* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Gene expression wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Protein folding wikipedia , lookup
Interactome wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein moonlighting wikipedia , lookup
Western blot wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Transcript
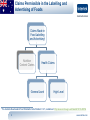
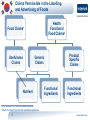
Health Claims for Whey (Protein) Products – A Global Evaluation of Current and Future Opportunities Nigel Baldwin BSc, Csci Director, Scientific and Regulatory Consulting, Europe Intertek Scientific and Regulatory Consultancy 1 www.intertek.com Agenda Claim Categories and Examples of Protein-related Claims • European Union • United States • Canada • Australia/New Zealand • China • South Korea 2 www.intertek.com Claims Permissible in the Labelling and Advertising of Foods Claims Made in Food Labelling and Advertising1 Nutrition Claims Article 13.3 Health Claims Article 13.5 Health Claims Article 14.1 Health Claims (a) Disease Risk Reduction 1 (b) Children’s Health and Development Regulation (EC) No 1924/2006 of the European parliament and of the council of 20 December 2006 on nutrition and health claims made on foods, available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:012:0003:0018:EN:PDF 3 www.intertek.com Examples of Health Claims for Protein* Claim Type Claim Wording Condition for Use of the Claim Protein contributes to a growth in muscle mass Article 13.3 Protein contributes to the maintenance of muscle mass Protein contributes to the maintenance of normal bones Article 14.1(b) Protein is needed for normal growth and development of bone in children Used only for food which is at least a source of protein (i.e., at least 12% of the energy value of the food is provided by protein) *As of September 4, 2014. From the EU Register on nutrition and health claims, available at: http://ec.europa.eu/nuhclaims/ 4 www.intertek.com Claims Permissible in the Labelling of Foods Claims Made in Food Labelling Nutrient Content Claims Structure/ Function Claims1 Authorized3 Health Claim2 Qualified4 1 Dietary Supplement Health and Education Act of 1994 (DSHEA) allows structure/function statements. General and specific requirements for health claims are laid out in 21 CFR 101.14 and 21 CFR 101 Subpart E, respectively. 3 The Nutrition Labeling and Education Act of 1990 (NLEA) introduced the use of Authorized Health Claims. 4 Due to a litigation that raised First Amendment challenges (i.e., free-speech) to the significant scientific agreement (SSA) standard, which authorized claims are held to, the U.S FDA announced in 2000 for dietary supplements and in 2002 for conventional foods to exercise its enforcement discretion with certain health claims (i.e., qualified health claims). 2 5 www.intertek.com Examples of Claims for Protein Claim Type Claim Wording Structure/Function Protein contributes to the maintenance of muscle mass. Authorized Health Claim1 25 grams of soy protein a day, as part of a diet low in saturated fat and cholesterol, may reduce the risk of heart disease. A serving of [name of food] supplies __ grams of soy protein. Qualified Health Claim2 For healthy infants who are not exclusively breastfed and who have a family history of allergy, feeding a 100% Whey-Protein Partially Hydrolyzed infant formula from birth up to 4 months of age instead of a formula containing intact cow's milk proteins may reduce the risk of developing atopic dermatitis throughout the 1st year of life. FDA has concluded that the relationship between 100% Whey-Protein Partially Hydrolyzed infant formulas and the reduced risk of atopic dermatitis is uncertain, because there is little scientific evidence for the relationship." 1 Authorized health claim for soy protein and risk of heart disease (21 CFR 101.82), available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.82 2 Letter of Enforcement Discretion for 100% whey-protein partially hydrolzed infant formula and reduced risk of atopic dermatitis, available at: http://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm256731.htm 6 www.intertek.com Claims Permissible in the Labelling and Advertising of Foods Claims Made in Food Labelling and Advertising1 Nutrient Content Claims Nutrient Function Health Claims Other Function Disease RiskReduction and Therapeutic 1 Food and Drug Regulations B.01.600 to B.01.603, available at: http://lawslois.justice.gc.ca/eng/regulations/C.R.C.%2C_c._870/FullText.html; and Canada Food Inspection Agency’s Food Labelling for Industry, available at: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/healthclaims/eng/1392834838383/1392834887794 7 www.intertek.com Examples of Claims for Protein Claim Type Nutrient Function1 Claim Wording - Protein helps build and repair body tissues. - Protein helps build antibodies. - Protein helps build strong muscles. - Food must be at least a source of protein (protein rating ≥20). Other Function - None specific to protein have been endorsed by Health Canada. - Possibilities exist if claims can be scientifically substantiated (e.g., satiety, physical performance, body weight/composition, sarcopenia). Disease RiskReduction and Therapeutic - None specific to protein have been authorized by Health Canada. - Possibilities exist if claims can be scientifically substantiated (e.g., reduced risk of certain diseases, treatment of obesity). 1 Acceptable nutrient function claims, available at: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/healthclaims/eng/1392834838383/1392834887794?chap=8 8 www.intertek.com Claims Permissible in the Labelling and Advertising of Foods Claims Made in Food Labelling and Advertising1 Nutrition Content Claims Health Claims General Level 1 High Level The Australia New Zealand Food Standards Code Standard 1.2.7, available at: http://www.comlaw.gov.au/Details/F2013L00054 9 www.intertek.com Examples of Health Claims for Protein Claim Type General Level1 Claim Wording Conditions for Use • Protein is necessary for tissue building and repair • Protein contributes to the growth of muscle mass • Protein contributes to the maintenance of muscle mass • Protein contributes to the maintenance of normal bones Food must meet the general conditions for making a nutrition content claim for protein (at least 5 g of protein per serving) • Protein is necessary for normal growth and development of bone • Food must meet the general conditions for making a nutrition content claim for protein (at least 5 g of protein per serving) • For children and adolescents aged 4 years and over • Protein is necessary for normal growth and development • Food must meet the general conditions for making a nutrition content claim for protein (at least 5 g of protein per serving) • For children aged 4 years and over • Protein is necessary for normal growth and development • Food must be a food for infants • For infants aged 6 to 12 months 1 From the Australia New Zealand Food Standards Code Standard 1.2.7, available at: http://www.comlaw.gov.au/Details/F2013L00054 10 www.intertek.com Claims Permissible in the Labelling and Advertising of Functional Foods • According to the Guidelines for Registration for Functional Foods, the functional (health) food referred to a food that has special health functions or is able to supply vitamins and minerals. It is suitable for consumption by special groups of people, aiming to regulate human body functions, but is not used for therapeutic purposes. • Must demonstrate safety and efficacy • Two categories: • Function Healthy Foods include substances such as fish oil, ginseng, etc. and can make health claims or structure/function claims. • Nutritional supplements include vitamins and minerals and can only make nutrient content claims. • Functional food claims are product-specific (not ingredient-specific). • Function Healthy Foods can bear 1 or several of the 27 established functions. • Product-specific testing must be conducted according to pre-defined research protocols at a qualified testing agency in China. 11 www.intertek.com Functional Claims for Function Healthy Foods • 27 categories of product-specific health claims • Function-related or refers to reduction of disease risk • Maximum of 2 claims per product • Those in red likely to be cancelled • Those in green likely to be combined 12 www.intertek.com Example of Claim for Protein Product Product Name Protein Powder 13 Image Price Key Claims 160 RMB 450g Enhancing Immune Function Unit Sales in 30 Days 23,519 www.intertek.com Nutrient Content and Nutrient Function Claims • Nutrient content claim criteria for protein are: • Source of protein: ≥10 % NRV per 100 g; ≥5 % NRV or per 100ml; ≥5 % NRV per 420 kJ • High, or rich in protein: ≥20 % NRV per 100 g; ≥10 % NRV or per 100ml; ≥10 % NRV per 420 kJ • Approved nutrient function claims are: • Protein is the main component of body and could provide various kinds of amino acids. • Protein is essential to human life activities, as well as contributing to tissue formation and growth. • Protein helps constituting or repairing human tissue. • Protein helps with tissue formation and growth. • Protein is an essential nutrient for tissue formation and growth. 14 www.intertek.com Claims Permissible in the Labelling and Advertising of Foods Food Health Functional Food Claims2 Claims1 Usefulness Claims Generic Claims Nutrient Functional Ingredients Product Specific Claims Functional Ingredients 1 Food 2 Sanitation Act and subordinate legislations. Health Functional Food Act and subordinate legislations. 15 www.intertek.com Examples of Claims for Protein Products Claims Generic Nutrient Claims & Usefulness Claims1 Description • Protein is a component of physical tissues such as muscle and connective tissue • Protein is required for the normal formation of enzymes, hormones and antibodies Conditions Single-dosage forms: 12 g/day Foods: at least a source of protein • Protein is required for the transport and storage of essential nutrients or active materials in the body • Protein is required for the maintenance of fluid balance and acidbase balance • Protein is required for the synthesis of energy, glucose, and lipids 1 From the Health Functional Food Code. 16 www.intertek.com Examples of Claims for Protein Products Claims Generic Functional Ingredient Claim1 Description • Soy protein helps maintain blood cholesterol Conditions Single-dosage forms: 15 g/day Product • Lactoferrin helps reduce body Specific Claims fat Single-dosage forms: 300 mg/day • Milk protein hydrosylate (Lactium®) helps reduce stressinduced anxiety Single dosage forms: 150mg/day 1 From the Health Functional Food Code. 2 From the Approved Product-Specific Ingredient register, available at: http://www.foodnara.go.kr/hfoodi/industry/ 17 www.intertek.com Thank you! Email: [email protected] Website: www.intertek.com/food/consulting NOTE: "Intertek Cantox" is now "Intertek Scientific & Regulatory Consultancy". 18 www.intertek.com