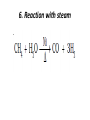* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download o-chem - WordPress.com
Survey
Document related concepts
Fischer–Tropsch process wikipedia , lookup
Homoaromaticity wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Aromaticity wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
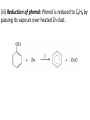
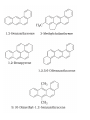
UNIT 13-HYDROCARBONS B K Sharma PGT K.V.BHEL Jagdishpur ALKANES • alkanes are saturated open chain hydrocarbons containing carbon - carbon single bonds. Methane (CH4) is the first member of this family. Preparation • 1.From unsaturated hydrocarbons • H2 gas adds to alkenes and alkynes in the presence of finely divided catalysts like Pt, Pd or Ni to form alkanes. • This process is called hydrogenation. 2. From alkyl halides • i) Alkyl halides (except fluorides) on reduction with zinc and dilute hydrochloric acid give alkanes. Wurtz reaction • ii) Alkyl halides on treatment with Na metal in dry ethereal (free from moisture) solution give higher alkanes. • Uses :- preparation of higher alkanes containing even number of carbon atoms. What will happen if two different alkyl halides are taken? 3. From carboxylic acids • i) Sodium salts of carboxylic acids on heating with soda lime (NaOH & CaO) give alkanes containing one carbon atom less than the carboxylic acid. • This process of elimination of CO2from a carboxylic acid is known as decarboxylation. ii) Kolbe’s electrolytic method Physical properties • almost non-polar molecules Chemical properties • 1. Substitution reactions • One or more hydrogen atoms of alkanes can be replaced by halogens, nitro group etc. Halogenation 2. Combustion • Alkanes on heating in the presence of air or • O2 are completely oxidized to CO2and H2Owith the evolution of large amount of heat. 3. Controlled oxidation • Alkanes on heating with a regulated supply of O2 or air at high pressure and in the presence of suitable catalysts give a variety of oxidation products. 4. Isomerisation • n-Alkanes on heating in the presence of anhydrous AlCl3and HCl gas isomerise to branched chain alkanes. 5. Aromatization (reforming) • n-Alkanes having six or more carbon atoms on heating to 773K at 10-20 atm. pressure in the presence of oxides of V,Mo or Cr gives benzene and its homologues. • This reaction is known as aromatization . 6. Reaction with steam 7. Pyrolysis • Higher alkanes on heating to higher temp. decompose into lower alkanes, alkenes etc. Such a decomposition reaction into smaller fragments by the application of heat is called pyrolysis or cracking. ALKENES • Alkenes are unsaturated hydrocarbons containing at least one double bond. Preparation • 1. From alkynes • Alkynes on partial reduction with H2in the presence of Pd/C (Lindlar’s catalyst) partially deactivated with poisons like S give cis alkenes. • However, alkynes on reduction with Na in liq NH3 form trans alkenes. 2. From alkyl halides (dehydrohalogenation)/β-elimination reaction • Alkyl halides (R-X) on heating with alc KOH eliminate one molecule of halogen acid to form alkenes. This reaction is known as dehydrohalogenation 3. From vicinal dihalides (dehalogenation.) 4. From alcohols by acidic dehydration: • Alcohols on heating with conc.H2SO4form alkenes with the elimination of one H2O molecule. • this reaction is known as acidic dehydration of alcohols. Physical properties of alkene Chemical properties • 1. Addition of dihydrogen 2. Addition of halogens 3. Addition of hydrogen halides: • (Markovnikov Rule) • Negative part of the addendum gets attached to that double bonded carbon atom which possesses lesser number of hydrogen atoms. Mechanism Anti Markovnikov addition or peroxide effect or Kharash effect Mechanism the peroxide effect is not observed in addition of HCl and HI. H–Cl bond stronger (430.5 kJ mol–1) than H–Br bond (363.7 kJ mol–1), is not cleaved whereas the H–I bond is weaker (296.8 kJ mol–1) and iodine free radicals combine to form iodine molecules instead of adding to the double bond. Problem 13.12 Write IUPAC names of the products obtained by addition reactions of HBr to hex-1-ene (i) in the absence of peroxide and (ii) in the presence of peroxide. Addition of water : Oxidation:-(a)Alkenes on reaction with cold, dil(aq)solution of KMnO4(Baeyer’s reagent) produce vicinal glycols. Decolonization of KMnO4 solution is used as a test for unsaturation. b) Acidic KMnO4 or acidic KMnO4 oxidises alkenes to ketones and/or acids depending upon the nature of the alkene and the experimental conditions =CO2 CO2 +H2O = CH-CH3 HOOC-CH3 or (CH3COOH) (CH3)2C= (CH3)2C= O • Ozonolysis : Addition of ozone molecule to alkene to form ozonide, and then cleavage of the ozonide by Zn-H2O to smaller molecules. Uses:- detecting the position of the double bond in alkenes or other unsaturated compounds. Polymerisation:-Combination of large number of alkenes molecules at high temp, high pressure and in the presence of a catalyst is known as polymerisation. obtained molecule is called polymers. compounds from which polymers are made are called monomers. ALKYNES Preparation 1. From calcium carbide 2. From vicinal dihalides:-Vicinal dihalides on treatment with alcoholic KOH undergo dehydrohalogenation. One molecule of hydrogen halide is eliminated to form alkenyl halide which on treatment with NaNH2 gives alkynes. Physical properties of alkynes Physical properties of alkynes follow the same trend of alkenes and alkanes. First three members are gases, the next eight are liquids and the higher ones are solids. All alkynes are colourless. Ethyene has characteristic odour.Other members are odourless. Alkynes are weakly polar in nature. They are lighter than water and immiscible with water but soluble in organic solvents like ethers, carbon tetrachloride and benzene. Their melting point, boiling point and density increase with increase in molar mass. Chemical properties of alkynes A. Acidic character of alkyne:- Na metal and NaNH2 are strong bases. They react with ethyne to form sodium acetylide with the liberation of H2 gas. These reactions have not been observed in case of ethene and ethane. B. Addition reactions (i) Addition of dihydrogen (ii) Addition of halogens:- Reddish orange colour of the solution of Br2 in CCl4 is decolourised. This is used as a test for unsaturation. (iii) Addition of hydrogen halides (iv) Addition of water:- Like alkanes and alkenes, alkynes are also immiscible and do not react with water. However, one molecule of water adds to alkynes on warming with HgSO4 and dilute H2SO4 at 333 K to form carbonyl compounds. (b) Cyclic polymerisation:- Ethyne on passing through red hot iron tube at 873K undergoes cyclic polymerization to form benzene. AROMATIC HYDROCARBONS are also known as ‘arenes’. Benzenoids:- Aromatic compounds containing benzene ring are known as Benzenoids. non-benzenoids (do it) FRIEDRICH AUGUST KEKULÉ (7th September 1829–13th July 1896) Aromaticity:- (Hückel Rule) rules are (i) Planarity (ii) Complete delocalisation of the π electrons in the ring (iii) Presence of (4n + 2) π electrons in the ring where n is an integer (n = 0, 1, 2, . . .) This is often referred to as Some examples of aromatic compounds. Preparation of Benzene (i) Cyclic polymerisation of ethyne: (ii) Decarboxylation of aromatic acids:-Sodium salt of benzoic acid on heating with sodalime gives benzene. (iii) Reduction of phenol: Phenol is reduced to C6H6 by passing its vapours over heated Zn dust. Physical properties of C6H6 usually colourless liquids or solids with a characteristic aroma. You are also familiar with naphthalene balls which are used in toilets and for preservation of clothes because of unique smell . immiscible with water but are readily miscible with organic solvents. They burn with sooty flame. Chemical properties of C6H6 1.Electrophilic substitution reactions (i) Nitration: A nitro group is introduced into benzene ring when benzene is heated with a mixture of concentrated nitric acid and concentrated sulphuric acid (nitrating mixture). (ii) Halogenation: Arenas react with halogens in the presence of a Lewis acid like anhydrous FeCl3, FeBr3 or AlCl3 to yield haloarenes. (iii) Sulphonation: The replacement of a hydrogen atom by a sulphonic acid group in a ring is called sulphonation. It is carried out by heating benzene with fuming sulphuric acid (oleum). (iv) Friedel-Crafts alkylation reaction: When benzene is treated with an alkyl halide in the presence of anhydrous aluminium chloride, alkylbenene is formed. (v) Friedel-Crafts acylation reaction: The reaction of C6H6 with an acyl halide or acid anhydride in the presence of Lewis acids (AlCl3) yields acyl benzene. When benzene on treatment with excess of Cl2 in the presence of anhydrous AlCl3 in dark yields hexachlorobenzene (C6Cl6) Addition reactions:-Under vigorous conditions, i.e., at high temperature and/ or pressure in the presence of nickel catalyst, hydrogenation of benzene gives cyclohexane. Under utra-violet light, three chlorine molecules add to benzene to produce benzene hexachloride, C6H6Cl6 which is also called gammaxane. Ortho and para directing groups: The groups which direct the incoming group to ortho and para positions are called ortho and para directing groups. As an example, let us discuss the directive influence of phenolic (–OH) group. Phenol is resonance hybrid of following structures: due to resonance. Therefore, –OH group activates the benzene ring for the attack by an electrophile. Other examples of activating groups are –NH2, –NHR, – NHCOCH3, –OCH3, –CH3, –C2H5, etc. In the case of aryl halides, halogens are moderately deactivating. Because of their strong – I effect, overall electron density on benzene ring decreases. It makes further substitution difficult. However, due to resonance the electron density on o– and p– positions is greater than that at the mposition. Hence, they are also o– and p– directing groups. Meta directing group: The groups which direct the incoming group to meta position are called meta directing groups. Some examples of meta directing groups are –NO2, – CN, –CHO, –COR, –COOH, –COOR, –SO3H, etc. Let us take the example of nitro group. Nitro group reduces the electron density in the benzene ring due to its strong–I effect. Nitrobenzene is a resonance hybrid of the following structures. CARCINOGENICITY AND TOXICITY:-Benzene and polynuclear hydrocarbons containing more than two benzene rings fused together are toxic and said to possess cancer producing (carcinogenic) property. Such polynuclear hydrocarbons are formed on incomplete combustion of organic materials like tobacco, coal and petroleum. They enter into human body and undergo various biochemical reactions and finally damage DNA and cause cancer. Some of the carcinogenic hydrocarbons are