* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download DNA - Chemistry Courses
DNA repair protein XRCC4 wikipedia , lookup
DNA sequencing wikipedia , lookup
Messenger RNA wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
DNA profiling wikipedia , lookup
Genomic library wikipedia , lookup
Metalloprotein wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
Restriction enzyme wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
Transformation (genetics) wikipedia , lookup
SNP genotyping wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Biochemistry wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Molecular cloning wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Epitranscriptome wikipedia , lookup
Gene expression wikipedia , lookup
Community fingerprinting wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Genetic code wikipedia , lookup
Point mutation wikipedia , lookup
Real-time polymerase chain reaction wikipedia , lookup
Non-coding DNA wikipedia , lookup
DNA supercoil wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
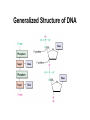
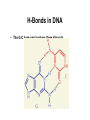
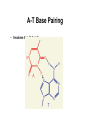
Chapter 28 Biomolecules: Heterocycles and Nucleic Acids Based on McMurry’s Organic Chemistry, 6th edition Heterocycles • Cyclic organic compounds are carbocycles or heterocycles – Carbocycle rings contain only carbon atoms – Heterocycle rings atoms in addition to carbon (N,S,O are common) • Heterocycles include many important natural materials as well as pharmaceuticals 28.1 Five-Membered Unsaturated Heterocycles • Pyrrole, furan, and thiophene are common fivemembered unsaturated heterocycles • Each has two double bonds and N, O, or S Pyrrole • Commercially from coal tar or by treatment of furan with ammonia over an alumina catalyst at 400°C. Furan • Made commercially by extrusion of CO from furfural, which is produced from sugars Thiophene • From coal tar or by cyclization of butane or butadiene with sulfur at 600°C Unusual Reactivity • Pyrrole is an amine but it is not basic • Pyrrole, furan, and thiophene are conjugated dienes but they undergo electrophilic substitution (rather than addition) 28.2 Structures of Pyrrole, Furan, and Thiophene • Pyrrole, furan, and thiophene are aromatic (Six electrons in a cyclic conjugated system of overlapping p orbitals) • In pyrrole electrons come from C atoms and lone pair on sp2-N Why Pyrrole is Not a Base • The nitrogen lone pair is a part of the aromatic sextet, protonation on nitrogen destroys the aromaticity, giving its conjugate acid a very low pKa (0.4) • The carbon atoms of pyrrole are more electron-rich and more nucleophilic than typical double-bond carbons (see comparison with cyclopentadiene) 28.3 Electrophilic Substitution Reactions of Pyrrole, Furan, and Thiophene • The heterocycles are more reactive toward electrophiles than benzene Position of Substitution • Electrophilic substitution normally occurs at C2, the position next to the heteroatom, giving more stable intermediate 28.4 Pyridine, a Six-Membered Heterocycle • Nitrogen-containing heterocyclic analog of benzene • Lone pair of electrons on N not part occupies an sp2 orbital in the plane of the ring and is not involved in bonding (Figure 28.3). Electronic structure of pyridine • Pyridine is a stronger base than pyrrole but a weaker base than alkylamines • The sp2-hybridized N holds the lone-pair electrons more tightly than the sp3-hybridized nitrogen in an alkylamine 28.5 Electrophilic Substitution of Pyridine • The pyridine ring undergoes electrophilic aromatic substitution reactions with great difficulty, under drastic conditions Low Reactivity of Pyridine • Complex between ring nitrogen and incoming electrophile deactivates ring with positive charge • Electron-withdrawing nitrogen atom deactivates causes a dipole making positively polarized C’s poor Lewis bases 28.6 Nucleophilic Substitution of Pyridine • 2- and 4-substituted (but not 3-substituted) halopyridines readily undergo nucleophilic aromatic substitution Mechanism of Nucleophilic Substitution on Pyridine • Reaction occurs by addition of the nucleophile to the C=N bond, followed by loss of halide ion Addition-Elimination • Addition favored by ability of the electronegative nitrogen to stabilize the anionic intermediate • Leaving group is then expelled 28.7 Fused-Ring Heterocycles • Quinoline, isoquinoline, and indole are fused-ring heterocycles, containing both a benzene ring and a heterocyclic aromatic ring Quinoline and Isoquinoline • Quinoline and isoquinoline have pyridine-like nitrogen atoms, and undergo electrophilic substitutions • Reaction is on the benzene ring rather than on the pyridine ring Indole • Has pyrrole-like nitrogen (nonbasic) • Undergoes electrophilic substitution at C3 of the electron-rich pyrrole Purine and Pyrimidine • Pyrimidine contains two pyridine-like nitrogens in a six-membered aromatic ring • Purine has 4 N’s in a fused-ring structure. Three are basic like pyridine-like and one is like that in pyrrole 28.8 Nucleic Acids and Nucleotides • Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), are the chemical carriers of genetic information • Nucleic acids are biopolymers made of nucleotides, aldopentoses linked to a purine or pyrimidine and a phosphate Sugars in DNA and RNA • RNA is derived from ribose • DNA is from 2-deoxyribose – (the ' is used to refer to positions on the sugar portion of a nucleotide) Heterocycles in DNA and RNA • Adenine, guanine, cytosine and thymine are in DNA • RNA contains uracil rather than thymine Nucleotides • In DNA and RNA the heterocycle is bonded to C1 of the sugar and the phosphate is bonded to C5 (and connected to 3’ of the next unit) The Deoxyribonucleotides The Ribonucleotides 28.9 Structure of Nucleic Acids • Nucleotides join together in DNA and RNA by as phosphate between the 5-on one nucleotide and the 3 on another • One end of the nucleic acid polymer has a free hydroxyl at C3 (the 3 end), and the other end has a phosphate at C5 (the 5 end). Generalized Structure of DNA Nucleic Acid Sequences • Differences arise from the sequence of bases on the individual nucleotides Describing a Sequence • Chain is described from 5 end, identifying the bases in order of occurrence, using the abbreviations A for adenosine, G for guanosine, C for cytidine, and T for thymine (or U for uracil in RNA) • A typical sequence is written as TAGGCT 28.10 Base Pairing in DNA: The Watson–Crick Model • In 1953 Watson and Crick noted that DNA consists of two polynucleotide strands, running in opposite directions and coiled around each other in a double helix • Strands are held together by hydrogen bonds between specific pairs of bases • Adenine (A) and thymine (T) form strong hydrogen bonds to each other but not to C or G • (G) and cytosine (C) form strong hydrogen bonds to each other but not to A or T H-Bonds in DNA • The G-C base pair involves three H-bonds A-T Base Pairing • Involves two H-bonds The Difference in the Strands • The strands of DNA are complementary because of H-bonding • Whenever a G occurs in one strand, a C occurs opposite it in the other strand • When an A occurs in one strand, a T occurs in the other Grooves • The strands of the DNA double helix create two continuous grooves (major and minor) • The sugar–phosphate backbone runs along the outside of the helix, and the amine bases hydrogen bond to one another on the inside • The major groove is slightly deeper than the minor groove, and both are lined by potential hydrogen bond donors and acceptors. 28.11 Nucleic Acids and Heredity • Processes in the transfer of genetic information: • Replication: identical copies of DNA are made • Transcription: genetic messages are read and carried out of the cell nucleus to the ribosomes, where protein synthesis occurs. • Translation: genetic messages are decoded to make proteins. 28.12 Replication of DNA • Begins with a partial unwinding of the double helix, exposing the recognition site on the bases • Activated forms of the complementary nucleotides (A with T and G with C) associate two new strands begin to grow The Replication Process • Addition takes place 5 3, catalyzed by DNA polymerase • Each nucleotide is joined as a 5-nucleoside triphosphate that adds a nucleotide to the free 3hydroxyl group of the growing chain 28.13 Structure and Synthesis of RNA: Transcription • RNA contains ribose rather than deoxyribose and uracil rather than thymine • There are three major kinds of RNA - each of which serves a specific function • They are much smaller molecules than DNA and are usually single-stranded Messenger RNA (mRNA) • Its sequence is copied from genetic DNA • It travels to ribsosomes, small granular particles in the cytoplasm of a cell where protein synthesis takes place Ribosomal RNA (rRNA) • Ribosomes are a complex of proteins and rRNA • The synthesis of proteins from amino acids and ATP occurs in the ribosome • The rRNA provides both structure and catalysis Transfer RNA (tRNA) • Transports amino acids to the ribosomes where they are joined together to make proteins • There is a specific tRNA for each amino acid • Recognition of the tRNA at the anti-codon communicates which amino acid is attached Transcription Process • Several turns of the DNA double helix unwind, exposing the bases of the two strands • Ribonucleotides line up in the proper order by hydrogen bonding to their complementary bases on DNA • Bonds form in the 5 3 direction, Transcription of RNA from DNA • Only one of the two DNA strands is transcribed into mRNA • The strand that contains the gene is the coding or sense strand • The strand that gets transcribed is the template or antisense strand • The RNA molecule produced during transcription is a copy of the coding strand (with U in place of T) Mechanism of Transcription • DNA contains promoter sites that are 10 to 35 base pairs upstream from the beginning of the coding region and signal the beginning of a gene • There are other base sequences near the end of the gene that signal a stop • Genes are not necessarily continuous, beginning gene in a section of DNA (an exon) and then resume farther down the chain in another exon, with an intron between that is removed from the mRNA 28.14 RNA and Protein Biosynthesis: Translation • RNA directs biosynthesis of peptides and proteins which is catalyzed by mRNA in ribosomes, where mRNA acts as a template to pass on the genetic information transcribed from DNA • The ribonucleotide sequence in mRNA forms a message that determines the order in which different amino acid residues are to be joined • Codons are sequences of three ribonucleotides that specify a particular amino acid • For example, UUC on mRNA is a codon that directs incorporation of phenylalanine into the growing protein Codon Assignments of Base Triplets The Parts of Transfer RNA • There are 61 different tRNAs, one for each of the 61 codons that specifies an amino acid • tRNA has 70-100 ribonucleotides and is bonded to a specific amino acid by an ester linkage through the 3 hydroxyl on ribose at the 3 end of the tRNA • Each tRNA has a segment called an anticodon, a sequence of three ribonucleotides complementary to the codon sequence The Structure of tRNA Processing Aminoacyl tRNA • As each codon on mRNA is read, tRNAs bring amino acids as esters for transfer to the growing peptide • When synthesis of the proper protein is completed, a "stop" codon signals the end and the protein is released from the ribosome 28.15 DNA Sequencing • The order of the bases along DNA contains the genetic inheritance. • Determination of the sequence is based on chemical reactions rather than physical analysis • DNA is cleaved at specific sequences by restriction endonucleases • For example, the restriction enzyme AluI cleaves between G and C in the four-base sequence AG-CT Note that the sequence is identical to that of its complement, (3)-TC-GA-(5) • Other restriction enzymes produce other cuts permitting partially overlapping sequences of small pieces to be produced for analysis Analytical Methods • The Maxam–Gilbert method uses organic chemistry to cleave phosphate linkages at with specificity for the adjoining heterocycle • The Sanger dideoxy method uses enzymatic reactions • The Sanger method is now widely used and automated, even in the sequencing of genomes The Sanger Dideoxy Method • The fragment to be sequenced is combined with: – A small piece of DNA (primer), whose sequence is complementary to that on the 3 end of the restriction fragment – The four 2-deoxyribonucleoside triphosphates (dNTPs) The Dideoxy Nucleotides • The solution also contains small amounts of the four 2,3-dideoxyribonucleoside triphosphates (ddNTPs) • Each is modified with a different fluorescent dye molecule The Dideoxy Method - Growing the and Stopping the Copied Chains • DNA polymerase is added and a strand of DNA complementary to the restriction fragment begins to grow from the end of the primer • Whenever a dideoxyribonucleotide is incorporated, chain extension cannot continue Dideoxy Method - Analysis • The product is a mixture of dideoxy-terminated DNA fragments with fluorescent tags • These are separated according to weight by electrophoresis and identified by their specific fluorescence 28.16 DNA Synthesis • DNA synthesizers use a solid-phase method starting with an attached, protected nucleotide • Subsequent protected nucleotides are added and coupled • After the final nucleotide has been added, the protecting groups are removed and the synthetic DNA is cleaved from the solid support • The bases are protected from reacting DNA Synthesis: Attachment • Attachment of a protected deoxynucleoside to a polymeric or silicate support as an ester of the 3 OH group of the deoxynucleoside • The 5 OH group on the sugar is protected as its pdimethoxytrityl (DMT) ether DNA Synthesis: DMT Removal • Removal of the DMT protecting group by treatment with a moderately weak acid DNA Synthesis: Coupling • The polymer-bound (protected) deoxynucleoside reacts with a protected deoxynucleoside containing a phosphoramidite group at its 3 position, catalyzed by tetrazole, a reactive heterocycle DNA Synthesis: Oxidation and Cycling • Phosphite is oxidized to phosphate by I2 • The cycle is repeated until the sequence is complete DNA Synthesis: Clean-up • All protecting groups are removed and the product is released from the support by treatment with aqueous NH3 28.17 The Polymerase Chain Reaction (PCR) • Copies DNA molecules by unwinding the double helix and copying each strand using enzymes • The new double helices are unwound and copied again • The enzyme is selected to be fast, accurate and heatstable (to survive the unwinding) • Each cycle doubles the amount of material • This is exponential template-driven organic synthesis PCR: Heating and Reaction • The subject DNA is heated (to separate strands) with – Taq polymerase (enyzme) and Mg2+ – Deoxynucleotide triphosphates – Two, oligonucleotide primers, each complementary to the sequence at the end of one of the target DNA segments PCR: Annealing and Growing • Temperature is reduced to 37 to 50°C, allowing the primers to form H-bonds to their complementary sequence at the end of each target strand PCR: Taq Polymerase • The temperature is then raised to 72°C, and Taq polymerase catalyzes the addition of further nucleotides to the two primed DNA strands PCR: Growing More Chains • Repeating the denature–anneal–synthesize cycle a second time yields four DNA copies, a third time yields eight copies, in an exponential series. • PCR has been automated, and 30 or so cycles can be carried out in an hour