* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biomolecules PPT
Gene regulatory network wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Photosynthesis wikipedia , lookup
Genetic code wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Signal transduction wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biosynthesis wikipedia , lookup
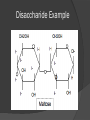
What is a Biomolecule? Organic molecule made by living organisms Consist mostly of carbon (C), hydrogen (H), and oxygen (O) But wait…What is an Organic Molecule? Organic Molecules: Contain carbon Considered the “chemicals of life” Inorganic Molecules: Do not contain carbon Monomers vs. Polymers Monomers: Molecules that may react with similar molecules to form a chain Polymers: A chain of many monomers that are chemically bonded together Formation of Polymers Animation How are polymers formed? Dehydration Synthesis (Condensation): Two hydrogen atoms and one oxygen atom are removed from the monomers to form water, and the two monomers are joined together. Breakdown of Polymers Animation How are polymers broken down? Hydrolysis—the reverse of dehydration synthesis (condensation) Water added to the polymer, un-linking the chain and breaking it back down to its original monomer units Carbohydrates What are they? Group of organic molecules that includes sugars, starches and cellulose. Carbohydrates Structure: Carbon, hydrogen and oxygen in a 1:2:1 ratio (CH2O)n – n is an integer such as 5 (C5H10O5) Subunits: Monosaccharides, such as glucose or fructose Most often in a ring shape Subunits are connected with covalent bonds Monosaccharide Example Disaccharide Example Polysaccharide Example Carbohydrates Function: Energy Structural Support Cell Wall Cell Membrane Marker Lipids What are they? Organic molecule group including fats and phospholipids Lipids Structure: Subunits: ○ Glycerol and fatty acids ○ Glycerol and fatty acids plus phosphate group Insoluble in water Do not form large polymers (2 or 3 fatty acids with glycerol) ○ Examples: diglyceride and triglyceride Triglyceride Example Phospholipid Example Lipids Function: Energy storage Insulation Part of cell membrane (phospholipids) Hormones Proteins What are proteins? Group of organic molecules that provides structure and facilitates chemical reactions. Proteins Structure: Subunits: Amino acids Amino acids connect via peptide bonds Very large molecules Globular or structural Amino Acid Proteins Function: Lots of functions! Enzymes (speed rate of chemical reactions) Structural components in cells Mechanical functions in muscles and cytoskeleton (internal cell framework) Cell signaling Immune response Nucleic Acids What are Nucleic Acids? Group of organic molecules including DNA and RNA Nucleic Acids Structure: Subunits are nucleotides—5-Carbon sugar, Nitrogen base and one or more Phosphate groups Nucleic Acids Function: Storage and retrieval of information: ○ Encode genes ○ Gene expression