* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cellular Respiration
Survey
Document related concepts
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Biosynthesis wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Mitochondrion wikipedia , lookup
Butyric acid wikipedia , lookup
Electron transport chain wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Transcript
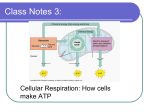
CELLULAR RESPIRATION Biology 30 Mrs. S. Pipke-Painchaud CELLULAR RESPIRATION SUMMARIZED “An active cell in the body requires millions of molecules of ATP per second to drive its biochemical machinery.” (Purves, Orian and Heller) What is it? Where? is the process by which cells release energy from food molecules by a type of controlled burning (food and oxygen enter the cell through the plasma membrane by diffusion, passive transport, or active transport) the series of reactions involved are controlled by Enzymes Inside the mitochondria of a cell Why? C.R. releases the energy stored in the form of sugars (it is the opposite of photosynthesis) It is an energy releasing reaction C.R. tries to release the greatest amount of energy possible; therefore, C.R. tries to capture Energy in the form of ATP rather than heat which is a waste (unusable form of energy) To avoid burning up this process captures energy in small, manageable steps to avoid over heating and killing the cell. What organisms go through this process? Animals Plants THE GENERAL EQUATION . . . enzymes C6H12O6 + 6O2 6 CO2 + 6 H2O + energy (glucose) I: GLYCOLYSIS The First Step . . . GLYCOLYSIS The Reaction: C6H12O6 + 2 ATP 2 C3H4O3 + 4 ATP + 4 NADH it occurs in the Cytoplasm it is an anaerobic reaction it is a Reduction/Oxidation Reaction C6H12O6(glucose) splits to form 2 3 carbon units = C3H4O3 Glucose Pyruvic Acid (pyruvate) C6H12O6 C3H4O3 in order for the 6 C sugar to split it requires energy 2 ATP if a reaction requires energy the energy is called Activation Energy the breaking of the 6 C molecule and the several other reactions that occur in this step produce a total of 4 ATP. Therefore, there is a net gain of 2 ATP per reaction GLYCOLYSIS . . . C6H12O6 + 2 ATP 2 C3H4O3 + 4 ATP + 4 NADH If the glucose molecule is split in half, what would you expect the formula to be? C6H12O6 split in half = C3H6O3 Actually = 2 C3H4O3 ** thus, we are missing _2__ hydrogens since matter can be neither created nor destroyed, the cell uses H acceptors (carriers) to keep the H from escaping. Hydrogen carriers are called NAD+ (Nicotinamide Adenine Dinucleotide) Purpose: to function as a hydrogen acceptor and store the hydrogen for later use (just like NADPH in photosynthesis) GLYCOLYSIS REACTION SUMMARY: II. INTERMEDIATE REACTION ~ OXIDATIVE DECARBOXYLATION II. INTERMEDIATE REACTION 2 C3H4O3 + 2 O2 2 C2H3O3 + 2 CO2 + 2 NADH If you examine the reactants what do you notice? 1) Oxygen is now involved 2) The carbon molecule in the reactants is the product of the last reaction. This reaction occurs in the Mitochondria (because this organelle has enzymes that enable them to use oxygen safely) This is an aerobic reaction. INTERMEDIATE REACTION Focus on . .. .CARBONS C C C C C C oxygen is used to remove Carbons from the pyruvate How many oxygens are used to do this? 2 How many Carbon Dioxides does this produce? 2 INTERMEDIATE REACTION Focus on . . . HYDROGEN The number of Hydrogen in the reactants 8 : the number of Hydrogens in the carbon molecule on the products side = 6 = a difference of 2 Thus, we need 2 hydrogen acceptors. 2 NADH INTERMEDIATE REACTION SUMMARY: III: KREB’S CYCLE OR THE CITRIC ACID CYCLE III. KREB’S CYCLE Is a complex series of reactions, simplified for our discussion to …. 2 C2H3O3 + 4 O2 4 CO2 + 2 ATP + 6 NADH This reaction occurs in the Mitochondria (because this organelle has the enzymes that enable them to use oxygen) This is an aerobic reaction. III. KREB’S CYCLE 2 C2H3O3 + 4 O2 4 CO2 + 2 ATP + 6 NADH Focus on the ….. CARBONS What element is used to remove the Carbons? How many Oxygen are used to remove the Carbons? Oxygen 4 As a result carbon dioxide is released to the atmosphere through the stomata in the leaves or when animals exhale. III. KREB’S CYCLE 2 C2H3O3 + 4 O2 4 CO2 + 2 ATP + 6 NADH Focus on the . . . HYDROGENS The number of Hydrogen in the reactants 6: the number of Hydrogens in the carbon molecule on the product side = 0. Thus, we need 6 hydrogen acceptors III. KREB’S CYCLE 2 C2H3O3 + 4 O2 4 CO2 + 2 ATP + 6 NADH Focus on . . . Oxygen How many oxygens are on the reactants side of the equation? How many were used up in the formation of CO2? 14 8 How many oxygen are left? 6 These oxygen are immediately used in the next reaction. FADH – (Flavin adenine dinucleotide) acts as a hydrogen carrier of the free energy produced by other reactions that happen within the cell. Depending on the textbook or website that you read, they will incorporate this molecule as well. We have just simplified. INTERMEDIATE REACTION SUMMARY: IV. ELECTRON TRANSPORT CHAIN OR OXIDATIVE The Final Stage PHOSPHORLATION IV: ELECTRON TRANSPORT CHAIN 12 H + 6 O 6 H2O or 6 H2 + 3 O2 6 H2O basically this transports H ions against the concentration difference from the inner membrane to the outer membrane of the mitochondria. It acts as a battery charger because the movement of H+ ions creates a pH difference and causes an electrical charge to build up The inner membrane has the enzyme (ATP synthase) which allows protons back across the nucleus and catalyzes the production of ADP to ATP. Collects the most energy IV: ELECTRON TRANSPORT CHAIN 12 H + 6 O 6 H2O OR 6 H2 + 3 O2 6 H2O all of the carbons were lost in the last stage; therefore, only Hydrogen and Oxygen remain. At the end of the last reaction 6 oxygen were set free In order to prevent them from escaping, the oxygen are immediately reused in the final reaction. 12 H + 6O IV: ELECTRON TRANSPORT CHAIN 12 H + 6 O 6 H2O OR 6 H2 + 3 O2 6 H2O Total of Hydrogen Acceptors: RXN 1: __4__ NADH 2: __2__ NADH 3: __6__ NADH TOTAL = 12 IV: ELECTRON TRANSPORT CHAIN 12 H + 6 O 6 H2O OR 6 H2 + 3 O2 6 H2O If you combine hydrogen and oxygen at a 2:1 ratio you end up with water. *** This reaction releases 34 ATP*** ATP SUMMARY FOR CELLULAR REPIRATION How much energy is released in total? RXN 1: __2__ ATP 2: __0_ ATP 3: __2_ ATP 4: __34_ ATP TOTAL = _________38___________ATP 1 molecule of glucose produces 38 ATP FINAL THOUGHTS If you release energy all at once it is similar to burning glucose. Just burning glucose all at once would release -686 kcal/mol of energy. Burning a substances creates a lot of heat energy Too much heat in the body will raise the body temperature and cause some cells to be destroyed. (consider enzymes) However, if each mole of ATP stores 12 Kcal of energy (36 X 12= 432 kcal eukaryotic respiration or 38 X 12 – 456 kcal prokaryotic respiration) Thus, the body uses a series of small manageable reactions, in order to capture as much energy as possible without harming the cell. The body is able to capture 44%of the energy as ATP, but the other 56% is lost as heat. ENERGY CONVERSIONS: if you look at conversion efficiencies, different phyla use different amounts of energy. Amphibians use 50-75% of the energy from food that they eat Reptiles use 50% Birds use 1% Mammals use 1.5% Why the difference? Why are reptiles and amphibians 50 times more efficient? Where do they live? Usually warm, tropical areas or very controlled environments Humans & birds live everywhere. Why? Reptiles and amphibians are cold blooded “A poikilotherm is a plant or animal whose internal temperature varies along with that of the ambient environmental temperature. Most, but not all, terrestrial ectotherms are poikilothermic. The opposite of poikilothermy is homeothermy, referring to animals that maintain a constant body temperature” (Wikipedia – Poikilotherm) WHICH IS MORE EFFICIENT AEROBIC RESPIRATION OR ANAEROBIC RESPIRATION? Anaerobic = Glycolysis = 2 ATP Aerobic = 34 – 36 ATP WHAT HAPPENS WITHOUT OXYGEN IN OUR BODIES? if we are deprived of oxygen for to long we will die because the cellular respiration reactions are dependent on the oxygen carrying molecule. Thus, without oxygen acceptors for carbon our bodies can’t get rid of the electrons that are bound to certain compounds. All compounds quickly use up the oxygen which leaves all the reduced compounds waiting to be oxidized. The chain reaction stops we lack ATP die Except for muscles which can get rid of their H atoms during glycolysis because the H are passed back to pyruvate and lactic acid is formed. Glycolysis occurs at a faster pace when there is no oxygen It will continue until the lactic acid reaches toxic levels which will kill the cell. Nerve cells cannot do this; therefore, brain damage occurs very quickly. The anaerobic production of ATP is called fermentation. FERMENTATION ~ producing energy without oxygen ANAEROBIC RESPIRATION ~ FERMENTATION Occurs in the cytoplasm Is similar to the first stage of cellular respiration, Glycolysis, and does not require oxygen. (therefore it is anaerobic. It produces 2 ATP per molecule of glucose If the body is severely taxed and it cannot supply enough oxygen to carry out the next two steps of cellular respiration. The molecules of pyruvic acid are still being produced Instead of continuing on to the next stage, Hydrogen is added to the pyruvic acid, which in turn converts it to lactic acid. A lactic acid build up in muscles inhibits the muscle’s ability to contract which causes fatigue (burning in the muscles) HUMANS . . . Many organisms will also ferment pyruvic acid into, other chemicals, such as lactic acid. Humans ferment lactic acid in muscles where oxygen becomes depleted, resulting in localized anaerobic conditions. This lactic acid causes the muscle stiffness couch-potatoes feel after beginning exercise programs. The stiffness goes away after a few days since the cessation of strenuous activity allows aerobic conditions to return to the muscle, and the lactic acid can be converted into ATP via the normal aerobic respiration pathways. “Fermentation also occurs in some muscle cells, which are also called twitch muscles, because these muscles cannot store or use much oxygen in comparison to the other muscles. When we run the oxygen, supply of these muscles gets short as a result of which the twitch muscles starts using the fermentation of lactic acid. Through this process, the muscles can go on functioning as ATP is produced by the Glycolysis.” Anaerobic Respiration http://www.anaerobicrespiration.net/ LACTIC ACID FERMENTATION FERMENTATION This process is also called anaerobic respiration. The same process occurs in yeast except enzymes within the yeast extract carbon dioxide and alcohol is produced as a result. What does carbon dioxide do? What industry uses fermentation? (Humans cannot ferment alcohol in their own bodies, we lack the genetic information to do so) Other Examples: bread dough rises from ____________________ (the alcohol evaporates during the cooking process) Bubbles in champagne ALCOHOL FERMENTATION NOTE: Anaerobic Respiration – works through the cellular respiration pathway (Glycolysis) Fermentation – follows a similar format but ends with the production of an alcohol which cannot be transformed back. http://www.anaerobicrespiration.net/ FERMENTATION EXAMPLES . . . In a general sense, fermentation is the conversion of a carbohydrate such as sugar into an acid or an alcohol. More specifically, fermentation can refer to the use of yeast to change sugar into alcohol or the use of bacteria to create lactic acid in certain foods. Fermentation occurs naturally in many different foods given the right conditions, and humans have intentionally made use of it for many thousands of years. The earliest uses of fermentation were most likely to create alcoholic beverages such as mead, wine, and beer. These beverages may have been created as far back as 7,000 BCE in parts of the Middle East. The fermentation of foods such as milk and various vegetables probably happened sometime a few thousand years later, in both the Middle East and China. While the general principle of fermentation is the same across all of these drinks and foods, the precise methods of achieving it, and the end results, differ. Beer is made by taking a grain, such as barley, wheat, or rye, germinating and drying it, and pulping it into a mash. This mash is then mixed with hot water, and some fermentation begins. After being further treated, the liquid is transferred to a fermentation vessel, where yeast is added to the mixture. This yeast “eats” the sugar present in the mash and converts it into carbon dioxide and alcohol. After a few weeks of fermentation and a further period of conditioning, the beer is ready to be filtered and consumed. Wine is created using a similar method that also involves fermentation. Grapes are crushed to release the sugar-rich juices, which are then either transferred quickly away from the skins or left to rest for a time to absorb some of the flavor, tannins, and color of the skins. Yeast is then added, and the grape juice is allowed to ferment for a number of weeks, at which point it is moved to different containers and fermented at a slower rate, and eventually aged or bottled. Pickling foods, such as cucumbers, may be accomplished by submerging the vegetable one wants to pickle in a salty water solution with vinegar added. Over time, bacteria create the lactic acid that gives the food its distinctive flavor and helps to preserve it. Other foods can be pickled simply by packing them in dry salt and allowing a natural fermentation process to occur. Milk can also be cultured, and people have been using fermentation with dairy products for nearly 5,000 years. It is speculated that early fermented dairy, such as yogurt, was the result of a natural process of fermentation that occurred when the milk was cultured by bacteria that dwelt in skin sacks used to store dairy. Yogurt these days is made by adding a number of special bacteria, such as L. acidophilus and L. bulgaricus to milk and keeping it at the proper temperature. The bacteria begin converting the sugar in the dairy to lactic acid, eventually creating what we know as yogurt. WiseGeek: http://www.wisegeek.com/what-is-fermentation.htm ARE YOU STILL ON THE BUS? Any Questions