* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Citric acid cycle wikipedia , lookup
Western blot wikipedia , lookup
Gene expression wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Peptide synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Metalloprotein wikipedia , lookup
Point mutation wikipedia , lookup
Genetic code wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Transcript
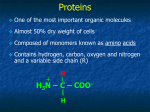
Four Major Types of Biological Macromolecules Type of Polymer Monomers making up Polymer I. Carbohydrates (Polysaccharides) Monosaccharides II. Lipids Fatty acids and glycerol III. Proteins Amino acids IV. Nucleic Acids Nucleotides Example Sugars, Starch, Cellulose Fats, steroids, cholesterol Enzymes, structural components DNA, RNA I. Carbohydrates • Made from monomers called monosaccharides (simple sugars) – Short term energy – Glucose: most common, used directly in cellular respiration to make ATP (energy) Figure 5.3 The structure and classification of some monosaccharides Disaccharides Polysaccharides: Complex Carbohydrates • 3 major types made from monomers of glucose: – Starch: energy storage in plants – Glycogen: energy storage in animals – Cellulose: structural molecules in plants Figure 5.6 Storage polysaccharides Figure 5.7a Starch and cellulose structures Figure 5.7b,c Starch and cellulose structures Figure 5.8 The arrangement of cellulose in plant cell walls II. Lipids • Not true polymers • Composed of mostly glycerol and fatty acids • Includes – Fats: energy storage – Phospholipids: membranes – Steroids: hormones, cholesterol Fats are made of one glycerol and three fatty acids Double bonds between carbons cause kinks in hydrocarbons. H2C CH2 H2C CH2 H2C CH Kink CH H2C H2C H2C CH2 CH2 Unsaturated fatty acid Double bonds, fewer H atoms Saturated fatty acid No Double bonds, maximum H atoms Figure 5.11 Examples of saturated and unsaturated fats and fatty acids Phospholipids are made of one phosphate group and 2 fatty acids Phospholipids are amphipathic Figure 5.13 Two structures formed by self-assembly of phospholipids in aqueous environments Steroids consist of a complex carbon ring structure Figure 5.14 Cholesterol, a steroid Figure 4.8 A comparison of functional groups of female (estradiol) and male (testosterone) sex hormones III. Proteins • Made from monomers called amino acids • Very different structures, very different functions H Amino group H2N C R Side chain O C Carboxyl group OH The R groups of an amino acid may be hydrophobic or hydrophilic Amino acids are joined together by a dehydration reaction H H2N C Carboxyl group O C OH Amino group H + N C CH3 H2O H2N C C C OH H H O H H O H H O N C C H CH3 Peptide bond OH Many amino acids joined together = Polypeptide chain N-terminus H C-terminus H H O H H O H H O H H O H N C N C C C C C H C CH3 N N C CH2 CH2 OH C OH H O H H O H H O H H O N C C N C C N C N C C CH2 CH H3C CH3 C CH2 CH2 SH O OH OH The sequence of amino acids in the polypeptide chain = the primary structure of a protein Hydrogen bonds between amino acids leads to the secondary structure of a protein Two common secondary structures are the -helix and pleated sheet Further folding of the polypeptide chain contributes to the tertiary structure of a protein The joining of more than one polypeptide chain leads to the quaternary structure of proteins Heat (energy) can break up the structure of a protein Table 5.1 An Overview of Protein Functions Type Role Example Enzyme s Quickens chemi cal r eact ions Over 10 0 0 ty pes o f enzyme s e xist Hormones Chemical me sseng ers Human Growt h hormone Tr ansport Move oth er molecules Hemoglobin- m ov es O 2 Cont ract ile Movement Prot ect ive Healing, d ef ense against predato rs Struct ural Mechanical supp ort Storage Stores nu t rients Ovalbumin ( egg wh it es) To xi ns Def ense, pre dat ion Snake ve no m Communicat ion Cell signaling Recept ors o n cell sur fa ce Myosin & a ct in- a llow muscle contr act ion Fibrino gen (b lood c lott ing) Anti bodies Kerat in (h air), collagen ( carti lidge) IV. Nucleic Acids • Nucleic acids (DNA and RNA) are made of monomers called nucleotides RNA Nucleot ide DNA Nucleot ide OH OH 5’ HO – P --- O –CH 2 A, G, T, or C O O P 1’ Phosphate 3' 2' OH H Sugar Nitrogenous base CH2 O A, G, U, or C O O 4’ group HO 4' Phosphate group 1' 2' 3' OH OH Sugar Nitrogenous base Figure 5.29 The components of nucleic acids Figure 3.17b 3´ 5´ DNA is a double helix. 5´ 5´ 3´ T T A G C C G T A A T A A C G G C A T T A C G C T A T G C T A G C T A Cartoon of base pairing 5´ 3´ A G C C G T T C G G A T A T A A T G C A 3´ G T 3´ 5´ Cartoon of double helix 5´ 3´ Space-filling model of double helix Nucleic acids store the information to make proteins Figure 5.30 The DNA double helix and its replication