* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download practice oxidative phosphorylation worksheet11
Metalloprotein wikipedia , lookup
Epitranscriptome wikipedia , lookup
Magnesium in biology wikipedia , lookup
Butyric acid wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Mitochondrion wikipedia , lookup
Biochemistry wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
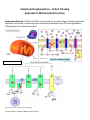
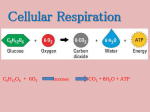
Oxidative Phosphorylation: Critical Thinking Approach to Mitochondrial Function Background Material: NADH and FADH2 made during the previous steps of aerobic respiration of glucose, fatty acids, or amino acids are ultimately transformed into ATP during Oxidative Phosphorylation in the mitochondria! Mcatzone.com Dept. Biology Penn State ©2004 https://www.bioscience.org/2009/v14/af/3509/fig5.jpg © Kristin Rosler, Johnson & Wales University 2014 Passive Transport is the flow of particles from a region of high concentration to a region of low concentration & uses NO energy to do so (spontaneous). Active Transport is the flow of particles from a region of low concentration to a region of high concentration & USES energy to do so (not spontaneous). The intermembrane space (aka outer matrix) is acidic (or high [H+]) The inner matrix is basic (or low [H+]) NADH & FADH2 are in the inner matrix and now are utilized for the production of ATP via oxidative phosphorylation. A series of proteins in the inner membrane will be used to do so. Active transport utilized to move H+ against concentration gradient in the Electron Transport Chain! A series of redox reactions are employed as H+ ions are moved against a concentration gradient via active transport. Protein Complexes I, III, & IV are H+ protein complex ““pump”s” which use the energy harnessed from the oxidation of NADH and FADH2 (electron movement = energy!) in the Electron Transport Chain (ETC) to ““pump”” H+ against a concentration gradient. Passive transport is coupled to the synthesis of ATP during chemiosmosis! Protein Complex V (ATP Synthase) couples the passive flow of H+ back into the inner matrix to the formation of ATP. This process can be likened to a water wheel at an old time mill. H+ pour from the high concentration of H+ on one side of the mitochondria membrane to the low concentration of H+ on the other. As the H+ pour passively down the concentration gradient through Protein Complex V, the inner domain of the protein complex “rotates.” This energy that is released from the passive movement of H+ (exergonic reaction) is now coupled to the endergonic synthesis of ATP, ADP + Pi ATP. Oxidative Phosphorylation (occurs in the inner membrane of mitochondria) I. Electron Transport Chain in inner mitochondrial membrane A. NADH oxidation: NADH → Complex I → Q → Complex III → Cytochrome c → Complex IV → O2 where Complexes I, III and IV are active proton ““pump”s,” while Q and Cytochrome c are mobile electron carriers. The final electron acceptor is molecular oxygen total of 3 H+ ““pump”s” move 12 H+ against concentration gradient B. FADH2 oxidation: FADH2 Complex II Q Complex III Cytochrome c Complex IV O2 where Complexes III and IV are active proton ““pump”s,” while Q and Cytochrome C are mobile electron cariers. The final electron acceptor is molecular oxygen total of 2 H+ ““pump”s” move 8 H+ against concentration gradient © Kristin Rosler, Johnson & Wales University 2014 B. Protein Complexes Involved a. Complex I: NADH dehydrogenase, oxidizes NADH and reduces FMN (Flavin mononucleotide) FMNH2, & passes its 2 e- to Q QH2; energy released upon electron oxidation used to actively moves 4 H+ across membrane, against gradient b. Q: Ubiquinone Q, mobile electron carrier that passes the 2 e- from FMNH2 via Q QH2 from Complex I to Complex III for NADH and from Complexes II to III for FADH2 c. Complex II: Succinate-CoQ reductase, oxidizes FADH2 and reduces Q QH2 d. Complex III: CoQH2-cytochrome c reductase, oxidizes QH2 and reduces 2 Cytochrome C/Fe+3 Cytochrome C/Fe+2; active transport of 4 H+ across membrane, against gradient e. Cyt C: Cytochrome C, water-soluble e- carrier that passes its 2 e- from Complex III to Complex IV f. Complex IV: Cytochrome C oxidase, passes 2 e- from Cyt C to oxygen 1/2 water; it is important to note that the final electron acceptor in aerobic organisms is oxygen and the endproduct is water. Each NADH or FADH2 1/2 H2O * Interestingly, many animals (camels, hibernating bears) rely upon the water being produced at the end of the ETC as its source of water while metabolizing fatty acids that lead to the formation of many NADH and FADH2. * Anaerobes, on the other hand, can use alternative terminal oxidases in an inducible manner depending upon cellular environments. Final electron acceptors can be nitrites, nitrates, DMSO, and fumarates to mention a few. II. Chemiosmosis in the inner mitochondrial membrane: V: ATP synthase couples the passive flow of H+ from the outer matrix back into the inner matrix to the production of ATP. So, for each H+ ““pump”” that was active during the electron transport chain, the H+ flow back, 1 ATP is made. For each NADH, 3H+ ““pump”s” are actively utilized, therefore 3 ATP are made. For each FADH2, 2H+ ““pump”s” are actively utilized, therefore 2 ATP are made. Sources Used: Nelson DL; Cox MM (April 2005). Lehninger Principles of Biochemistry (4th ed.). W. H. Freeman. ISBN 978-0-7167-4339-2. White D. (September 1999). The Physiology and Biochemistry of Prokaryotes (2nd ed.). Oxford University Press. ISBN 978-0-19-512579-5. © Kristin Rosler, Johnson & Wales University 2014 Tally Sheet for Glucose Aerobic Respiration Overall Equation: C6H12O6 + 6 O2 6 CO2 + 6 H2O + 38 ATP ATP PRODUCTION I. GLYCOLYSIS: - 2 ATP in energy investment phase + 4 ATP in energy pay-off phase *substrate-level phosphorylation +2 NADH X 3 ATP/NADH + 2 ATP = + 6 ATP = +6 ATP II. PREP PHASE, PYRUVATE GROOMING + 2 NADH X 3 ATP/NADH III. CITRIC ACID CYCLE + 3 NADH X 2 rotations per glucose X 3 ATP/NADH = 18 ATP + 1 FADH2 X 2 rotations per glucose X 2 ATP/FADH2 = 4 ATP + 1 ATP X 2 rotations per glucose = 2 ATP WATER PRODUCTION Each NADH and FADH2 ½ H20 at the end of the ETC Therefore, for the 10 NADH + 2 FADH2 made = 6 H2O © Kristin Rosler, Johnson & Wales University 2014 + 38 ATP Critical Thinking Time: Based upon your understanding of the pathways, determine the values in the blanks below. Under NORMAL + O2 Circumstances: For each ACETYL-co A ________ NADH X _____ turn X _______ ATP/NADH = _______ ATP ________ FADH2 X______ turn X _______ ATP/FADH2 = _______ ATP ________ ATP X _____ turn + = ______ ATP Total yield _______ ATP A. Imagine a drug blocks H+ passive diffusion back through protein V, ATP synthase. How many ATP would each Acetyl-coA make in this scenario? ________________ 1 Acetyl-co A turns CAC _____ NADH X _____ turn X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ turn X _____ ATP/FADH2 = ________ATP _____ ATP X _____ turn = + ________ATP Total yield = ___________ATP B. Imagine a drug blocks FADH2 binding to succinate dehydrogenase, protein II. How many ATP would each Acetyl-coA make in this scenario? _______________ 1 Acetyl-co A turns CAC _____ NADH X _____ turn X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ turn X _____ ATP/FADH2 = ________ATP _____ ATP X _____ turn = + ________ATP Total yield = ___________ATP C. Imagine a drug damages the inner cell membrane of the mitochondria and causes “pores” to form between these two domains. How many ATP would each Acetyl-coA make in this scenario? _________________ 1 Acetyl-co A turns CAC _____ NADH X _____ turn X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ turn X _____ ATP/FADH2 = ________ATP _____ ATP X _____ turn = + ________ATP Total yield = ___________ATP © Kristin Rosler, Johnson & Wales University 2014 Fatty Acid Oxidation: Fatty acids yield an immense amount of ATP! See how below! A. Step 1: Fatty Acid Primed (-1 ATP per fatty acid) in cytoplasm - 1 ATP Fatty acid + Coenzyme A Fatty acyl-co A (now imported into mitochondria) B. Step 2: Fatty Acyl-co A “cleavage” 2-C Acetyl-co As (# Carbons / 2) * Cleaves = (# carbons/ 2) -1 * Each “cleave” 1 FADH2 + 1 NADH C. Step 3: Acetyl-coAs enter CAC Acetyl-co A (2-C) Turns 1 X per Acetyl-coA CAC Each turn of the CAC yields: 3 NADH 1 FADH2 1 ATP © Kristin Rosler, Johnson & Wales University 2014 Calculate the number of ATP made from the following molecules. Stearic Acid: 18-C fatty acid (use the tally sheet below to determine the number of total ATP I. Fatty acid priming? (how many ATP needed?) _______ ATP II. 18-C chain produces _______ cleaves. Each cleave produces: _____ NADH X _____ cleaves X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ cleaves X _____ ATP/FADH2 = ________ ATP III. 18-C chain forms ______ Acetyl-co As (2-C) Each Acetyl-co A turns CAC: _____ NADH X _____ turns X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ turns X _____ ATP/FADH2 = ________ATP _____ ATP X _____ turns = ________ATP Total yield = I. ________ATP II. ________ ATP + III. ________ ATP NET: ____________ ATP IV. A triglyceride is made up of ____ fatty acids. Just from the fatty acid components, how many ATP are made? _________ © Kristin Rosler, Johnson & Wales University 2014 ALIEN MITOCHONDRIAL OXIDATIVE PHOSPHORYLATION Imagine additional proteins in the electron transport chain evolved in an organism’s mitochondria as illustrated below. Assume protein I is the site of NADH oxidation, and protein III is the site of FADH 2 oxidation. Also assume that proteins I, II, IV, V and VI “pump” 1 Hydrogen against the concentration gradient. A. How many ATP would be generated for the oxidation of 1 Acetyl-co A for this organism? 1 Acetyl-coA turns CAC _____ NADH X _____ turn X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ turn X _____ ATP/FADH2 = ________ATP _____ ATP X _____ turn = + ________ATP Total yield = ___________ATP B. How many ATP would be generated for the oxidation of 1 Acetyl-co A if treated with a toxin that blocked NADH binding to protein I, NADH dehydrogenase? 1 Acetyl-co A turns CAC _____ NADH X _____ turn X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ turn X _____ ATP/FADH2 = ________ATP _____ ATP X _____ turn = + ________ATP Total yield = ___________ATP C. How many ATP would be generated upon the oxidation of 1 Acetyl-co A if treated with a toxin that blocked protein VII function? 1 Acetyl-coA turns CAC _____ NADH X _____ turn X _____ ATP/NADH = ________ ATP _____ FADH2 X _____ turn X _____ ATP/FADH2 = _____ ATP X _____ turn = ________ATP + ________ATP Total yield = ___________ATP © Kristin Rosler, Johnson & Wales University 2014 Answers: Under NORMAL + O2 Circumstances: For each ACETYL-co A __3_NADH X _1___ turn X ___3____ ATP/NADH = ___9____ ATP ___1_FADH2 X_1__ turn X ____2___ ATP/FADH2 = ____2___ ATP ___1_ ATP X _1__ turn + Total yield = ____1__ ATP __12__ ATP A. Imagine a drug blocks H+ passive diffusion back through protein V, ATP synthase. How many ATP would each Acetyl-coA make in this scenario? 1 ATP (all ATP made from oxidative phosphorylation negated, just from substrate-level phosphorylation during the CAC) 1 Acetyl-co A turns CAC __3___ NADH X ___1__ turn X ___0__ ATP/NADH = ____0____ ATP __1___ FADH2 X __1___ turn X __0___ ATP/FADH2 = ____0____ATP ___1__ ATP X __1___ turn = + _____1___ATP Total yield = _____1______ATP B. Imagine a drug blocks FADH2 binding to succinate dehydrogenase, protein II. How many ATP would each Acetyl-coA make in this scenario? ____10 ATP (all the ATP made from FADH2 oxidation negated!) 1 Acetyl-co A turns CAC ___3__ NADH X __1___ turn X __3___ ATP/NADH = _____9___ ATP __1___ FADH2 X __1___ turn X __0___ ATP/FADH2 = ___0_____ATP __1___ ATP X __1___ turn = + _____1___ATP Total yield = ___10____ATP C. Imagine a drug damages the inner cell membrane of the mitochondria and causes “pores” to form between these two domains. How many ATP would each Acetyl-coA make in this scenario? 1 ATP, again if there is no difference in H+ concentration between the two sides of the mitochondrial matrices, there will be no chemiosmosis specifically 1 Acetyl-co A turns CAC __3___ NADH X ___1__ turn X ___0__ ATP/NADH = ____0____ ATP __1___ FADH2 X __1___ turn X __0___ ATP/FADH2 = ____0____ATP ___1__ ATP X __1___ turn = + _____1___ATP Total yield = _____1______ATP Stearic Acid: 18-C fatty acid (use the tally sheet below to determine the number of total ATP I. Fatty acid priming? (how many ATP needed?) ___- 1 ATP____ ATP II. 18-C chain produces ___8____ cleaves. Each cleave produces: __1___ NADH X __8___ cleaves X ___3__ ATP/NADH = ___24_____ ATP __1___ FADH2 X __8___ cleaves X ___2__ ATP/FADH2 = ___16_____ ATP © Kristin Rosler, Johnson & Wales University 2014 III. 18-C chain forms __9____ Acetyl-co As (2-C) Each Acetyl-co A turns CAC: ___3__ NADH X __9___ turns X __3___ ATP/NADH = ___27_____ ATP ___1__ FADH2 X __9___ turns X __2___ ATP/FADH2 = __18______ATP ___1__ ATP X __9___ turns = ____9____ATP Total yield = I. __-1______ATP II. ___40_____ ATP + III. ___54_____ ATP NET: ______93______ ATP IV. A triglyceride is made up of __3__ fatty acids. Just from the fatty acid components, how many ATP are made? _279________ CRITICAL THINKING TIME! ALIEN MITOCHONDRIAL OXIDATIVE PHOSPHORYLATION A. How many ATP would be generated for the oxidation of 1 Acetyl-co A for this organism? 19 ATP, If 5 H are “pump”ed per NADH 5 ATP/NADH; if 3 H+ are “pump”ed per FADH2, then 3 ATP per FADH2 1 Acetyl-coA turns CAC __3___ NADH X __1___ turn X __5___ ATP/NADH = ___15_____ ATP __1___ FADH2 X __1___ turn X __3___ ATP/FADH2 = __3______ATP __1___ ATP X ___1__ turn = + ___1_____ATP B. How many ATP would be generated for the oxidation of 1 Acetyl-co A if treated with a toxin that blocked NADH binding to protein I, NADH dehydrogenase? 4 ATP 1 Acetyl-coA turns CAC __3___ NADH X __1___ turn X __0___ ATP/NADH = ___0_____ ATP __1___ FADH2 X __1___ turn X __3___ ATP/FADH2 = __3______ATP __1___ ATP X ___1__ turn = + ___1_____ATP C. How many ATP would be generated upon the oxidation of 1 Acetyl-co A if treated with a toxin that blocked protein VII function? 1 ATP 1 Acetyl-coA turns CAC __3___ NADH X __1___ turn X __0___ ATP/NADH = ___0_____ ATP __1___ FADH2 X __1___ turn X __0___ ATP/FADH2 = _0_____ATP __1___ ATP X ___1__ turn = © Kristin Rosler, Johnson & Wales University 2014 + ___1_____ATP