* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Stereochemistry of E2 Reactions
Woodward–Hoffmann rules wikipedia , lookup
Elias James Corey wikipedia , lookup
Marcus theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
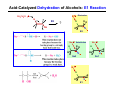
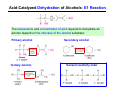
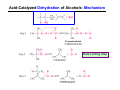
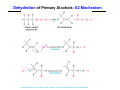
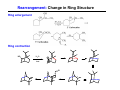
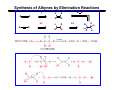
The Stereochemistry of E2 Reactions . . . . Dehydrohalogenation: Zaitsev’s Rule Hammond-Leffler Postulate: the transition state for a step that is downhill in energy should show a strong resemblance to the reactant of that step. . . . . . . . . . . . . . The Stereochemistry of E2 Reactions . . . . . . The Stereochemistry of E2 Reactions . . . . . . . . Acid-Catalyzed Dehydration of Alcohols: E1 Reaction B CH3CH2O H E2 Elimination RR H ? OH δ R Nu R Nu Substitution RR H R E2 Xδ H δ R R Xδ SN 2 B H R R R R X E1 R R R X S N1 Acid-Catalyzed Dehydration of Alcohols: E1 Reaction The temperature and concentration of acid required to dehydrate an alcohol depend on the structure of the alcohol substrate: Primary alcohol Tertiary alcohol Secondary alcohol General reactivity order Acid-Catalyzed Dehydration of Alcohols: E1 Reaction Some primary and secondary alcohols also undergo rearrangements of their carbon skeletons during dehydration. CH3 . H3C C rearranged to . CH3 C H CH2 not formed Acid-Catalyzed Dehydration of Alcohols: Mechanism Rate Limiting Step Dehydration of Alcohols: E1 Mechanism Rate Limiting Step Carbocation Stability . . . . Dehydration of Primary Alcohols: E2 Mechanism . . . . . . . . . Dehydration of Primary Alcohols: E2 Mechanism . . . . . . . . . . . . . Rearrangement during Dehydration A (80%) . B (20%) . . . . . . Path B B H CH3 H2C C C CH3 CH3 H B Path A The formation of the more stable alkene is the general rule (Zaitsev's rule) in the acid-catalyzed dehydration reactions of alcohols. Rearrangement: Change in Ring Structure Ring enlargement Ring contraction HO H3O+ + + heat B H + + + Synthesis of Alkynes by Elimination Reactions ? R R R H H R R Br Br Br R E2 R H R E2 Br H R R H Br The Acidity of Terminal Alkynes Relative Acidity Relative Basicity