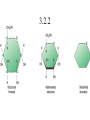
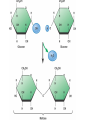
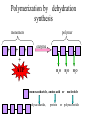
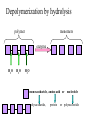
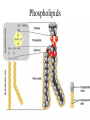
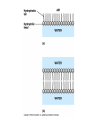
TOPIC 3.2 MOLECULES OF CELLS ORGANIC and INORGANIC MOLECULES 3.2.1 ORGANIC MOLECULES: molecules containing C and H in their structure INORGANIC MOLECULES : molecules which do not have C in its structure. A few chemical groups are key to the functioning of biological molecules • An organic compound has unique properties that depend upon the – size and shape of the molecule and – groups of atoms (functional groups) attached to it. • A functional group affects a biological molecule’s function in a characteristic way. • Compounds containing functional groups are hydrophilic (water-loving). © 2012 Pearson Education, Inc. A few chemical groups are key to the functioning of biological molecules • The functional groups are – hydroxyl group—consists of a hydrogen bonded to an oxygen, – carbonyl group—a carbon linked by a double bond to an oxygen atom, – carboxyl group—consists of a carbon doublebonded to both an oxygen and a hydroxyl group, – amino group—composed of a nitrogen bonded to two hydrogen atoms and the carbon skeleton, and – phosphate group—consists of a phosphorus atom bonded to four oxygen atoms. © 2012 Pearson Education, Inc. Table 3.2 A few chemical groups are key to the functioning of biological molecules • An example of similar compounds that differ only in functional groups is sex hormones. – Male and female sex hormones differ only in functional groups. – The differences cause varied molecular actions. – The result is distinguishable features of males and females. © 2012 Pearson Education, Inc. ORGANIC MOLECULES 3.2.2 Monomer: smallest unit of an organic molecules e.g: glucose Dimer: two monomers Polymer: many monomers are linked to each other to form macromolecules. How are they linked to each other? condensation How are they broken down? hydrolysis CONDENSATION 3.2.5 HYDROLYSIS 3.2.5 3.2.2 CARBOHYDRATES 3.2.3 FUNCTIONS: 1- main energy source for cells 2- structural elements STRUCTURE: consist of C, H,O Monosaccharide, Disaccharides, Polysaccharides 3.2.3. Monosaccharides Hexoses (6C sugars) Glucose Fructose Galactose Pentoses (5C sugars) Ribose Deoxyribose They are soluble in water. Why? 3.2.3. Disaccharides Glucose + Glucose Fructose + Glucose Galactose + Glucose Maltose Sucrose Lactose They are less soluble than monosaccharides. Polysaccharides 3.2.4 Starch Glucose + Glucose + Glucose +..... Glycogen Cellulose Chitin is a N containing polysaccharide which is found in the exoskeleton of insects and cell wall of fungi. They are not soluble or slightly soluble. 3.2.4 Polymerization by dehydration synthesis monomers + + polymer + enzyme + ATP H2O monosaccharide, amino acid or polysaccharide, protein H2O H2O nucleotide or polynucleotide Polymerization 1. Happens by dehydration synthesis 2. Monomers are joined by covalent bonds to form polymers 3 different covalent bonds can be formed: Glycosidic bond between monosaccharides Peptide bond between amino acids Phosphodiester bonds between nucleotides Polymerization 4. Enzymes are used 5. ATP is required 6. 1 H2O molecule is released for each bond made between monomers Depolymerization by hydrolysis polymer monomers enzyme H2O H2O + + H2 O monosaccharide, amino acid or polysaccharide, protein nucleotide or polynucleotide + Depolymerization 1. Happens by hydrolysis 2. Polymers are broken down into monemers by adding a water. 3. Energy is not used 4. Enzymes are required. Example: digestion LIPIDS 3.2.6 • Do all living things have lipids? • Which elements do lipids contain? • Are lipids hydrophilic? What are the functions of lipids? 3.2.6 • Storage of energy • Structural elements (cell membrane, hormones, myelin sheat) • Thermal insulation. How? Fatty acids Essential fatty acids Saturated fats vs. Unsaturated fats • 3.2.6 • Triglyceride (oil or fat) Natural fats, found in the fat tissue of animals, seed and fruit of plants. • Phospholipids Main component of the cell membrane • Steroids – Cholesterol, sex hormones • Wax Water proof material around fruits, leaves. Triglycerides Cholesterol • Essential component of animal cell membranes • Precursor for the synthesis of vitamin D and steroid hormones such as testosterone and estrogen • Important for the metabolism of fat soluble vitamins (A, D, E, K) Phospholipids