* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download lec4-5-biosynthesis_specificity
Ultrasensitivity wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Peptide synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Restriction enzyme wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Epitranscriptome wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Genetic code wikipedia , lookup
Western blot wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Metalloprotein wikipedia , lookup
Catalytic triad wikipedia , lookup
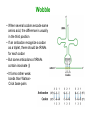
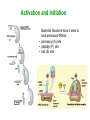
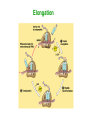
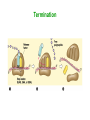
Biosynthesis of Enzymes • They must be • synthesized when needed • transported to appropriate cellular location • degraded when no longer needed • Research in ribosome structure reveals that proteins are synthesized by a gigantic RNA enzyme (ribozyme) Wobble • When several codons encode same amino acid, the difference is usually in the third position • If an anticodon recognize a codon as a triplet, there should be tRNAs for each codon • But some anticodons of tRNAs contain inosinate (I) • It forms rather weak bonds than WatsonCrick base pairs Wobble hypothesis 1. First 2 bases of codon form strong bonds and determine the specificity 2. First base of anticodon determines the number of codons recognized by tRNA The wobble (or third) base of the codon also permits rapid dissociation of tRNA during protein synthesis So accuracy and speed of reaction is balanced by this way Protein Synthesis 1. Activation of amino acids: amino acids are attached to corresponding tRNA by aminoacyl-tRNA synthetase (ATP) 2. Initiation: mRNA binds to small subunit of ribosome and initiating tRNA, then large subunit binds -> initiation complex (GTP, initiation factors) 3. Elongation: succesive covalent attachment of amino acids (GTP, elongation factors) 4. Termination and release: completion is signaled by a termination codon (release factors) 5. Folding and posttranslational processing: removal of one or more amino acid, addition of acetyl, phosphoryl, methyl, carboxyl or other groups, attachment of oligosccharides or prosthetic groups Activation and Initiation Bacterial ribosome have 3 sites to bind aminoacyl-tRNAs: • aminoacyl (A) site • peptidyl (P) site • exit (E) site Elongation Termination Polypeptide chains undergo folding and processing Post-translational modifications: 1. Amino or carboxy terminal modifications (amino terminal Met residue is generally removed) 2. Loss of signal sequences (sequences to direct polypeptide to its ultimate location) 3. Modification of individual amino acids: phosphorylation, carboxyl group addition, methylation 4. Attachment of carbohydrates 5. Addition of isophenyl groups (helps to anchor protein to the membrane) 6. Addition of prosthetic groups (e.g. heme group) 7. Proteolytic processing (from large inactive precursors...) 8. Formation of disulphide xlinks (protect from denaturation) Protein synthesis is inhibited by many antibiotics and toxins Puromycin: binds to A site, participate in peptide bond formation and dissociate with unfininished peptide... Some other antibiotics: • Tetracyclins (block A site), • Chloramphenicol (block peptidyl transferase), • Streptomycin (dose dependent action) Toxins (to mammalians): Diphtheria toxin and ricin (from castor bean) Specificity of enzyme action-1 • Most enzymes are highly specific to their substrate and reaction catalysed – Bond specificity: e.g peptidase, phosphatase – Group specificity: e.g hexokinase – Absolute or near-absolute specificity: e.g. glucokinase • Stereospecificity: – Dehydrogenases catalyst the transfer of hydrogen from the substrate to a particular side of nicotinamide ring in NAD+ or NADP+ – Phenylalanine hydroxylase uses L-Phe not D-Phe – NZ-catalysed rxns may yield stereospecific products even when substrate possesses no asymmetric carbon atom... • Importance of specificity in DNA replication and protein synthesis proofreading Specificity of enzyme action-2 The active site • Orgston (1948) at least three different points of interaction... • These interactions can have: – Binding function binding sites – Catalytic function catalytic sites • A.acid residues in the active site which do not have either of these functions may also contribute to specificity... – Side-chains must be of suitable size, shape and character – Creation of a specific microenvironment... Specificity of enzyme action-3 The active site • The active site takes up a relatively small portion of the total volume of an enzyme • The active site is a 3-D entity – In lysozyme, the important groups in the active site are contributed by residues numbered 35, 52, 62, 63, and 101 in their linear sequence of 129 a.acids • The specificity of binding depends on the precisely defined arrangement of atoms in an active site • Substrates are bound to enzymes by relatively weak forces • Active sites are clefts or crevices – In all enzymes of known structure, substrate molecules are bound to a cleft or crevice from which water is largely excluded Specificity of enzyme action-4 The Fischer “lock-and-key” hypothesis • • Fischer (1890) According to this model, structures do not change their shape during the binding process The Koshland “induced-fit” hypothesis • • Koshland (1958) X-ray diffraction analysis and NMR data have revealed differences in structure btw free and substrate-bound NZs Conformational change • Such mechanism could help to achieve high degree of specificity.... – e.g. yeast hexokinase D-hexose + ATP D-hexose-6-P + ADP Induced-fit Specificity of enzyme action-5 Hypothesis involving strain or transition-state stabilization • Both lock-and-key and induced-fit can explain NZ specificity BUT what is the mechanism to drive the reaction??? • Energy is often spent in substrate-binding and further energy must be supplied for reaction to proceed • Haldane (1930) and Pauling (1948) Binding energy is used to distort the substrate in such a way to facillate the subsequent reaction – Little clear-cut evidence on distorted binding • Transition state stabilization is more likely – Substrate is bound in an undistorted form, but ES complex possesses various unfavorable interaction yielding: ES complex transition state products Specificity of enzyme action-6 Specificity of enzymes and drug design • In addition to exploiting a binding event, a highly specific chemical events takes place in the enzyme's active site • There are 317 FDA approved drugs that target enzymes (human, bacterial, viral, fungal and protazoal) • The majority of these drugs (~65%) rely on some type of substrate mimicking (direct interaction with cofactor, structural resemblance to substrate, transition state analogue, etc.). • In order to exploit these highly specific chemical interactions, one must fully understand the enzyme mechanism, and perhaps even obtain a detailed transition state structure Monomeric and oligomeric enzymes-1 Monomeric Enzymes • Only a single polypeptide chain.... • Very few monomeric NZs are known and all catalyse hydrolytic rxns (e.g. Proteases) • 100-300 a-acid with Mw: 13-35 kDa • Most act without a cofactor • They are often synthesized in inactive form: proenzyme or zymogen Monomeric and oligomeric enzymes-2 The serine proteases: • Chymotrypsin, trypsin and elastase • Only 40% of the 1O structure is common but – Most catalytically important residues are same – Their 3O structures are very similar • All are endopeptidases but their specificity different: – Chymotrypsin: hydrophobic side chains – Trypsin: specificity for basic side chains – Elastase: small non-polar side chains Some other monomeric NZs • Pepsin (an acid protease) in mammals • Thiol proteases (e.g. papain, ficin) in plants • Exopeptidases (e.g. carboxypeptidase A and B) • Ribonucleases and lysozymes (act on non-protein substrates...) Monomeric and oligomeric enzymes-3 Oligomeric Enzymes • Two or more polypeptide chains • Linked usually by non-covalent interactions and NEVER by peptide bond • May be identical or different (subunits) • Mw >35 kDa • Not synthesized as inactive zymogens Monomeric and oligomeric enzymes-4 Some examples Lactate dehydrogenase (LDH): • Tetramer catalysing the rxn: Lactate + NAD+ pyruvate + NADH + H+ • A tetramer of 140 kDa • Two subunit types (from seperate genes), M- and H-form, predominating different tissues – Monomers are catalytically inactive – All five isoenzymes of LDH can exist...... – They all catalyse the same reaction but with different characteristics Monomeric and oligomeric enzymes-5 Lactose synthase • Non-functional subunit modifies behavior of the functional subunit -lactalbumin + galactosyl transferase • (-) -lactalbumin involve in the synthesis of the carbohydrate components of glycoproteins (+) -lactalbumin lactose production Monomeric and oligomeric enzymes-6 Tryptophan synthase • Two different functional subunits (2 and ): indole-3-glycerol-P indole + glyceraldehyde-3-P indole + L-serine L-tryptophan • ( subunit) ( subunit) The rate of these partial reactions are less than 5% of the rate of the reaction catalysed by intact 22 enzyme... Pyruvate dehydrogenase • • • • A multienzyme complex.... Enable pyruvate to enter the TCA cycle acetyl-CoA production E.coli enzyme: 60 polypeptide chains with a MW: ca 4600 kDa.... Three catalytic activities are present Engineered enzymes-1 AIM • to alter the reaction mechanism of the enzyme to catalyse new reactions • to expand substrate specificity • to switch or increase substrate specificity, such as improvement of the enantioselectivity • to increase stability Alteration of enzyme reaction chemistry is an attractive strategy to obtain new catalysts for the synthesis of fine chemicals Engineered enzymes-2 Directed evolution: • It is a powerful strategy that does not rely on knowledge of the 3-D structure of the enzyme – Usage of molecular biological methods such as random mutagenesis or gene shuffling for generating large diverse DNA libraries – Gene expression – High-throughput screening for the identification of highly selective mutant enzymes Rational redesign: • a good 3-D structure should be available • It is often based on molecular graphics or more advanced molecular modeling (structure-based computational design techniques) • Such models place an emphasis on enthalpic interactions and omit entropic contributions Rational redesign-1 Reshaping enzyme specificity • reshaping a substrate-binding site or cofactor specificity or positioning charged residues to favor one substrate relative to another one Re-engineering catalytic mechanisms • The current view is that chemistry, not binding specificity, is the dominant factor in the evolution of new enzymatic activities. As a consequence, proteins with similar folds can support very different chemical reactions after the incorporation of new catalytic groups Engineering by molecular assembling • assembling the necessary components, that is, the catalytic machinery, a substrate-binding site and so on, on a selected macromolecular template Rational redesign-2 Reshaping enzyme specificity e.g. linoleate 13-lipoxygenase 9-lipoxygenase • by a single substitution, H608V, at the level of the residue that is supposed to be responsible for the positional specificity of the substrate • Bulky His608 replaced a basic residue at the bottom of the active pocket becomes accessible • The carboxylate group of the lipid substrate, which is normally outside the pocket, becomes able to interact with this basic residue Different oxygenation site in the lipid Rational redesign-3 Re-engineering catalytic mechanisms • 4 substitutions an oleate-hydroxylase changed on an oleatedesaturase • Butyrylcholinesterase (BChE) enzyme is transformed to take a potent inhibitor as a substrate • The mechanism of BChE can be redirected to favor the hydrolysis of the strong irreversible inhibitors (e.g. Soman), as opposed to irreversible modification of BChE Rational redesign-4 Engineering by molecular assembling-1 Inventory of robust catalytic machineries • A prerequisite to convergent enzyme redesign is the identification of the small number of catalytic devices that can work in various structural contexts • The TESS software searches through a dataset of PDB structures for user-defined combinations of atoms or residues • The results have been compiled in the PROCAT database of 3D active site coordinates • Another computational search for consensus catalytic devices, such as the Ser–His–Asp catalytic triad, the His–His heme coordination site Rational redesign-5 Engineering by molecular assembling-2 Searching for appropriate engineering templates • Nature seems to recruit a limited number of protein folds for building a large variety of functions • Some enzyme superfamilies display remarkably divergent properties in terms of functions, while conserving some specific chemical properties • However, the possibilities offered by nature to protein engineers for the redesign of enzyme function seem to go beyond this well-known subset of structures • For example, a soluble and functional chimaeric bacterial– human cytochrome P450 is engineered: The resulting hybrid enzyme exhibits mammalian enzyme active site characteristics, with the solubility property of the bacterial enzyme Rational redesign-6 Engineering by molecular assembling-3 Grafting catalytic machineries • It is possible to engineer catalytic antibodies by grafting functional residues • A protease that is able to cleave a small bacterial protein was engineered from an immunoglobulin single-chain variable fragment after introducing three residues: – a lysine to increase the polarizability of the carbonyl group – a glutamate to increase the nucleophilicity of a nearby water molecule – a histidine to provide a proton to convert the amine into a better leaving group Abzymes-1 • Catalytic antibody, abzyme (mid-80s) • Abzymes which are able to hydrolyze proteins, DNA, RNA, or polysaccharides have been found in the sera of patients with autoimmune and also viral pathologies • Possible applications – As sequence-specific cleaving agents to destroy peptides or carbohydrates associated with viral particles or tumor cells – The genetic deficiency of an extracellular enzyme could be cured by immunization with an appropriate hapten to elicit catalytic antibodies that would substitute the missing enzyme – Catalytic antibodies with the capacity to degrade cocaine into the resulting nontoxic products for cocain addiction – Detoxification after accidental exposure to insecticides or the intentional poisoning by nerve gas Abzymes-2 • One of the most fascinating potential applications is in the area of prodrug activation in cancer treatment using a strategy called Antibody Directed Abzyme Prodrug Therapy (ADAPT) Ribozymes • In the early 1980s, Sidney Altman and Thomas Cech independently found that RNAs can also act as catalysts for chemical reactions • This class of catalytic RNAs are known as ribozymes (1989 Nobel Prize in Chemistry) – Ribozome – Group I and II introns – «5S rRNA Is a Leadzyme. A Molecular Basis for Lead Toxicity» M.Z. Barciszewska et al (2003) – «Hammerhead RNA motif» In the natural state, is a single strand of RNA: the cleavage is autocatalytic but not a true enzyme in its natural state, as it cannot catalyze multiple turnovers BUT in vitro hammerheads can be engineered – Artificial ribozymes