* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download © John Congleton, Orange Coast College Organic Chemistry 220
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
George S. Hammond wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Petasis reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Bottromycin wikipedia , lookup
Stille reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
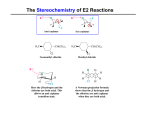
Organic Chemistry 220 Final Exam Preparation Do all homework, WebAssign problems, and worksheets until you no longer have to look at notes, books, or other problems to answer them correctly. Work extra problems to further concretize any material if needed (ha! Are there any extra problems left after all of the work I have given you? ☺). This study guide is only a rough guide (this is my legal disclaimer…). You are responsible for all of the material that I have covered in chapters 10, 11, 13, 14 (and all of the old stuff too: chapters 1-9) and the class lecture notes. The Final Exam will have: • Nomenclature • Short synthesis reaction (reactants to products) • Mechanisms • Multi-step synthesis • Conceptual questions Big concepts to keep in mind: • You need to know all of the reagents required for the reactions that are outlines below. • You need to know the mechanisms that I have outlined below. • Know all sterochemical outcomes of the reactions that we covered. • You need to be able to carry out multi-step synthesis reactions. Some Basics to Review • Nomenclature • pKa stuff • Resonance • SN1, SN2, E1, E2 • Stereochemistry Chapter 1 • Lewis structures • Electronegativity • Bond polarities and dipole moments • Formal charges • Resonance; drawing structures and predicting which resonance is best • Condensed structural formulas • Line-angle formulas • Acids and bases o Arrhenius acid/base o Bronsted-Lowry acid/base o Lewis acid/base o Acid strength o Predicting acid base reactions, do we favor reactants or products? (pKa’s) o Structural effects of acidity Electronegativity Size Resonance Chapter 2 • s-orbital • p-orbital • Molecular orbital theory (MO) o Bonding and antibonding mo’s o s-s overlap © John Congleton, Orange Coast College • • • • o s-p overlap o p-p overlap (head on) o pi-bonding Hybridization o sp3, sp2, sp o where are the unhybridized p-orbitals relative to the hybridized orbitals? o Be able to draw organic compounds showing the orbital overlap (single, double, triple bonds etc…) Isomers o Be able to draw constitutional isomers o Cis/trans isomers Intermolecular forces o LDF o D-D o H-bonding o Predicting BP o Predicting solubility Know the functional groups Chapter 3 • Alkane nomenclature o Names to structure o Structure to names o Complex substituents o Common names (isopropyl, etc…) • Physical properties of alkanes o MP, BP of branched and straight chain alkanes • Reactions o Combustion o Cracking • Structure of alkanes o Newman projections o Potential energy diagrams & Newman projections o Totally eclipsed o Partially eclipsed o Gauche o Anti o Dihedral angle o Be able to draw Newman projections for alkanes • Cycloalkane nomenclature • Stability of cycloalkanes o Heats of combustion: what do they mean? o Angle strain and torsional strain • Cyclohexane o Chair and boat conformations o Twist boat conformation o Half-chair conformation o Axial and equatorial positions o Be able to flip the chair (as in figure 3-23) o Be able to draw and predict which chair structure is more stable for monosubstituted cyclohexanes o Be able to draw and predict which structure is more stable for disubstituted cyclohexanes o Be able to draw and predict which chair structure is more stable for cyclohexanes with bulky substituents • Nomenclature of bicyclic alkanes © John Congleton, Orange Coast College Chapter 4 • Free radical chain mechanism (all of the sterps) • ΔGo and equilibrium constants • Enthalpy, entropy, Gibbs • BDE • Magnitude of BDE and stability of free-radical • Activation energy • Reaction energy diagrams o Activation energy o Exothermic o Endothermic o Multistep reactions energy diagrams o How does temperature influence reaction rates? • Selectivity of chlorination o Can you determine the percents for products • Selectivity of bromination o Can you determine the percents for products • Free-radical stability o Hypercongjugation and inductive effects… • Why is bromination so much more selective? • Allylic bromination • What is the Hammond Postulate o What does the transition state have to do with this postulate? • Be able to determine chlorination and bromination products taking stereochemistry into consideration Chapter 5 • Definitions: o Chiral, achiral, enantiomer, diastereomer, meso, R, S, plane of symmetry • Be able to determine if a molecule is chiral • Be able to determine if a molecules are enantiomers, diastereomers, different molecules, etc… • Determine R and S for all types of molecules o Fischer projections o Line structures o Newman projections • Be able to name compounds using R/S stereochemistry • Draw Fischer projections from line structures and vice-versa • Optical activity o What is it? o Are racemic solutions optically active? o What does ± mean in front of a compound? o Can you determine R and S from + and - ? Chapter 6 • Know the detailed mechanisms for: SN1, SN2, E2, and E1. • Be able to predict products (including stereochemistry) of: o S N1 o S N2 o E2 o E1 © John Congleton, Orange Coast College • • • • • • • • • • • • Be able to predict whether a reaction will proceed via o SN1 and E1 o S N2 o SN2 and E2 o E2 What makes a good nucleophile? What makes a good base? What makes a good leaving group? What is meant by high and low polarizability? Allylic bromination Understand, be able to predict, and be able to complete mechanisms that involeve hydride and methyl shifts. During E1 and E2 reactions what will be the major alkenes? Zaitsev’s rule o When do we use this? Hoffman product (anti-Zaitsev’s rule) o When are these products favored? The summary table on page 251 should be memorized The summary table on page 264 should be memorized Chapter 7, Alkenes • Framework of the alkene • Calculation of elements of unsaturation & drawing possible isomers • Nomenclature of alkenes (including E/Z) • What are the stabilities of alkene? • Bredt’s rule • Alkene synthesis o Dehydrohalogenatation, know mechanism, stereochemistry, etc… o Bulky bases and Hoffman products… o E2 reactions (anti-coplanar!, know mechanism, stereochemistry, etc…) o E1 reactions (know mechanism, stereochemistry, etc…) • Alkene synthesis via dehydration reactions o Know this mechanism Chapter 8: • What is an electrophile? • What is a nucleophile? • What is an anti-addition? • What is a syn-addition? • Addition of hydrogen halides to alkenes (8-3) o Know mechanism, stereochemistry, etc… o Know Markavnikov’s rule o What are the products in the presence of peroxides? Can it work will all hydrogen halides? Know the mechanism, stereochemistry, etc… (8-3b). • Addition of water to alkenes (8-4) o Acid-catalyzed hydration Know the mechanism, stereochemistry, etc… • Oxymercuration-demurcuration (8-5, 8-6) o Know the mechanism, stereochemistry, etc… • Hydroboration (8-7) o Know the mechanism, stereochemistry, etc… o Don’t worry about the second step of the mechanism (page 340, H2O2, NaOH) • Addition of Halogens (8-10) o Know the mechanism, stereochemistry, etc… © John Congleton, Orange Coast College • • • • • • • • o Can aromatic double bonds react? Formation of halohydrins (8-9) o Know the mechanism, stereochemistry, etc… Catalytic hydrogenation o Know the stereochemistry and the required types of catalysts… Skip 8-11 Epoxidation (8-12) o Know the mechanism, stereochemistry, etc… Skip 8-13 Syn Hydroxylation (8-14) o Know the reagents and the stereochemistry of this reaction Oxidative cleavage (8-15) o What is the difference between ozonolysis and adding warm KMnO4? o What products are formed? Skip 8-16 Chapter 9: • Nomenclature of alkynes • How do you name compounds with a double bond and a triple bond? And an alcohol? • Structure of the triple bond • Acidity pf alkynes • Synthesis of alkynes from acetylides o Know the mechanism, stereochemistry, etc… o Know the additions to ketones and Aldehydes too • Synthesis of alkynes via elimination o What are the differences between the addition of: KOH, 200oC and 1) NaNH2, 150oC, 2) H2O • Addition reactions of alkynes o Hydrogenation Know the difference when you add: • H2 and Pt, Pd, or Ni • Lindlar’s catalyst • Na, NH3 (Know the mechanism, stereochemistry, etc… for this one) • Skip 9-9D • Skip 9-9E o Hydration of alkynes (9-9f) Know the mechanism, stereochemistry, etc… for the addition of an alkyne with HgSO4/ H2SO4 Know the mechanism, stereochemistry, etc of acid-catalyzed keto-enol tautomerism Know the mechanism, stereochemistry, etc… for the addition of an alkyne with 1) SiaBH.THF 2) H2O2, NaOH Know the mechanism, stereochemistry, etc of base-catalyzed keto-enol tautomerism. • Skip 9-10 Chapter 10 • Classification of alcohols (primary, etc…) • Alcohol nomenclature o Diols, multifunctional groups, etc… • Naming phenols o Ortho, meta, para phenols © John Congleton, Orange Coast College • • • • • • • Physical properties of alcohols Acidity of alcohols Why is phenol more acidic than cyclohexanol? Synthesis of alcohols (know mechanisms & stereochemistry) o SN2 reactions (chp 6) o Acid-catalyzed hydration (8-4) o Oxymercuration-demurcuration (8-5) o Hydroboration-oxidation (8-7) o Synthesis of diols (8-13, 8-14) o Addition of acetylides to carbonyls (9-7) Grignard reaction (know mechanism & stereochemistry) with carbonyl containing compounds (10-9) Organolithium reactions (know mechanism & stereochemistry) with carbonyl containing compounds (10-9) Reduction of carbonyl using NaBH4 and LAH (know mechanism & stereochemistry) o Know the stereochemistry here… o Which reagent is more selective? Chapter 11 • What is meant by oxidation and reduction? • Oxidation of alcohols with Na2Cr2O7/H2SO4 o Know mechanism o Where does oxidation stop? • Oxidation of alcohols with pyridinium chlorochromate (PCC) o Know mechanism o Where does oxidation stop? • Don’t worry about the other many methods of alcohol oxidation in section 11-3. • Biological oxidation o Fate of ethanol, methanol, ethylene glycol in the body… o What is the biological source of hydride? • Formation of tosylate esters from alcohols. o Know structure of para-toluenesulfonyl chloride o Know mechanism & stereochemistry o Why is OTs- a good leaving group? o Review SN1, SN2, E1, and E2 reactions, since any of these can occur with a good leaving group. • Alcohols with hydrohalic acids (H-Br and H-I) o Know mechanism & stereochemistry o 2o, 3o alcohols react via SN1 o 1o, 2o alcohols react via SN2 • Alcohols with hydrochoric acid/ ZnCl2 (Lucas reagent) o Know mechanism & stereochemistry (formation of a carbocation) o 1o alcohols do not react o 2o, 3o alcohols react through SN1 mechanism • Reaction of alcohols with phosphorus halides o Know mechanism & stereochemistry o Can use PBr3, PCl3, and P/I2 o Reaction does not work on tertiary alcohols (see mechanism to understand why…) • Reaction of alcohols with thionyl chloride o Know mechanism & stereochemistry o Reaction does not work well on tertiary alcohols (see mechanism to understand why…) o Retention of configuration • Dehydration of alcohols o Know mechanism & stereochemistry o What needs to be in excess in order to shift the equilibrium to the alkene? © John Congleton, Orange Coast College • • • Bimolecular dehydration to form ethers (see also chapter 14-7) o Know mechanism o Low temp usually needed here. Skip 11-11, 11-12, 11-13 How can you form an alkoxide? (section 11-14) o Na, K, or NaH o Williamson-Ether synthesis (know mechanism) Chapter 13: Nuclear Magnetic Resonance Spectroscopy • Upfield, downfield, chemical shifts • Splitting, (n+1) rule • Integration • Calculate the index of unsaturation • Number of peaks expected • Be able to determine a structure given a 1H-NMR and a molecular formula • Skip all 13C-NMR material Chapter 14; Ethers and Epoxides • Physical properties of ethers • Nomenclature of ethers o Common names o IUPAC names • Nomenclature of cyclic ethers o Epoxides Name as oxirane Name as epoxy o Know structure of oxetane, furan, THF, pyran, THP • Williamson-ether synthesis (know mechanism) • Synthesis of ethers via oxymercuration-demurcuration o Know mechanism & stereochemistry o Know reagents used o Know stereochemistry too… • Bimolecular dehydration of alcohols o Know mechanism (we did this in chapter 11 also) • Cleavage of ethers using H-Br and H-I o Know mechanism o What happens if one of the ether substituents is a phenyl? • Skip section 14-9, 14-10, 14-16 • Synthesis of epoxides o Know peroxyacid mechanism & stereochemistry (review chapter 8) o What is MCPBA? o Know base-promoted epoxide formation What orientation is needed here? • Acid-catalyzed ring opening of epoxides o Using H2O and an acid: Know mechanism & stereochemistry o Using alcohol and an acid: Know mechanism & stereochemistry o Using H-Cl, H-Br, or H-I: Know mechanism & stereochemistry o What is the orientation of ring opening here? • Base-catalyzed ring opening of epoxides o Know mechanism (alkoxide) & stereochemistry o Know mechanism (Grignard reagent) & stereochemistry o What is the orientation of ring opening here? © John Congleton, Orange Coast College