* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download LE - 2 - Organic Molecules
Western blot wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Signal transduction wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Metalloprotein wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Point mutation wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Proteolysis wikipedia , lookup
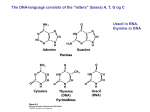
Elements in Life • To put it simply, all life contains the same basic materials. Living organisms are made up of the following elements with the following percentages: Elements in Life • How do we know if something is living? It contains the elements C and H together. We call this organic. • Inorganic molecules do NOT contain carbon and hydrogen together. Elements in Life • Water! Our bodies are 65% water, but water is inorganic • Organic molecules can be broken down into 4 main categories. These substances are needed for life to grow and function properly. Proteins Carbohydrates Lipids Nucleic Acids How Do We Get These ORGANIC Molecules? • We eat to take in these substances. – Food for building materials - proteins • to make more of us (cells) • for growth • for repair – Food to make energy - carbs • calories • to make ATP Plants don’t need to eat to get these molecules. They make them! Does anyone know HOW? Breaking Down and Building Up • How does the food turn into molecules that run and build our body? • The food gets broken down into simple units. Then those units are used or assembled in our bodies and turn into something we need! Monomers and Polymers We call the building blocks monomers: The complex units are called polymers: Breaking it Down • The food we eat gets broken down into simple units (monomers). We call this process digestion. – We can break it down physically – We can break it down chemically Hydrolysis • Literally means “water – cutting”. Water is used to break down various polymers. + = Example of Digestion ATP ATP ATP ATP ATP starch ATP glucose ATP Starch is digested to Glucose Building it Up • Once we have these monomers in our bodies, we use them for various functions. • Synthesis – building bigger molecules from smaller molecules – building cells & bodies • repair • growth • reproduction Dehydration Synthesis • Dehydration = lose water • Synthesis = put together - = Example of Synthesis amino acids protein Proteins are synthesized by bonding amino acids amino acids = building block protein = polymer Life Substances • Now we’ll be talking about these life substances in detail. Remember, all living things contain these substances. • Our bodies contain them, and the food we eat contains them too…this makes sense since the food we eat comes from living material as well. Life Substances in Detail Life Substance Protein Carbohydrate Lipid Nucleic Acid Usually ends in... Monomer Polymer Elements it Contains Indicator Chemical Structure What it Does Food Examples Examples in Our Bodies Unique Properties Monomer/Polymer • Usually ends in –in, or –ine. • Monomer – Amino Acids – 20 different kinds of amino acids • Polymers – Protein, Peptide, Polypeptide amino amino amino amino amino acid – acid – acid – acid – acid About Proteins • How many amino acids are linked together to make this protein? • Is this a dipeptide of a polypeptide? Elements/Indicator • Elements: C, H, O. N • Indicator: Biuret solution DID YOU KNOW? It’s the Nitrogen in proteins that make our urine YELLOW? The more N, the greater the color… Chemical Structure • The function of a protein depends on its SHAPE. • Proteins fold into different shapes because of certain bonds in the amino acids. • Different shape = different function! growth hormone hemoglobin pepsin It’s SHAPE that matters! • Proteins do their jobs, because of their shape • Unfolding a protein destroys its shape – wrong shape = can’t do its job – unfolding proteins = “denature” • temperature • pH (acidity) unfolded “denatured” folded What it Does • What do proteins do? – many, many functions • hormones – signals from one body system to another – insulin • movement – muscle • immune system – protect against germs • enzymes – help chemical reactions Examples in Food/Bodies Body Examples – muscle – skin, hair, fingernails, claws • collagen, keratin – pepsin • digestive enzyme in stomach – insulin • hormone that controls blood sugar levels Food Examples - meat - beans - egg - cheese Monomer/Polymer • Usually ends in –ose • Monomer = simple sugar, monosaccharide • Polymer = carbohydrate, polysaccharide sugar sugar sugar sugar sugar sugar sugar sugar About Carbohydrates • Looking at the molecules below, identify the: – Monosaccharide (glucose) – Disaccharide sugar sugar – Polysaccharide MONOSACCHARIDE sugar sugar sugar DISACCHARIDE sugar sugar POLYSACCHARIDE Elements/Indicator • Elements: C, H, O • Indicator: – Glucose – Benedicts Solution – Starch – Iodine Chemical Structure • Monosaccharides have a simple ring structure. • When they are bonded in a chain they make a polysaccharide. What it Does • Function: - quick energy • from simple sugars, like fruits - energy storage • from complex carbs, like starch - structure • cell wall in plants What it Does Examples in Food/Bodies • Body Examples • Food Examples – Glycogen – Bread – Glucose – Fruit – Cellulose (plants only) – Veggies – Ice Cream Plants need cellulose! When we eat it, we can’t digest it. Digesting Starch vs. Cellulose starch easy to digest cellulose hard to digest enzyme enzyme Cellulose • Cell walls in plants – herbivores can digest cellulose well – most carnivores cannot digest cellulose • that’s why they eat meat to get their energy & nutrients • cellulose = roughage – stays undigested – keeps material moving in your intestines Different Diets of Herbivores Cow can digest cellulose well; no need to eat other sugars Gorilla can’t digest cellulose well; must add another sugar source, like fruit to diet Helpful bacteria • How can cows digest cellulose so well? – BACTERIA live in their stomachs & help digest cellulose-rich (grass) meals Eeeew… Chewing cud? http://www.youtube.com/watch?v=_qf_r5EVP6U Monomer/Polymer • Don’t end in anything specific • Monomer = fatty acid • Polymer = Lipid Elements/Indicator • Elements: C, H, O • Indicator: Brown Paper Towel Chemical Structure • Very long chains. Made up of a “head” and “tail” • Hydrophobic “water-hating” tail • Hydrophilic “waterloving” head What it Does • Function: – energy storage • very concentrated • twice the energy as carbohydrates! – cell membrane – cushions organs – insulates body • think whale blubber! Examples in Food/Bodies • Body Examples • Food Examples - Hormones - Fats - Sex Hormones - Oils - estrogen - Waxes - testosterone Saturated fats • Most animal fats – solid at room temperature • Limit the amount in your diet – contributes to heart disease – deposits in arteries Unsaturated fats • Plant, vegetable & fish fats – liquid at room temperature • the fat molecules don’t stack tightly together • Better choice in your diet Saturated vs. Unsaturated saturated unsaturated Other Lipids in Biology • Cholesterol – good molecule in cell membranes – make hormones from it • including sex hormones – but too much cholesterol in blood may lead to heart disease Other Lipids in Biology • Cell membranes are made out of lipids – phospholipids – heads are on the outside touching water • “like” water – tails are on inside away from water • “scared” of water – forms a barrier between the cell & the outside http://www.youtube.com/watch?v=NmcLCpXVTrY Monomer/Polymer • Ends in –ine or –cil • Monomer = Nucleotide • Polymer = Nucleic Acid nucleotide – nucleotide – nucleotide – nucleotide Elements/Indicator • Elements: C, H, O, N, P, S • Indicator: Everything has DNA, but if we wanted to compare DNA we can use Gel Electrophoresis. Chemical Structure • Double strand twists into a double helix – weak bonds between nitrogen bases join the 2 strands • A pairs with T – A :: T • C pairs with G – C :: G – the two strands can separate when our cells need to make copies of it What it Does • Function: – genetic material • stores information – genes – blueprint for building proteins » DNA RNA proteins DNA • transfers information – blueprint for new cells – blueprint for next generation proteins Examples in Organisms Examples in Organisms: – DNA • DeoxyriboNucleic Acid – RNA • RiboNucleic Acid Copying DNA • Replication – copy DNA – 2 strands of DNA helix are complementary • they are matching • have one, can build other • have one, can rebuild the whole DNA Replication • Copying DNA – pairing of the bases allows each strand to serve as a pattern for a new strand Newly copied strands of DNA http://www.youtube.com/watch?v=xZaMi6OhsSU