* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture
Survey
Document related concepts
Bottromycin wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Polythiophene wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Petasis reaction wikipedia , lookup
Aromaticity wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Transcript
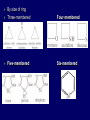
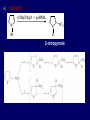
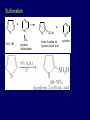
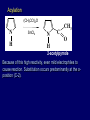
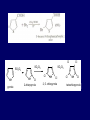
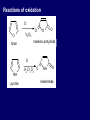
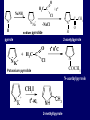
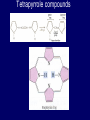
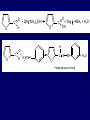
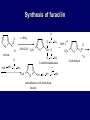
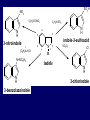
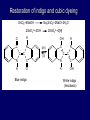
LECTERE 5 THEME: Classification, structure and biological role of heterocyclic compounds. Lecturer: Yevheniya B. Dmukhalska Plan 1. 2. 3. 4. Classification and nomenclature of heterocycles compouns. Five-membered heterocycles compouns with one heteroatom. Physical and chemical properties of furan, pyrrole, thiophene. The important derivatives of pyrrole, furan and thiophene. Six-membered heterocycles compouns with one heteroatom. Physical and chemical properties of indole. Heterocyclic compounds are cyclic compounds in which one or more ring atoms are not carbon (that is, hetero atoms). As hetero atom can be N, О, S, В, Al, Si, P, Sn, As, Cu. Bat common is N, О, or S. Classification Heterocycles are conveniently grouped into two classes, nonaromatic: and aromatic By size of ring Three-membered Four-membered Five-membered Six-membered Nonaromatic heterocycles Saturated monocyclic rings are named according to ring size as 3-, iran; 4-, -etan; 5-, -olan; and 6-, -ane. Even this system does not apply to nitrogencontaining rings and finds only limited use in common practice. Fivemembered heterocycles compounds with one heteroatom. The structures of these three heterocycles would suggest that they have highly reactive diene character. These heterocycles have characteristics associated with aromaticity. From an orbital point of view, pyrrole has а planar pentagonal structure in which the four carbons and the nitrogen have sp² hybridization. Each ring atom forms two sp²—sp² bonds to its neighboring ring atoms, and each forms one sp² – s bond to а hydrogen. Methods of synthesis of five-membered heterocycles compounds with one heteroatom. 1. Cyclization of 1,4-dicarbones compounds (Paale-Knorr synthesis ) H H C C H 2C CH 2 R C C R R C C R OH HO H SO c. 2 4 O O P 2S 5 NH3 R O R R R N H R S R Substituted furans, pyrroles, and thiophenes may be prepared by electrophilic substitution on one of the available materials discussed or by а variety of cyclization reactions. The most general is the PaalКлоrr synthesis, in which а 1,4-dicarbonyl compound is heated with а dehydrating agent, ammonia, or an inorganic sulfide to produce the furan, pyrrole, or thiophene, respectively. Reciprocal transformation of furan, pyrrole, thiophene (Yurie`s cycle reactions) NH3 H2O O H2S Al2O3 H2O NH3 S N H H2S Formation of furan: In laboratory conditions furan is produce by dry distillation of mucic acid. OH OH CH CH CH CH CO OH OH OH t COOH t - 3 H2O O COOH O furan furoic acid dehydromucic acid mucic acid - CO2 - CO2 COOH COOH O t In the industry furan derived from aldopentozes H H HO nH2O (C5H8O4)n t0 polypentoze nC5H10O5 pentoza H - CO2 O furoic acid COOH C O furan H C C t O C H OH OH HO C t0 C O H O furfural O H + 3H2O Formation of thiophene Thiophene is prepared industrially by passing а mixture of butane, butenes,or butadiene and sulfur through а reactor heated at 600' for а contact time of about 1 sec n- C4H10 + S = + H2 S Physical properties of furan, pyrrole, thiophene At room temperature, thiophene is a colorless liquid with a mildly pleasant odor reminiscent of benzene, with which thiophene shares some similarities. Like benzene, thiophene forms an azeotrope with water. Furan is typically derived by the thermal decomposition of pentose-containing materials, cellulosic solids especially pine-wood. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is toxic and may be carcinogenic. Pyrrole is a heterocyclic aromatic organic compound. Substituted derivatives are also called pyrroles. Porphobilinogen is a trisubstituted pyrrole, which is the biosynthetic precursor to many natural products Chemical properties of furan, pyrrole, thiophene The typical reaction of furan, pyrrole, and thiophene is electrophilic substitution. All three heterocycles are much more reactive than benzene. The reactivity order being is: To give some idea of the magnitude of this reactivity order, partial rate factors (reactivities relative to benzene) for tritium exchange with fluoroacetic acid. 1. Interaction with dilute mineral acids Pyrroles are polymerized by even dilute acids, probably by a mechanism such as the following . 2. Reactions of electrophilic substitution: This orientation is understandable in terms of the mechanism of electrophilic aromatic substitution. The / ratio is determined by the relative energies of the transition states leading to the two isomers. As in the case of substituted benzenes, we may estimate the relative energies of these two transition states by considering the actual reaction intermediates produced by attack at the -or -positions. a) Nitration (CH3CO)2O + р.HNO3 N N H H NO2 2-nitropyrrole Further substitution on 2-substituted furans tends to осcur at the other -position. With 2-substituted pyrroles and thiophenes, attack can occur at С-4 or С-5 when the group present is meta directing, or at С-3 and С-5 when the group present is ortho, раrа directing. Sulfonation + X X=O, NH + N SO3 pyridine sulfotrioxide X SO3H furan-2-sulfoacid pyrrole-2-sulfoacid N pyridine Acylation (CH3CO)2O N H SnCl4 N H C CH3 O 2-acetylpyrrole Because of this high reactivity, even mild electrophiles to cause reaction. Substitution occurs predominantly at the αposition (С-2). Of these structures, the most important are the two with the positive charge on sulfur because, in these two sulfonium cation structures, all atoms have octets of electrons. Nevertheless, as the sets of resonance structures show, the charge on the cation resulting from attack at the -position is more extensively delocalized than that for the cation resulting from attack at the -position. The following examples further demonstrate the generality of -attack. Halogenation In the last example, note that 2-iodothiophene is the sole product of iodination, eyeu though the reaction is carried out in benzene as solvent; that is, thiophene is so much more reactive than benzene that no significant amount of iodobenzene is formed. Cl Cl SO2Cl2 SO2Cl2 NH pyrrole NH Cl 2-chlorpyrrole SO2Cl2 Cl NH Cl 2, 5- chlorpyrrole Cl NH Cl tetrachlorpyrrole Reactions of reconstruction Thiophen are more stable and do not undergo hydrolysis. Reduction of pyrrole: Ni + 2 H2 O O tetrahydrofuran furan Pd + S thiophene 2 H2 S tetrahydrothiophene Reactions of oxidation O O V2O5 O O O maleinic anhydride furan O NH pyrrole H2Cr2O4 O NH O maleinmide O NaNH2 N H ;t H3C C N Cl + Na N -NaCl H sodium pyrrolide pyrrole C CH3 O 2-acetylpyrrole H3C C - + + NK O t 0C N Cl COCH3 Potassium pyrrolide N-acethylpyrrole CH 3I N K+ to -KI, NH CH3 2-methyllpyrrole Pyrrole compounds occur widely in living systems. One of the more important pyrrole compounds is the porphyrin hemin, the prosthetic group of hemoglobin and myoglobin. А number of simple alkylpyrroles have played an important role in the elucidation of the porphyrin structures. Thus, drastic reduction of hemin gives а complex mixture from which the four pyrroles, hemopyrrole, cryptopyrrole, phyllopyrrole, and opsopyrrole, have been isolated. For identification of pyrrole and furan used the method coloring of a pine chip. Couples of pyrrole painted a pine chip soaked in hydrochloric acid in the red colour and furan - in the green colour. Qualitative reaction on thiophene is indophenin`s reaction: a mixture of izathine with concentrated sulfuric acid painted in the blue colour. The important derivatives of pyrrole, furan and thiophene. Condensed of Pyrrole Pyrrole compounds occur widely in living systems. One of the more important pyrrole compounds is the porphyrin hemin, the prosthetic group of hemoglobin and myoglobin. А number of simple alkylpyrroles have played an important role in the elucidation of the porphyrin structures. Thus, drastic reduction of hemin gives а complex mixture from which the four pyrroles, hemopyrrole, cryptopyrrole, phyllopyrrole, and opsopyrrole, have been isolated. Tetrapyrrole compounds The function of hemoglobin in an organism is to transport oxygen. 1 g of hemoglobin absorbs 1.35 ml of oxygen at STP, corresponding to exactly one molecule of О2 per iron. Tetrapyrrole ancyclic compounds Vitamin B12 (cyanocobalamin), is an especially common vitamer of the vitamin B12 family. Cyanocobalamin is usually prescribed for the following reasons: after surgical removal of part, or all of the stomach or intestine to ensure there are adequate levels of vitamin B12 in the bloodstream; to treat pernicious anemia; vitamin B12 deficiency due to low intake from food; thyrotoxicosis, hemorrhage, malignancy, liver or kidney disease. Cyanocobamide is also used to perform the Schilling test to check your ability to absorb vitamin B12 Derivatives of furan Furfural is an industrial chemical compound derived from a variety of agricultural byproducts, including corncobs, oat and wheat bran, and sawdust. It is a colorless oily liquid with the odor of almonds, but upon exposure to air it quickly becomes yellow. Furfural's physical properties are summarized in the table at right. Furfural dissolves readily in most polar organic solvents, but is only slightly soluble in either water or alkanes. H H HO (C5H8O4)n polypentozes nH2O t0 C H nC5H10O5 pentoza C C O furfural O H H C C H OH OH HO + 3H2O O C H t0 Chemically, furfural participates in the same kinds of reactions as other aldehydes and other aromatic compounds. The aromatic stability of furfural is not as great as in benzene, and furfural participates in hydrogenation and other addition reactions more readily than many other aromatics. NaOH O + C O O CH2OH ONa furfurilic alcohol sodium salt of furoic acid O 2 O KCN C O H furfural CH C O O OH furoin O + 2 NH3 - 3H2O N O HC CH O N hydrofurfuramide HC O O + 2[Ag(NH ) ]OH 3 2 C H C O O + 2Ag +4NH3 + H2O OH H C O O + H H2N-NH C N NH O Phenylhydrazone furfural + H2O Synthesis of furacilin O O C O H c. HNO3 O (CH3CO)20 O N 2 furfural NH C CH3 CH O O NH2 O2N O CH N NH C semicarbazone of 5-nitrofurfural, furacilin + HOH, H O2N O C CH3 5-nitrofurfuraldiacetate O O H2N C NH2 O O C H 5-nitrofurfural Derivatives of thiophene Biotin (vitamin H) is a water-soluble B-complex vitamin which is composed of an ureido tetrahydroimidizalone) ring used with a tetrahydrothiophene ring. Physical and chemical properties of indole Indole (benzo [b] pyrrole) is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a solid at room temperature. The indole structure can be found in many organic compounds like the amino acid tryptophan and in tryptophan-containing protein, in alkaloids, and in pigments. Indole undergoes electrophilic substitution, mainly at position 3. SO3H NO2 C6H5COONO2 C5H5N SO3 N H 4 N H 3 5 3-nitroindole indole-3-sulfoacid + [C6H5NCl- N=N-C6H5 N H 3-benzolazoindole 6 7 N1 H 2 indole SO2Cl2 Cl N H 3-chlorindole The important derivatives of indole. a) indoxyl Indoxyl is isomeric with oxindol and is obtained as an oily liquid. Indoxyl is obtained from indican, which is a glycoside. The hydrolysis of indican yields β-D-glucose and indoxyl. Indigo dye is a product of the reaction of indoxyl by a mild oxidizing agent such as atmospheric oxygen. O OH C C N N H H keto form enol form Indigo is a powder, insoluble in water, with a melting point higher than 300C. It absorbs light in the yellow region of the spectrum (maximum at 602 nm), which gives it its intense blue colour. indoxyl O O H C C N O C C N N C H H O indigo Restoration of indigo and cubic dyeing SnCl2+4NaOH Na2SnO2+2NaCl+2H2O 2SnO22-+2OHO H C N C C 2SnO32-+2[H] 2[H] OH H C N C [O] C N C N C H O H OH Blue indigo White indigo (leicobasic) Tryptophan, serotonin, β-indolyl acetic acid For many organisms (including humans), tryptophan is an essential amino acid. This means that it cannot be synthesized by the organism and therefore must be part of its diet. Amino acids, including tryptophan, act as building blocks in protein biosynthesis. In addition, tryptophan functions as a biochemical precursor for the following compounds. Serotonin HO СH 2 СH 2 NH 2 N H Serotonin [5-hydroxy-3-( -aminoethyl) іndole] Serotonin is a monoamine neurotransmitter synthesized in serotonergic neurons in the central nervous system (CNS) and enterochromaffin cells in the gastrointestinal tract of animals including humans. Serotonin is also found in many mushrooms and plants, including fruits and vegetables. CH 2-COOH N H - indolyl acetic acid (heteroauxin) It is a heterocyclic compound that is an phytohormone called auxins. This colourless solid is probably the most important plant auxin. Structure, classification, nomenclature, izomery, methods of getting and chemical properties of pyridine. Heterocycles containing as a heteroatom atom of nitrogen, behave to the most widespread representatives of this group of connections (azynes): pyridine quinoline isoquinoline acridine Pyridine is an analog of benzene in which one of the СН units is replaced by nitrogen. The nitrogen lone pair is located in an sp2 hybrid orbital which is perpendicular to the system of the ring. Various values have been deduced for the empirical resonance energy of pyridine, but it would appear to be roughly comparable to benzene. The resonance stabilization is shown by the two equivalent Kekule structures and the three zwitterionic forms with negative charge on nitrogen. Derivatives of pyridine are biological active compounds, such as nicotine amide, nicotinic acid (vitamin PP). nicotinic acid Characteristic for pyridine reactions can be divided into three groups: Reactions which followings with participation of heteroatom. Reactions of substituting for the hydrogen atoms of pyridines ring. Reactions of reduction and oxidization. Reactions which followings with participation of heteroatom. 1. Cooperating with acids. Due to the indivisible pair of electrons atom of nitrogen of pyridine shows weak basic properties. At cooperating with strong mineral and organic acids he forms soluble salt of pyridine. pyridine bromide 2. Reaction with the oxide of sulphur (VI). Reactions of substituting for the hydrogen atoms of pyridines ring. 1. Reactions of electrophilic substitution (SE). The reactions of nitration, sulphonation and halogenation pass slowly drastic and with low exits. Thus an electrophilic reagent is direct in position 3. 3-nithropyridine 3-pyridinesulphure acid Reactions of nucleophilic substitution. The substitution goes on positions 2,4,6, most easy of nucleophilic reagent is entered in position of 2,6 (α-position ). The prime example of reaction of this type is an amination of pyridine with sodium of amide on Chychybabyne. The reaction flows to the mechanism SN2: σ- complex 2- aminopyridine Reactions of reduction and oxidization. 1. Reduction . pyperedine 2. Oxidization . nicotinic acid These ring systems, particularly that of pyrimidine, occur commonly in natural products. The pyrimidines, cytosine, thymine, and uracil are especially important because they are components of nucleic acids, as are the purine derivatives adenine and guanine. Diazines In this section, we shall take а brief look at another class of heterocycles, the diazines. The three types of diazabenzenes are: In addition to these three diazines, the bicyclic tetraaza compound, purine, is an important heterocyclic system. Aminopurines aminoderivatives of purine – adenine and guanine present in nucleinic acids as purine’s bases. Maine The рininе nucleus also occurs in such compounds as caffeine (coffee and tea) and theobromine (cacao beans). Hypoxantine and xantine have the same chemical properties as uric acid N-methyl derivatives of hypoxantine and xantine widely used in pharmacy Murexidne’s reaction is the qualitative reaction on uric acid By heating uric acid with nitrate acid and next adding of ammonium observe purpur-violet color Thanks you for attention!!!