* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Article Full Text PDF
Patch clamp wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Subventricular zone wikipedia , lookup
Apical dendrite wikipedia , lookup
Resting potential wikipedia , lookup
Signal transduction wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Action potential wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Optogenetics wikipedia , lookup
Node of Ranvier wikipedia , lookup
Neuroregeneration wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
End-plate potential wikipedia , lookup
Biological neuron model wikipedia , lookup
Neuroanatomy wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Axon guidance wikipedia , lookup
Multielectrode array wikipedia , lookup
Synaptic gating wikipedia , lookup
Neurotransmitter wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Nervous system network models wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Evoked potential wikipedia , lookup
Synaptogenesis wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Electrophysiology wikipedia , lookup
Single-unit recording wikipedia , lookup
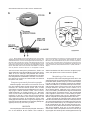
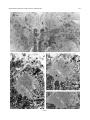
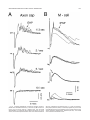
THE JOURNAL OF COMPARATIVE NEUROLOGY 395:493–509 (1998) Physiological Properties of the Mauthner System in the Adult Zebrafish KOHEI HATTA* AND HENRI KORN Institut Pasteur, Biologie Cellulaire et Moléculaire du Neurone, INSERM U261, Département des Biotechnologies, 75015 Paris, France ABSTRACT We investigated the morphological and electrophysiological properties of the Mauthner (M-) cell and its networks in the adult zebrafish (Danio rerio) in comparison with those in the goldfish (Carassius auratus). The zebrafish M-cell has an axon cap, a high resistivity structure which surrounds the initial segment of the M-axon, and accounts for an unusual amplification of the fields generated within and around it. Second, extra- and intracellular recordings were performed with microelectrodes. The resting potential was ,280 mV with an input resistance of ,0.42 MV. The M-cell extracellular field was large (10-20 mV), close to the axon hillock, and the latency of antidromic spikes short (,0.4 milliseconds), confirming a high conduction velocity in the M-axon. The extrinsic hyperpolarizing potential (EHP), which signals firing of presynaptic cells and collateral inhibition, was markedly lower at frequencies of spinal stimulation . ,5/second, suggesting an organization of the recurrent collateral network similar to that in the goldfish. Inhibitory postsynaptic potentials (IPSPs) were highly voltage-dependent; their decay time constant was increased by depolarizations. The presynaptic neurons which are numerous could be identified by their passive hyperpolarizing potential (PHP) produced by the M-spike current. Auditory responses, mediated via mixed synapses (electrical and chemical), had short delays and hence are well suited to trigger the escape reaction. The similarities of their properties indicate that the wealth of information generated over decades in the goldfish can be extrapolated to the zebrafish. J. Comp. Neurol. 395:493–509, 1998. r 1998 Wiley-Liss, Inc. Indexing terms: axon cap; field effects; electrical inhibition; chemical inhibition; auditory response; electrical excitation The zebrafish (Danio rerio), a small teleost, is a useful vertebrate model for genetic studies of development (Streisinger et al., 1981). It has a relatively short generation time, produces a large number of eggs, and can be easily raised in the laboratory. The embryos develop externally and are transparent such that it is easy to observe their phenotypes. Genetic screening and analysis are facilitated by the availability of genetic methods, including those for generating haploids and gynogenetic diploids and the viability of frozen sperm. Several laboratories have screened g-ray- or ethylnitrosourea (ENU)-induced mutants and have reported thousands of mutations in hundreds of gene loci which cause ontogenetic deficiencies in morphology (e.g., Kimmel et al., 1991; Haffter et al., 1996; Driever et al., 1996) or in behavior (Westerfield et al., 1990; Brockerhoff et al., 1995; Granato et al., 1996). Some of the neurons which develop early (primary neurons) are identifiable individually (Kimmel and Westerfield, 1990). Examples include the pair of Mauthner (M-) cells, the largest neurons in the nervous system. They belong to the set of reticulospinal neurons located in the r 1998 WILEY-LISS, INC. hindbrain. The development of their morphology and sensory inputs has been well described (Kimmel and Model, 1978; Kimmel et al., 1982; Metcalfe et al., 1986; Kimmel et al., 1990; Lee and Eaton, 1991), and their functions in other teleosts and amphibians have been elucidated. Zebrafish mutants which show abnormal pathfinding of M-axons have been described (Hatta et al., 1991, 1996a; Hatta, 1992; Jiang et al., 1996), and consequently, the zebrafish can now be used as a model for genetic analysis of M-cell function. However, in contrast to the wealth of general information on this neuron’s morphology and development, there have been few electrophysiological studies in the zebrafish. It would therefore be useful to Grant sponsor: INSERM. *Correspondence to: Dr. Kohei Hatta, Institut Pasteur, Biologie Cellulaire et Moléculaire du Neurone, INSERM U261, Départment des Biotechnolgies, 25 rue du Dr. Roux, 75015 Paris, France. E-mail: [email protected] Received 30 September 1997; Revised 26 January 1998; Accepted 6 February 1998 494 K. HATTA AND H. KORN determine whether the zebrafish M-cell and its synaptic inputs can be analyzed reliably. The paired M-cells in teleosts and amphibians initiate a quick escape (or startle) response which removes them from a dangerous situation and thereby contributes to survival. M-cells are highly integrative cells for motor behavior, and a part of the ‘‘brainstem escape network’’ (Eaton et al., 1991). M-cells are unique in the vertebrate central nervous system because they are readily identifiable both morphologically and physiologically (Faber and Korn, 1978). In goldfish they display all four physiological types of synapses: electrical inhibitory, electrical excitatory, chemical inhibitory, and chemical excitatory (Furshpan and Furukawa, 1962; Furukawa and Furshpan, 1963; Korn et al., 1990). They receive afferent sensory inputs from a variety of sources, notably auditory, and also many inhibitory inputs. These inhibitory signals determine the threshold and the input-output relations of the cell, and are a major factor in regulation of its function (Faber and Korn, 1978; Burnod and Korn, 1989; Korn and Faber, 1990). Another important feature is that some of the inhibitory presynaptic interneurons can be identified electrophysiologically. This facilitates paired recordings from the pre- and postsynaptic neurons in vivo (Korn and Faber, 1975), often difficult in other vertebrates. There have been few electrophysiological investigations of the zebrafish M-cell and all at juvenile stages. The activity of M-cells in the embryo and larva, monitored extracellularly, exhibits features that probably reflect the immaturity of the cell (Eaton and Farley, 1975). For example, its axon fatigues at relatively low rates (1/second) of antidromic stimulation. Nevertheless, the transparency of the embryonic and larval zebrafish brain has enabled both patch clamp studies of glycinergic currents at synaptic and single channel levels (Legendre and Korn, 1994, 1995), and intracellular Ca21 imaging studies of the reticulospinal neurons during the escape behavior (O’Malley et al., 1996). We studied the electrophysiological properties of mature zebrafish M-cells. Their properties suggest that this preparation will be useful for multidisciplinary studies and for identifying electrophysiologically the cellular sites affected by mutations. Preliminary results from this study have already been published in abstract form (Hatta et al., 1996b; Korn et al., 1996). MATERIALS AND METHODS Adult zebrafish (Danio rerio) were obtained from Charles River Aquatics (Someren, The Netherlands). Animal care procedures were in accordance with current requirements in France. Physiology In the course of these experiments, we found that the adult zebrafish is considerably more fragile than the goldfish. Thus, it was necessary to modify methods previously used for the studies of other teleosts (Korn and Faber, 1975; Faber and Korn, 1978) and to minimize the impact of surgery. Therefore, the lower half of the fish was first embedded in agarose (4%, w/v) as shown in Figure 1A. The holding chamber consisted of a Petri dish, two tubes for perfusion inflow and outflow and a bed of agarose (dark bottom layer) with an oval depression (not shown) to hold the fish temporarily. The animal (3-4 cm body length) was anesthetized in MS 222 (3-aminobenzoic acid ethyl ester, methanesulfonate salt, Sigma, St. Louis, MO; 0.2 g/l), placed in the chamber, and an inflow tube, with a mouthpiece made from a pipetteman tip, was placed in its mouth. A small tissue paper ribbon was inserted into the drainage tube and wrapped around the opercula. Melted agarose was cooled until about to solidify, and was poured into the chamber (gray top layer). Once it had solidified, the ribbon and the agarose near the opercula were removed to create a drainage pathway. The animal was continuously perfused through the mouth during the operation with 0.2 g/l MS 222 in commercially available mineral water (Evian, BSN-GD, Paris, France) at the rate of ,20 ml/hour. A ground electrode (chlorided silver wire) was placed next to the body of the fish. To stimulate the M-cell from the spinal cord antidromically, bipolar electrodes made of two stainless steel insect pins, insulated except at their tip with nail polish, were inserted with a micromanipulator into both sides of the vertebral column near the posterior end of the dorsal fin. The satisfactory placement of these electrodes was confirmed by testing for the ‘‘M-reflex’’ in response to electrical stimulations (typically 2–30 V). This reflex consists of a generalized contraction of the trunk muscles, accompanied by a tail flip, a forward flick of the eyes, a rapid protrusion of mouth, and opercular and pectoral fin movements (Zottoli, 1978; Faber and Korn, 1978). A muscular relaxant Pavulon (Pancuronium bromide, Organon Teknika, Fresnes, France; ,1 µg/g body weight) was then injected into the body muscles. A hole (around 0.5 mm radius) was cut in the posterior dorsal skull with a sharp knife and a portion of the cerebellum was exposed with a fine pair of forceps. Broken tip glass pipettes for recordings were filled with 3 M KCl or 4 M potassium acetate (KAc); their impedence was 3-7 MV. The microelecrodes were implanted into the M-cell at an angle of about 5 degrees posterior from the vertical. The M-cell soma was typically found at a depth of 0.7–1.2 mm below the cerebellar surface and 100-150 µm lateral from the midline. The discontinuous single electrode voltage clamp (SEVC) technique (Axoclamp-2A, Axon instruments, Foster City, CA) was used with sampling rates of 16 to 23 kHz. To stimulate the M-cell by sound through the air, sinusoidal waves of different frequencies and durations were provided by a function generator (GX240, Metrix S.A., Annecy, France), and their intensities were controlled by an amplifier (Integrated Stereo Amplifier F245R, Sony, Tokyo, Japan). The loud speaker was placed to the left and above the fish at a distance of 50 cm. All experiments were performed at room temperature (18–25°C). Data were processed by a digitizer (PCM-4/8, Medical Systems Corp., Greenvale, NY) and stored on tape (VCR: HR-D158MS, JVC, Tokyo, Japan) for subsequent computer analysis. The programs we used were Trace analyzer (M. Volaski, Allegheny University of Health Sciences, Philadelphia, PA) for data collection, superimposition, averaging and subtraction of individual responses, and KaleidaGraph 3.0 (Synergy Software, Inc., Reading, PA) for plotting, curve fitting, and generating histograms. Morphology For light microscope observation, adult zebrafish were anesthetized in MS222 as described above and decapitated in 0.12 M phosphate-buffered saline (PBS, pH 7.4) on ice. MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH 495 Fig. 1. A: Top (above) and transverse (below) views of the chamber adapted to the adult zebrafish for electrophysiological recordings. The anesthetized animal is laid on a bed of agarose (dark bottom layer), a perfusion tube is placed into its mouth, and melted agarose (gray top layer) is then poured into the chamber. A drainage pathway is then cleared near the opercula for the perfusion flow (indicated by arrows). Bipolar electrodes made of two stainless steel needles are inserted near the posterior end of the dorsal fin. A micropipette is inserted through the cerebellum for recording. The ground electrode is placed next to the fish body. (See Materials and Methods for details.) B: Location and size of the Mauthner (M-) cells. Schematic representation of a thick transverse section containing the M-cells with their major dendrites, axon and axon cap (shaded area). Drawings according to Lee and Eaton (1991) and Wullimann et al. (1996). Scale bar 5 300 µm. The brain tissues were quickly dissected out, fixed in 1% glutaraldehyde-1% paraformaldehyde in PBS (Karnovsky, 1967) at 4°C for overnight, and postfixed with osmium tetroxide (2%) for 2 hours at room temperature. They were then dehydrated and embedded in Araldite, cut into semithin sections (1-µm-thick) and stained with 1% toluidine blue (Korn et al., 1973). determine whether their basic structure, integrative properties, and operations are similar to those in goldfish. Computer-generated photomicrographs Photomicrographs were taken on Ilford PanF Plus 50 film. The negatives were printed and scanned at 320 dpi resolution on a Horizon scanner (AGFA, Paris, France) controlled by a Power Macintosh 9500/180 using a Photoshop plug-in module (Fotolook, AGFA, Paris, France). Adobe Photoshop 4.0 (Adobe Systems, Inc., Mountain View, CA) was used to assemble the figures with a final resolution of 320 dpi. Linear contrast and brightness adjustments were made to the scanned image until the gray range and contrast of the electronic version most closely approximated that of the original photograph. The final figures were obtained with a Pictography 3000 photographic printer (Fuji, Bois d’Arcy, France). RESULTS The morphological and electrophysiological characteristics of the zebrafish M-cell network were studied to Morphology of the axon cap The position and overall morphology of the M-cells in the adult zebrafish brain have been described in a horseradish peroxidase (HRP) backfill study by Lee and Eaton (1991; Fig. 1B). The M-cells are bilateral, located in the medulla under the cerebellum (C) and behind the optic tectum (OT) near the point of entry of the VIIIth nerve. They have two principal dendrites, one lateral and one ventral. As in other teleosts (Zottoli, 1978) their axon crosses the midline, turns caudally, and extends through the contralateral side of the spinal cord toward the tail. The unmyelinated initial segment of the M-axon is surrounded by a specialized neuropil called the axon cap (AC; Bartelmez, 1915). We first analyzed the morphological features of the AC. A light micrograph of a semithin plastic section stained with toluidine blue shows the left M-cell soma (Ms) with its AC located dorsomedially and a middle portion of the right M-cell’s lateral dendrite (Fig. 2A). The adjacent sections at larger magnification (Fig. 2B1-3) reveal the typical structure of an AC which shares several common characteristics with that of goldfish. They are: (1) its outer border is covered with a wall-like epithelial structure consisting of layered glial cells called cap cells and designated as the cap 496 wall (Robertson et al., 1963; Nakajima and Kohno, 1978; Fig. 2B1); (2) it contains a dense neuropil comprised of numerous afferent fibers; (3) axons inside the AC lack myelin. Three types of axons were distinguished: (a) the M-axon runs dorsomedially and a myelinated portion (Fig. 2B1), an unmyelinated initial segment (Fig. 2B2), and an axon hillock (Fig. 2B3) were observed; (b) smaller myelinated afferent fibers (Fig. 2B1) probably part of the spiral axons (Bodian, 1937); and (c) large myelinated fibers (Fig. 2B1), presumably axons of inhibitory interneurons or ‘‘PHP (passive hyperpolarizing potential)-cells’’ (Faber and Korn, 1973; Korn and Faber, 1975), were also present. As in the goldfish (Korn et al., 1978), these axons dilate to form large bulbs or swellings at the border of the AC (Fig. 2B2). There are some differences between the ACs of the zebrafish and goldfish. In the goldfish, the neuropil can be resolved into two distinct regions: a central core portion which contains the spiral fibers, and a peripheral region which contains most inhibitory axons (Nakajima and Kohno, 1978). The central core of the AC stains more heavily than the peripheral part (Bartelmez, 1915). In contrast, in the adult zebrafish, the neuropil was relatively uniformly stained (Fig. 2B1-3), as already described in the larva (Eaton et al., 1977a; Kimmel et al., 1979). More major differences between other teleosts have been reported (Otsuka, 1964; Zottoli, 1978; Kimmel and Model, 1978). Generally the basic organization of the M-cell structure is very similar in the two species. The diameter of the AC is ,60 µm in the zebrafish assuming 5% to 10% shrinkage during histological preparation and ,100 µm in the goldfish (Nakajima, 1974). This is a small discrepancy given the 3- to 5-fold difference in body lengths. Generation of field effects and of chemical inhibition in the goldfish, a brief summary We found that as in the goldfish, the M-cell collateral inhibitory network of the zebrafish produces both electrical and chemical inhibitions mediated by the same set of interneurons. Before describing the results leading to this conclusion, we will summarize the major features of the M-cell inhibitory networks in goldfish and the various electrical events that they generate. Antidromic stimulation of the M-axon in the spinal cord activates the recurrent network (Fig. 3A). This results in a disynaptic excitation of PHP-cells which establish chemical inhibitory synapses on the M-cell, and paired electrophysiological recordings can be made intracellularly from the identified PHP-cells and the M-soma, or extracellularly. The sequences of responses evoked by this procedure are schematically represented in Figure 3B. Passive hyperpolarizing potentials in the interneurons. The network inhibitory interneurons are called PHP-cells because when the M-cell is excited they exhibit a passive hyperpolarizing potential or PHP, the latency and duration of which coincide with the M-spike (B1 versus B2-B3). This potential is generated by the M-spike action currents (Fig. 3C1). Figure 3C illustrates the intra- and extracellular distribution of current. As the extracellular resistance in the AC is relatively high, some current appears to be channeled through the parallel resistive path provided by the interneurons (iPHP). This PHP has an inhibitory effect on the interneurons because it hyperpolarizes their excitable region. It can also be followed by a postanodal rebound excitation, which has a role in synchro- K. HATTA AND H. KORN nizing the discharge of neighboring PHP-cells (Faber and Korn, 1983). Extrinsic hyperpolarizing potentials. In the axon cap the large negative M-spike field is followed by a series of positive peaks named the extrinsic hyperpolarizing potential, or EHP (Furukawa and Furshpan, 1963; Fig. 3B2). The latency and components of the EHP coincide with the action potentials in PHP-cells (B2 versus B1). Its size and electrical inhibitory effect on the M-cell are consequences of the high resistance of the AC (Fig. 3C2). Note that although PHPs are produced by the action of one neuron (i.e., the M-cell), at least 40-50 synchronously active cells contribute to the EHP (Korn and Faber, 1975). Chemical inhibition. Cl2-dependent inhibitory postsynaptic potentials (IPSPs) recorded in the goldfish M-cell are glycinergic (Furukawa et al., 1964; Faber and Korn, 1982; Korn et al., 1990) and appear as depolarizations when the neuron is chloride-loaded (Fig. 3B3). In goldfish, it has been directly shown (Korn and Faber, 1975, 1976) that chemical and electrical inhibitions are produced by the same set of interneurons. This is strongly supported by the observation of the presence of a PHP in interneurons near the M-cell and the fact that the latency of the IPSP following spinal stimulations is compatible with three synaptic delays. Localization of the M-cell in the zebrafish As in other species (Furukawa and Furshpan, 1963; Faber and Korn, 1978), extracellular fields helped localize the M-cell in the zebrafish. Multiple tracks were used to maximize the size of the M-spike and of the EHP. The former had a latency of about 0.2–0.7 milliseconds and its maximal amplitude was 210 to 220 mV at the center of the AC (Fig. 4B, bottom; also see Fig. 6A). Within this region, this negative component was followed by an EHP, which increased in magnitude as the electrode approached the ‘‘spike focus,’’ i.e., the center of the AC. Furthermore, when the electrode was moved laterally along the M-cell lateral dendrite, the field became biphasic, positivenegative, a waveform indicative of passive spike propagation along the dendrite (Furshpan and Furukawa, 1962). The amplitude of the extracellular M-spike was 210 to 220 mV. That of the corresponding action potential recorded intracellularly in the soma was ,20 to 30 mV. Thus, the full size of the transmembrane potential change can only be determined by calculating the difference between Fig. 2. Morphological features of the zebrafish M-cells and of their axon cap. Light micrographs of semithin (1 µm) plastic sections stained with toluidine blue. Transverse sections, dorsal, up. A: Photograph at low magnification showing the location of the left M-soma (Ms) and axon cap (AC), and the right lateral dendrite (LD). An arrow indicates the midline. B1–B3: Adjacent sections at larger magnification. Midline to the right. B1: The axon cap contains numerous small axons and glial processes, and densely packed of cytoplasmic membranes. Its outer border is covered by a wall-like structure consisting of a layer of glial cells (G; classified as astrocytes) or cap cells. Note the M-axon (M-ax), and the presence of several small myelinated fibers (crossed arrow) probably spiral fibers running in parallel with it. They lose their myelin sheath where they enter the axon cap. Large myelinated fibers, presumably axons of passive hyperpolarizing potential (PHP) inhibitory cells, also lose their myelin sheaths at their boundary (arrow). B2–B3: Initial segment (IS) and axon hillock (AH) of the M-axon. At this level, most of the large fibers dilate and form large spherical or ovoid bulbs (asterisks). Scale bars 5 50 µm in A, 10 µm in B1–B3. MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH 497 Figure 2 498 K. HATTA AND H. KORN Fig. 3. Correlation of electrical events recorded pre- and postsynaptically with the anatomical characteristics of the M-cell system in the goldfish. A: Recurrent inhibitory network. When the M-axon is stimulated antidromically, it activates a class of intermediate or cranial relay cells (CR). These cells excite a group of inhibitory interneurons (passive hyperpolarizing potential [PHP]-cells), the axons of which enter the axon cap (shaded area) and synapse on the M-cell. Arrows indicate the direction of impulse propagation. B1–B3: Consequences of M-cell activation. Scheme of the events recorded intracellularly from a PHP-cell (B1), extracellularly in the axon cap (B2), and intracellularly from a Cl2-loaded M-cell (B3). The field effects (shadowed) and spikes which generate the action currents providing them, are linked by dashed lines; note that the latencies of the PHP and of the M-cell spike recorded extracellularly (B2) or intracellularly (B3) are identical. Subsequently, the action potentials in the interneuron (B1) and the components of the extrinsic hyperpolarizing potential (EHP; B2) coincide with each other. The inhibitory postsynaptic potential (IPSP) produced by chemical transmission from the PHPcells is delayed (B3) because it occurs after three synaptic delays. C1–C2: Equivalent circuits accounting for the generation of the PHP (C1) and of the EHP (C2); intracellular channeling of action currents due to the high extracellular resistance (Re) results in reciprocal hyperpolarizing effects. Two adjacent neurons are shown in each diagram. They are the M-cell, with its soma and axon, and a PHP-cell (Int), that sends its axon towards M-cell’s axon hillock. These two units are coupled through Re. C1: The M-cell is active (battery) and it produces an action potential at the initial segment and axon hillock; action currents (arrows) leaving the cell at its soma return to the spike focus or pass through both Re and the interneuron (iPHP), hyperpolarizing the latter at the point of entry. C2: The interneuron is active (battery at its soma). Resultant currents flowing extracellularly to the interneurons return to the soma along either Re, or, more importantly, along the parallel resistive pathway provided by the M-cell (iEHP). This current produces a positive extracellular field near the initial segment and axon hillock of the M-cell, and causes hyperpolarization at this level. Note that both field effects are inhibitory because hyperpolarizations are imposed on the spike-generating membranes; in contrast, the channeled currents exit the affected cell across electrically inexcitable regions. (C1, C2 are modified from Faber and Korn, 1989.) the intracellular (Vm) and extracellular (Vout) potentials (Figs. 3B2-B3 and 4B). The true spike amplitude was thus calculated to be ,40 to 50 mV. In the axon, however, it was as high as 70 to 80 mV, and in the lateral dendrite, it decreased with distance from the soma to 10 mV or less. The M-axon conduction velocity in goldfish varies as a function of body length with a maximum of about 100 m/second for a 9.5 cm fish (Funch et al., 1981). This is about twice the value found in the adult zebrafish. The similarity of the conduction time in both species, i.e., 0.2–0.7 milliseconds versus 0.3-1.2 milliseconds in the goldfish (Furshpan and Furukawa, 1962), is consistent with the notion that the escape behavior has similar kinematics (Eaton et al., 1977b) in the two species. Axonal properties, conduction velocity of the M-axon As the M-axon is the largest fiber in the spinal cord (Furshpan and Furukawa, 1962), it presumably has the fastest conduction velocity. The conduction velocity was measured (Fig. 4A), from both intracellular and extracellular recordings (Fig. 4B). Both methods yielded the same results: an average conduction velocity of 45 m/second 6 9.6 (S.D., n 5 28; Fig. 4C). Membrane properties The resting potential of the recorded cells was in the range of 70 to 80 mV, similar to that in the goldfish. The M-cell’s instantaneous current-voltage relation obtained in the soma with the voltage clamp technique using a MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH 499 Fig. 4. Conduction velocity of the zebrafish M-axon. A: Diagram of the experimental set-up for antidromic stimulation (Stim) of the M-cell at the level of the caudal spinal cord and for recording (Rec) at the level of the M-soma or initial segment. d is the distance between the sites of recording and of stimulation (calculated from nine experi- ments). B: Antidromic M-spikes were recorded intracellularly (upper trace) or extracellularly in the AC (lower trace). Note that the measured latencies (t), indicated by the dashed lines, were identical. C: Histogram of the M-spike’s latency measured extracellularly (open) and intracellularly (shadowed), in 28 experiments. KAc-filled microelectrode was linear (Fig. 5A1-2, open circles). The mean input resistance calculated from the slope of this relationship for 17 cells was 0.42 MV 6 0.20 (Fig. 5B), which is about twice as large as that of the goldfish (Fukami et al., 1965; Faber and Korn, 1986). This difference is consistent with the difference in size (Faber and Korn, 1988; Edwards et al., 1990). There was a marked voltage- and time-dependent change in the leakage currents (Fig. 5A1). During the larger hyperpolarization, there was a pronounced rapid relaxation of the inward currents. When the cell was hyperpolarized by more than 20 mV, the current remaining after 15 milliseconds was less than 50% of the peak current. This confirms that the major component of the M-cell’s input conductance is voltage-dependent, as it is inactivated by hyperpolarization (Faber and Korn, 1986). Thus, the M-cell has a high resting K1 conductance, at least 50% of which can be readily inactivated by brief hyperpolarizations. In contrast, the low conductance for outward rectification present in the dendrites of the goldfish was not observed in zebrafish because recordings were somatic. Instead, there was a slight increase in the outward current during depolarizations, which may reflect activation of voltagesensitive K1 channels at the axon hillock and initial segment. Identification and properties of collateral inhibition External: Extrinsic hyperpolarizing potential (EHP). The EHP in the AC signals firing of collateral PHP neurons as explained above. Its waveform was similar to that in the goldfish in some cases, containing 2-4 components (n 5 6 of 16 recordings). Figure 6A illustrates a case in which it had three peaks and was abolished when the frequency of the spinal stimulation was increased from 5 to 10/second. Most often, however, it lasted longer and exhibited as many as 12–27 components (n 5 10 of 16). Figure 6B1 is representative of this situation where the number of the peaks fluctuated from 3 to 16 with a total duration of 2.7 to 14 milliseconds. The mean interval between the successive wavelets was 0.9 milliseconds, suggesting a frequency of about 1.1 kHz for presynaptic firing. In two cases the number of the components increased as the strength of the spinal stimulus was raised beyond the threshold, suggesting recruitment of spinal or other afferent inputs to the M-cell circuits (See Discussion). Conversely, an EHP was observed in some cases even when the stimuli failed to excite the M-axon of the recorded cell (Fig. 6B2,B3), which is to be expected, assuming activation of the contralateral M-axon with collaterals of networks converging onto com- 500 K. HATTA AND H. KORN Fig. 5. Current-voltage relations of the M-cell, obtained in one experiment with the single electrode voltage clamp (SEVC) technique, demonstrating hyperpolarizing inactivation of the instantaneous leakage current. A1–A2: Voltage clamp currents and corresponding currentvoltage plot, respectively. The soma was voltage-clamped at a chopping rate of 18 kHz with a 4 M KAc-filled electrode. A1: Family of currents (above) evoked by 15 millisecond command pulses (below) in the depolarizing (15, 110, 115, 120 mV) and hyperpolarizing (210, 215, 220, 225, 230, 235 mV) directions. Note that with the larger hyperpolarizations, there was a rapid decline in the inward currents from their initial peaks, and that there was a slow increase in the outward current with larger depolarizations, presumably due to the onset of delayed rectification at the axon hillock. A2: Plots of the voltage clamp currents (ordinate, I) as a function of membrane potential (abscissa, V). The peak currents (open circles) were linearly related to membrane potential (input conductance 5 3.38 µS) whereas the currents measured after 15 milliseconds (filled circles) did not increase in direct proportion to the voltage steps (corresponding symbols in A1 designate times at which the currents were measured). B: Histogram of the input resistance of the M-cell in 17 experiments. The leakage conductances used for calculation were measured at the beginning of the command pulses. mon presynaptic interneurons (Furukawa and Furshpan, 1963). Intracellular recordings: Presynaptic interneurons (PHP-cells). Presynaptic interneurons were identified by the presence of a PHP (Fig. 7A1,B1). The latency of the PHP coincided with that of the M-spike (Fig. 7A2 versus A3). The net value of this transmembrane potential (DV in A3,B2) was determined by comparing the intracellular potential with the corresponding extracellular potential. Its amplitude varied from 2 to 5 mV (3.3 mV 6 1.2, n 5 6) and was insensitive to the quality of the penetration. The PHP was often followed by an excitatory postsynaptic potential (EPSP; Fig. 7A3) the latency of which was 0.45 milliseconds 6 0.15 (n 5 7), relative to the peak of the PHP. This lag presumably reflects in part the synaptic delays between the M-collaterals and the cranial relay cells (CR) and between the CR-cells and the recorded neurons (see Discussion). As expected, these EPSPs could trigger action potentials. It should be noted, however, that although some PHP-cells were only briefly excited (2–3 spikes maximum, in 3 of 6 cells) as in the goldfish (Korn and Faber, 1975), others fired repetitively with 23 to 27 spikes, as expected from the shape of the EHP. The fact that the frequency of the presynaptic spikes (Fig. 7B1) coincides with that observed extracellularly for a population of interneurons indicates that this property of zebrafish inhibitory cells is not an artifact. Fatigability of the collateral network. The EHP is diminished and eventually abolished at relatively low stimulation frequencies in the goldfish (1–5/second; Furshpan and Furukawa, 1962), because M-cell axon synapses onto CR fatigue (see Fig. 3A for network diagram and Hackett and Faber, 1983; Faber et al., 1989). This was also the case in the zebrafish (Fig. 8A), although higher frequencies (no less than 10/second) were required to totally eliminate both the EHP and the corresponding IPSPs (Fig. 8B). Figure 8A and B shows that this decrease was gradual with both EHP and IPSPs becoming progressively smaller as the frequency of the stimulation increased. The similar frequency dependence of the electrical and chemical inhibitions is consistent with the notion that they are mostly generated by the same populations of presynaptic neurons (Korn and Faber, 1976). In intracellular recordings at high frequency (5 and 10/s in Figure 8B), a small depolarization often persisted, albeit with a delayed onset. This was probably a component of the IPSP generated in the lateral dendrite by another set of inhibitory cells (Furukawa et al., 1964; Faber and Korn, 1978). Chemical inhibition The kinetics of the chemical inhibition induced in the M-cell by the collateral network was studied by three methods. All gave similar results. First, the inhibitory conductance underlying the IPSP was revealed: it produced a reduction, or shunt, of the test antidromic spike with paired spinal stimulations, delivered at progressively increasing intervals, using KAc-filled electrodes. Figure 9A shows superimposed traces from one such experiment, the shunt being maximal at the shortest intervals, and gradually diminishing, to cease at around 20 to 30 milliseconds following the first stimulus. MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH 501 Fig. 6. Evidence that collaterals of both M-axons activate common inhibitory interneurons. A: Superimposed extracellular recordings obtained in the AC after spinal cord stimuli above threshold for firing the M-cell. The extrinsic hyperpolarizing potential (EHP) disappeared when the frequency of antidromic stimulation was raised from 5 to 10/second. B1–B3: Superimposed extracellular responses induced by stimuli at threshold for firing the M-cell at one-third second recorded in the AC of a different fish. B1: The antidromically evoked M-spike was followed by an EHP (arrow) or by a prolonged oscillatory field potential which may have been produced by orthodromic activation of spinal afferents. B2: The stimuli failed to excite the recorded M-cell but there remained an EHP resulting, presumably, from activation of the opposite M-axon. B3: Comparison of the EHPs with and without a preceding M-spike. Their identical waveform can be explained by the fact that collaterals of both M-axons and/or spinal inputs converge onto common interneurons. Second, with KCl-filled electrodes, the Cl2-inverted collateral IPSP was a frank depolarization (Fig. 9B), of as much as 20–40 mV. Third, the underlying inhibitory postsynaptic currents (IPSCs) were monitored by voltage-clamp at resting potential (Fig. 9C). The kinetics of the conductance change were the same with all three methods. The total duration was about 30 milliseconds, and the decay time course was relatively simple, suggesting that in the case of repetitive firing, the last spikes in presynaptic trains does not contribute significantly to the inhibition. Voltage dependency of Cl2 currents. M-cell’s Cl2 channels in the adult goldfish are activated by glycine and are strongly voltage-dependent (Faber and Korn, 1987). This may also the case in the adult zebrafish (Fig. 10). Specifically, the exponential decays of the collateral IPSCs were highly voltage-sensitive (Fig. 10A1-A2) in both Cl2loaded and nonloaded cells (Fig. 10B). The peak synaptic conductance was lower at stronger hyperpolarizations (e.g., at 240 mV in Fig. 10A2). However, the major effect was on the IPSC decay time constant tD, which increased logarithmically as a function of membrane potential, so that the IPSC was significantly prolonged with depolarizations. As postulated for goldfish, this may provide a mechanism for enhancing inhibitory responses in the face of increased excitations (Faber and Korn, 1987). Excitatory inputs: Responses to sounds The properties of the excitatory postsynaptic potentials (EPSPs) evoked in the M-cell by sounds of different intensities and tones were examined using KAc-filled electrodes. The top diagram in Figure 11 illustrates the afferent auditory network in the goldfish. Figure 11A1-B2 shows potentials evoked by tone bursts and recorded in the M-soma of the zebrafish. EPSPs were induced by sounds (500 Hz) of various intensity, the largest of which could fire the cell. The duration of the initial component of the EPSP (crossed arrow) was short (about 1 millisecond), suggesting the involvement of gap junctions. Its amplitude increased with sound intensity. It was followed by longer lasting components that could be mixed, i.e., chemical and electrotonic as, for example, in the lateral vestibular nucleus of the toadfish (Korn et al., 1977). A comparison of the traces superimposed in Figure 11A2 demonstrates that the latency of the auditory response is variable, decreasing 502 K. HATTA AND H. KORN Fig. 7. Passive hyperpolarizing potential (PHPs) evoked in presynaptic interneurons by M-cell antidromic spikes. Inset: Diagram of the experimental set-up. A microelectrode was inserted into a M-cell presynaptic interneuron and responses were evoked in the M-cell by spinal cord stimulation (Stim). A1: Superimposed intra- and extracellular recordings obtained with spinal stimuli that were above the threshold for M-cell activation. The stimulation evoked a hyperpolarization, or PHP, followed by a prolonged depolarization. A2: Recording from the axon cap during the same experiment. A3: Net transmem- brane hyperpolarization calculated as the difference (DV 5 22.5 mV) between the intra- and extracellular potentials illustrated in A1. Note that the latencies of the M-spike in the axon cap and in the PHP were identical (dashed line). B1: Recordings from another PHP-neuron and same presentation as above. The PHP was presumably followed by a postanodal rebound and a recurrent excitatory postsynaptic potential (EPSP) resulting in a high frequency repetitive firing of the cell. B2: Net hyperpolarization during the PHP (DV 5 22.0 mV) calculated as for A3. The black dot indicates a PHP in all records. with increasing sound stimulus: for sounds of 50, 60, and 70 dB, the latency was 3.8 6 0.3, 2.8 6 0.4, and 2.5 6 0.2 milliseconds, respectively (n 5 4). A similar relation has also been reported for the goldfish afferent eighth nerve fibers (Furukawa, 1981). Responses to tones of 500 Hz and 1 kHz were compared. Their respective latencies and time courses (50 and 70 dB) were the same (Fig. 11B1-B2). weaker than the goldfish, an impediment for neurophysiological studies. Nevertheless, with some caution, this in vivo preparation appears to be well suited for a variety of studies. For example, it may allow identification of the sites (network or synapses) affected by genetic mutations causing defective locomotion and behavior. DISCUSSION Our data for the zebrafish M-system are very similar to those reported for the goldfish. The few differences between the two systems are mostly quantitative, despite the fact that these two species belong to distinct subfamilies of Cyprinidae with different systematics and biogeography (Chen et al., 1984; Wu, 1987; Nelson, 1994). Thus, we conclude that the basic properties of the M-network and afferent synapses onto the M-cell are conserved in these two teleosts, and that the detailed knowledge which has been accumulated on the goldfish M-system and its afferent inputs (Korn et al., 1990) is valid for the zebrafish. Overall, the zebrafish preparation provides a useful genetic model. However, it is considerably smaller and Properties of the axon cap The structure of the AC is more uniform in the zebrafish than in the goldfish, possibly because its central core is larger and its peripheral part is less prominent. On the other hand, it has been estimated that the goldfish AC has at least a 2- to 2.5-fold greater resistivity than the surrounding tissues (Korn and Faber, 1975). This high extracellular resistivity can be attributed to the dense packing of the fine neuronal processes, to a restricted extracellular space (Nakajima and Kohno, 1978), and to the specialized glial barrier formed by the cap cells. It contributes to the large size of the extracellular spike recorded in the axon cap and the reciprocal electrical inhibitions between the M-cell and its presynaptic inhibitory interneurons (Furshpan and Furukawa, 1962; Korn and Faber, 1975) via characteristic field effects (Faber and Korn, 1989). We showed here that MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH Fig. 8. Frequency dependence of collateral inhibition. Superimposed traces recorded extracellularly in the axon cap (A) and intracellularly from the M-cell soma of a different fish, with KCl-filled electrodes (B). The responses were evoked antidromically by spinal stimuli, which were suprathreshold for the M-axon. Note that both the 503 extrinsic hyperpolarizing potential (EHP) in A and the inhibitory postsynaptic potential (IPSPs) in B became progressively smaller and more delayed as the stimulus frequency was increased, and that they almost completely disappeared at 10/second (see text). The crossed arrow indicates a late dendritic IPSP. Fig. 9. Time course of the M-cell collateral inhibition obtained from three different experiments. A: Multiple exposures of responses produced in a M-cell by paired spinal stimulations separated by progressively longer intervals, and recorded with a KAc-filled microelectrode. Note the reduced amplitude of the test spikes, due to the increased membrane conductance. B,C: Time course of the depolarizing postsynaptic potential assessed with a KCl-filled microelectrode (B) and of the inhibitory postsynaptic current monitored in the single electrode voltage clamp (SEVC) mode (chopping frequency, 18 kHz) by using a KAc-filled electrode, both inserted into the M-cell soma (resting potential, 280 mV; C). Note that the kinetics of the shunt, of the IPSP and of the inhibitory postsynaptic currents (IPSC) almost coincide with each other. MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH 505 Fig. 10. Voltage dependence of the inhibitory synaptic currents. A12A2: Collateral inhibitory postsynaptic currents (IPSCs) recorded in the single electrode voltage clamp (SEVC) mode using a KAc-filled microelectrode. A1: Superimposed currents recorded in the soma at the indicated potentials relative to the resting potential (RP) with and without antidromic stimulations. A2: Family of the net collateral synaptic currents determined by subtraction of the leak currents in A1. Note that the IPSC decays more rapidly as the cell is more hyperpolarized, and that the magnitude of the inward current does not increase in proportion to the level of hyperpolarization. B: Evidence that a single steep exponential relates tD to membrane potential, regardless of the internal Cl2 concentration. Semilogarithmic plot of the time constant (tD) of IPSC decay (ordinate) against the membrane potential (Vm, abscissa); data points were obtained under different experimental conditions, i.e.; IPSCs evoked in the presence (two experiments, filled symbols) and in the absence (four experiments, clear triangles, clear and quartered squares and crosses, respectively) of Cl2 loading. All recordings were from the M-cell soma, except for one, which was obtained from the proximal part of the lateral dendrite (filled triangles). The straight line fitting all data points was derived according to the expression tD 5 t0 exp(Vm/B); (n 5 22, r2 5 0.77, P , 0.01). all the anatomical features accounting for the high resistivity of the AC can be found in the zebrafish by light microscopy. As a consequence, we observed the typical electrical ‘‘signature’’ of M-cell activation, including its large extracellular spike followed by an EHP, and PHPs were also recorded in presynaptic neurons. more often repetitive than in the goldfish. The EHP was also prolonged when recorded extracellularly and thus the sustained firing of PHP-cells could not have been due to damage during intracellular recordings. In other cases, the number of peaks in the EHP and its duration were found to fluctuate, and/or to increase when the intensity of the spinal stimulations was raised. Such additional EHPs evoked by suprathreshold stimuli were obviously distinct in their origin from those induced by activation of the collateral network. They were due to the recruitment of additional afferents having higher thresholds than that of the M-axon, which has the lowest threshold of any axon at the spinal level (Furukawa and Furshpan, 1963). Chemical inhibition of the M-cell is chloride-dependent and lasts for about 20 to 30 milliseconds (t 5 ,8 milliseconds, RP 5 280 mV); it is largely glycinergic (and therefore strychnine-sensitive) in both goldfish and zebrafish (Furukawa et al., 1964; Faber and Korn, 1982; Korn et al., 1990; Legendre and Korn, 1994; this work, not shown). Furthermore, collateral IPSCs are highly voltage-dependent, with their decay time constant increased by depolarizations. A similar voltage dependency has been reported Similarities and differences in the M-system The design and flexibility of the recurrent networks are also remarkably similar in both species. For example, EHPs were detected even in the absence of an M-spike, suggesting that the collateral inhibition feeds back on each M-cell via the bilateral projection of CR neurons (Hackett and Faber, 1983; Faber et al., 1989). However, the collateral network fatigues at higher frequencies of antidromic stimulation in the zebrafish (responses disappear at 10/ second versus 5/second in goldfish), due presumably to the properties of the synapses between the M-cell’s axon collaterals and CR neurons (Hackett and Faber, 1983). Thus, both chemical and electrical inhibitions are more powerful in the zebrafish. In agreement with this notion, the EHP could last longer, and PHP-neuron firing was 506 K. HATTA AND H. KORN Fig. 11. Sound-evoked excitatory responses of the M-cell. Top: Diagram of the afferent auditory network. VIIIth nerve primary fibers (VIII n.) activated by the hair cells terminate on the lateral dendrite of the ipsilateral M-cell with mixed electrical (el.) and chemical (ch.) synapses. They also excite a class of commissural inhibitory interneurons (Comm. inhib.) which are presynaptic to both the ipsi- and the contralateral (not shown) M-cells. A1–B2: Potentials evoked by tone bursts (top traces, except for A2) of indicated intensities and recorded in the M-soma with KAc-filled electrodes (single or superimposed traces). A1: Excitatory postsynapatic potentials (EP- SPs) evoked by eleven cycles of 500 Hz. The higher intensity sound fired the cell (arrow). Note that the initial component of the excitatory potential (crossed arrows) is of short duration (about 1 millisecond), and is followed by long lasting components, suggesting transmission via electrical and chemical synapses, respectively. A2: Superimposition of the traces illustrated in A1 but shown at a faster sweep speed. Note that the onset of the synaptic potential (arrows) becomes quicker as the sound stimulus is stronger. B: Responses to tones of different frequencies (B1: 500 Hz, B2: 1 kHz) and of moderate intensities (50 and 70 dB). The M-cell reacted similarly to sounds of either tone. in the adult goldfish (Faber and Korn, 1987), and for the single channels in embryonic zebrafish (Legendre and Korn, 1995). Functionally, the increase in tD during depo- larization may provide an important inhibitory safety factor that would compensate for any reequilibration of Cl2 and diminution of the IPSP driving force. MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH The lateral dendrites also share common features. When the electrode was moved posteriolaterally from the M-cell soma and placed in the vicinity of this process, the antidromic extracellular field became positive-negative, as described for the goldfish (Furshpan and Furukawa, 1962). It was smaller and monophasic in more distal regions (not shown). This suggests that spikes generated at the initial segment of the M-axon propagate passively in the lateral dendrite. Intradendritically, we recorded additional components of the collateral IPSPs, with a latency ,1 to 1.5 milliseconds longer than that in the soma (Fig. 8B), which correspond to dendritic collateral IPSPs in goldfish (Furukawa et al., 1964; Faber and Korn, 1978). Furthermore, the responses to sound bursts contained both fast electrical and slow, presumably chemical, components, suggesting mixed transmission from primary VIIIth nerve afferents as in goldfish (Furshpan, 1964; Nakajima and Kohno, 1978; Lin and Faber, 1988). Zebrafish M-system as a model for studying motor control and neuronal diseases Functionally, the M-cell is a major integrating neuron that triggers the escape command to motor networks (Eaton and Bombardieri, 1978; Eaton et al., 1991; Korn and Faber, 1996). It is also involved in a variety of related motor behaviors in different contexts, such as hatching (Eaton et al., 1991) and prey capture (Canfield and Rose, 1993). Since the discovery of its large axon in the cord (Mauthner, 1859), the M-cell has been a valuable vertebrate model in diverse neurobiological disciplines (Piatt, 1950; Stefanelli, 1951). The M-cell’s afferent inputs also exhibit a strong history-dependent plasticity, including a long-term potentiation of the inhibitory synapses (Korn et al., 1992; Oda et al., 1995) and of both the electrotonic and chemical components of the excitatory VIIIth nerve junctions (Yang et al., 1990; Pereda and Faber, 1996). Inhibition in neural networks is one of the major mechanisms regulating motor behavior. In humans, point mutations within the glycine receptor a1 subunit gene have been found to underlie hypertonic motor disorders (hyperekplexia: startle disease such as ‘‘stiff baby syndrome’’). The substitution of arginine 271 with leucine or glutamine (Shiang et al., 1993) drastically reduces agonist-binding, glycine-induced currents and elementary chloride conductance (Langosch et al., 1994; Rajendra et al., 1994). Mutant mice with similar phenotypes, spasmodic (spd) and oscillator (spdot), carry recessive mutations of the a1 subunit gene (Glra 1): the substitution of the Alanine 52 lowering the agonist affinity, and a microdeletion resulting in a functional null allele, respectively (Ryan et al., 1994; Saul et al., 1994; Buckwalter et al., 1994; reviewed in Becker, 1995). Mutant zebrafish with motor defects have already been isolated (Granato et al., 1996), and cDNA encoding the a1 subunit of the zebrafish glycine receptor has been recently cloned (David-Watine et al., 1997) and expressed in oocytes (Bregestovski et al., 1997). Hence, the zebrafish M-system offers a particularly suitable model for studying the consequences of such mutations at the synaptic, cellular network, and behavioral levels. Results obtained in zebrafish models by combining electrophysiological studies with molecular tools may contribute significantly to our understanding of genetically acquired motor disorders, including those described in mammals and primates (Korn and Faber, 1996). 507 ACKNOWLEDGMENTS We are grateful to Dr. Faber (Allegheny University, Philadelphia, PA) for useful comments throughout this work, and to N. Ankri and J. Asmanis for computer and secretarial help. This work was sponsored by INSERM fellowship to K.H. LITERATURE CITED Bartelmez, G.W. (1915) Mauthner’s cell and the nucleus motorius tegmenti. J. Comp. Neurol. 25:87–128. Becker, C.-M. (1995) Glycine receptors: Molecular heterogeneity and implications for disease. The Neuroscientist 1:130–141. Bregestovski, P., D. De Saint Jan, J. Stinnakre, C. Goblet, B. David-Watine, and H. Korn (1997) Functional characterization of a glycine receptor a1 subunit from zebrafish Danio rerio. Soc. Neurosci. Abstr. 23:378. Bodian, D. (1937) The structure of the vertebrate synapse. A study of the axon endings on Mauthner’s cell and neighboring centers in the goldfish. J. Comp. Neurol. 68:117–159. Brockerhoff, S.E., J.B. Hurley, U. Janssen-Bienhold, S.C.F. Neuhauss, W. Driever, and J.E. Dowling (1995) A behavioral screen for isolating zebrafish mutants with visual system defects. Proc. Natl. Acad. Sci. USA 92:10545–10549. Buckwalter, M.S., S.A. Cook, M.T. Davisson, W.F. White, and S. Camper (1994) A frameshift mutation in the mouse a1 glycine receptor gene (Glra1) results in progressive neurological symptoms and juvenile death. Hum. Mol. Genet. 3:2025–2030. Burnod, Y. and H. Korn (1989) Consequences of stochastic release of neurotransmitters for network computation in the central nervous system. Proc. Natl. Acad. Sci. USA 86:352–356. Canfield, J.G. and G.J. Rose (1993) Activation of Mauthner neurons during prey capture. J. Comp. Physiol. A 172:611–618. Chen, X.-L., P.-Q. Lue, and R.-D. Lin (1984) Major groups with the family Cyprinidae and their phylogenetic relationships. Acta Zootaxon. Sin. 9:424–440. David-Watine, B., C. Goblet, M. Imboden, P. Bregestovski, and H. Korn (1997) Molecular cloning of an a1 homologous subunit of the glycine receptor of the zebra fish (Danio rerio): A natural chimera? Soc. Neurosci. Abstr. 23:378. Driever, W., L. Solnica-Krezel, A.F. Schier, S.C.F. Neuhauss, J. Malicki, D.L. Stemple, D.Y.R. Stainier, F. Zwartkruis, S. Abdelilah, Z. Rangini, J. Belak, and C. Boggs (1996) A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123:37–46. Eaton, R.C. and R.A. Bombardieri (1978) Behavioral functions of the Mauthner neuron. In D.S. Faber and H. Korn (eds): Neurobiology of the Mauthner Cell. New York: Raven Press, pp. 221–244. Eaton, R.C. and R.D. Farley (1975) Mauthner neuron field potential in newly hatched larvae of the zebra fish. J. Neurophysiol. 38:502–512. Eaton, R.C., R.D. Farley, C.B. Kimmel, and E. Schabtach (1977a) Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J. Neurobiol. 8:151–172. Eaton, R.C., R.A. Bombardieri, and D.L. Meyer (1977b) The Mauthnerinitiated startle response in teleost fish. J. Exp. Biol. 66:65–81. Eaton, R.C., R. DiDomenico, and J. Nissanov (1991) Role of the Mauthner cell in sensorimotor integration by the brain stem escape network. Brain Behav. Evol. 37:272–285. Edwards, F.A., A. Konnerth, and B. Sakmann (1990) Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: A patch-clamp study. J. Physiol. 430:213–249. Faber, D.S. and H. Korn (1973) A neuronal inhibition mediated electrically. Science 179:577–578. Faber, D.S. and H. Korn (1978) Electrophysiology of the Mauthner cell: Basic properties, synaptic mechanisms, and associated networks. In D.S. Faber and H. Korn (eds): Neurobiology of the Mauthner Cell. New York: Raven Press, pp. 47–131. Faber, D.S. and H. Korn (1982) Transmission at a central inhibitory synapse. I. Magnitude of unitary postsynaptic conductance change and kinetics of channel activation. J. Neurophysiol. 48:654–678. Faber, D.S. and H. Korn (1983) Field effects trigger post-anodal rebound excitation in vertebrate CNS. Nature 305:802–804. Faber, D.S. and H. Korn (1986) Instantaneous inward rectification in the Mauthner cell: A postsynaptic booster for excitatory inputs. Neuroscience 19:1037–1043. 508 Faber, D.S. and H. Korn (1987) Voltage-dependence of glycine-activated Cl2 channels: A potentiometer for inhibition? J. Neurosci. 7:807–811. Faber, D.S. and H. Korn (1988) Unitary conductance changes at teleost Mauthner cell glycinergic synapses: A voltage-clamp and pharmacologic analysis. J. Neurophysiol. 60:1982–1999. Faber, D.S. and H. Korn (1989) Electrical field effects: Their relevance in central neural networks. Physiol. Rev. 69:821–863 Faber, D.S., J.R. Fetcho, and H. Korn (1989) Neuronal networks underlying the escape response in goldfish. General implications for motor control. Ann. N. Y. Acad. Sci. 563:11–33. Fukami, Y., T. Furukawa, and Y. Asada (1965) Excitability changes of the Mauthner cell during collateral inhibition. J. Gen. Physiol. 48:581–600. Funch, P.G., S.L. Kinsman, D.S. Faber, E. Koenig, and S.J. Zottoli (1981) Mauthner axon diameter and impulse conduction velocity decrease with growth of goldfish. Neurosci. Lett. 27:159–164. Furshpan, E.J. (1964) Electrical transmission at an excitatory synapse in a vertebrate brain. Science 144:878–880. Furshpan, E.J. and T. Furukawa (1962) Intracellular and extracellular responses of the several regions of the Mauthner cell of the goldfish. J. Neurophysiol. 25:732–771. Furukawa, T. (1981) Effects of efferent stimulation on the saccule of goldfish. J. Physiol. 315:203–215. Furukawa, T. and E.J. Furshpan (1963) Two inhibitory mechanisms in the Mauthner neurons of goldfish. J. Neurophysiol. 26:140–176. Furukawa, T., Y. Fukami, and Y. Asada (1964) Effects of strychnine and procaine on collateral inhibition of the Mauthner cell of goldfish. Jap. J. Physiol. 14:386–399. Granato M., F.J.M. van Eeden, U. Schach, T. Trowe, M. Brand, M. Furutani-Seiki, P. Haffter, M. Hammerschmidt, C.-P. Heisenberg, Y.-J. Jiang, D.A. Kane, R.N. Kelsh, M.C. Mullins, J. Odenthal, and C. Nüsslein-Volhard (1996) Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123:399–413. Hackett, J.T. and D.S. Faber (1983) Relay neurons mediate collateral inhibition of the goldfish Mauthner cell. Brain Res. 264:302–306. Haffter, P., M. Granato, M. Brand, M.C. Mullins, M. Hammerschmidt, D.A. Kane, J. Odenthal, F.J.M. van Eeden, Y.-J. Jiang, C.-P. Heisenberg, R.N. Kelsh, M. Furutani-Seiki, E. Vogelsang, D. Beuchle, U. Schach, C. Fabian, and C. Nüsslein-Volhard (1996) The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123:1–36. Hatta, K. (1992) Role of the floor plate in axonal patterning in the zebrafish CNS. Neuron 9:629–642. Hatta, K., C.B. Kimmel, R. Ho, and C. Walker (1991) The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature 350:339–341. Hatta, K., C.E. Beattie, M.E. Halpern, H. Liu, J.S. Eisen and C.B. Kimmel (1996a) Genetic Dissection of Motoneuronal Induction and Axonal Patterning in the Zebrafish CNS. Cold Spring Harbor: Zebrafish Development and Genetics, p. 160. Hatta, K., G. Rao, R.C. Eaton, and H. Korn (1996b) Functional and morphological properties of the Mauthner system in the adult zebrafish. Soc. Neurosci. Abstr. 22:1846. Jiang, Y.-J., M. Brand, C.-P. Heisenberg, D. Beuchle, M. Furutani-Seiki, R.N. Kelsh, R.M. Warga M. Granato, P. Haffter, M. Hammerschmidt, D.A. Kane, M.C. Mullins, J. Odenthal, F.J.M. van Eeden, and C. Nüsslein-Volhard (1996) Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development 123:205–216. Karnovsky, M.J. (1967) The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J. Cell Biol. 35:213–236. Kimmel, C.B. and P.G. Model (1978) Developmental studies of the Mauthner cell. In D.S. Faber and H. Korn (eds): Neurobiology of the Mauthner Cell. New York: Raven Press, pp. 183–220. Kimmel, C.B. and M. Westerfield (1990) Primary neurons of the zebrafish. In: G.M. Edelman, W.E. Gall, M.W. Cowan (eds): Signal and Sense. New York: Wiley-Liss, pp. 561–588. Kimmel, C.B., S.K. Sessions, and R.J. Kimmel (1979) Target recognition in neurogenesis: Formation of the Mauthner axon cap. Proc. Natl. Acad. Sci. USA 76:4691–4694. Kimmel, C.B., S.L. Powell, and W.K. Metalfe (1982) Brain neurons which project to the spinal cord in young larvae of the zebrafish. J. Comp. Neurol. 205:112–127. Kimmel, C.B., K. Hatta, and W.K. Metcalfe (1990) Early axonal contacts during development of an identified dendrite in the brain of the zebrafish. Neuron 4:535–545. K. HATTA AND H. KORN Kimmel, C.B., K. Hatta, and J.S. Eisen (1991) Genetic control of primary neuronal development in zebrafish. Development,Suppl. 2:47–57. Korn, H. and D.S. Faber (1975) An electrically mediated inhibition in goldfish medulla. J. Neurophysiol. 38:452–471. Korn, H. and D.S. Faber (1976) Vertebrate central nervous system: Same neurons mediate both electrical and chemical inhibitions. Science 194:1166–1169. Korn, H. and D.S. Faber (1990) Transmission at a central inhibitory synapse IV. Quantal structure of synaptic noise. J. Neurophysiol. 63:198–222. Korn, H. and D.S. Faber (1996) Escape behavior - brainstem and spinal cord circuitry and function. Curr. Op. Neurobiol. 6:826–832 Korn, H., C. Sotelo, and F. Crepel (1973) Electronic coupling between neurons in the rat lateral vestibular nucleus. Exp. Brain Res. 16:255– 275. Korn, H., C. Sotelo, and M.V.L. Bennett (1977) The lateral vestibular nucleus of the toadfish Opsanus tau: Ultrastructual and electrophysiological observations with special reference to electrotonic transmission. Neuroscience 2:851–884. Korn, H., A. Triller, and D.S. Faber (1978) Structural correlates of recurrent collateral interneurons producing both electrical and chemical inhibitions of the Mauthner cell. Proc. R. Soc. Lond. B. 202:533–538. Korn, H., D.S. Faber, and A. Triller (1990) Convergence of morphological, physiological, and immunocytochemical techniques for the study of single Mauthner cells. In: A. Björklund, T. Hökfelt, F.G. Wouterlood, and A.N. van den Pol (eds): Handbook of Chemical Neuroanatomy Vol. 8. New York: Elsevier Science Publishers, pp. 403–480. Korn, H., Y. Oda, and D.S. Faber (1992) Long-term potentiation of inhibitory circuits and synapses in the central nervous system. Proc. Natl. Acad. Sci. USA 89:440–443. Korn, H., K. Hatta, N. Ankri, G. Rao, and R.C. Eaton (1996) Electrophysiological Characterization of the Mauthner Cell in the Adult Zebrafish. Cold Spring Harbor: Zebrafish Development and Genetics, p. 174. Langosch, D., B. Laube, N. Rundström, V. Schmieden, J. Bormann, and H. Betz (1994) Decreased agonist affinity and chloride conductance of mutant glycine receptors associated with human hereditary hyperekplexia. EMBO. J. 13:4223–4228. Lee, R.K.K. and R.C. Eaton (1991) Identifiable reticulospinal neurons of the adult zebrafish, Brachydanio rerio. J. Comp. Neurol. 304:34–52. Legendre, P. and H. Korn (1994) Glycinergic inhibitory synaptic currents and related receptor channels in the zebrafish brain. European J. Neurosci. 6:1544–1557. Legendre, P. and H. Korn (1995) Voltage dependence of conductance changes evoked by glycine release in the zebrafish brain. J. Neurophysiol. 73:2404–2412. Lin, J.W. and D.S. Faber (1988) Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I. Characteristics of electrotonic and chemical postsynaptic potentials. J. Neurosci. 8:1302– 1312. Mauthner, L. (1859) Untersuchungen über den Bau des Rückenmarkes der Fische. Sitz ber. Kgl. Preuss. Wiss. 34:31–36. Metcalfe, W.K., B. Mendelson, and C.B. Kimmel (1986) Segmental homologies among reticulospinal neurons in the hindbrain of the zebrafish larva. J. Comp. Neurol. 251:147–159. Nakajima, Y. (1974) Fine structure of the synaptic endings on the Mauthner cell of the goldfish. J. Comp. Neurol. 156:375–402. Nakajima, Y. and K. Kohno (1978) Fine structure of the Mauthner cell: synaptic topography and comparative study. In D.S. Faber and H. Korn (eds): Neurobiology of the Mauthner Cell. New York: Raven Press, pp. 133–166. Nelson, J.S. (1994) Fishes of the World, 3rd Edition, New York: John Wiley & Sons, Inc. Oda, Y., S. Charpier, Y. Murayama, C. Suma, and H. Korn (1995) Long-term potentiation of glycinergic inhibitory synaptic transmission. J. Neurophysiol. 74:1056–1074. O’Malley, D.M., Y.-H. Kao, and J.R. Fetcho (1996) Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Neuron 17:1145–1155. Otsuka, N. (1964) Weitere Vergleichend-anatomische Untersuchlungen an Mauthnerschen Zellen von Fischen. Z. Zellforsch. 62:61–71. Pereda, A.E. and D.S. Faber (1996) Activity-dependent short term enhancement of intercellular coupling. J. Neurosci. 16:983–992. Piatt, J. (1950) Further studies on the differentiation and growth of Mauthner’s cell in Amblystoma. J. Exp. Zool. 113:379–396. MAUTHNER SYSTEM IN THE ADULT ZEBRAFISH Rajendra, S., J.W. Lynch, K.D. Pierce, C.R. French, P.H. Barry, and P.R. Schofield (1994) Startle disease mutations reduce the agonist sensitivity of the human inhibitory glycine receptor. J. Biol. Chem. 269:18739– 18742. Robertson, J.D., T.S. Bodenheimer, and D.E. Stage (1963) The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J. Cell Biol. 19:159–199. Ryan, S.G., M.S. Buckwalter, J.W. Lynch, C.A. Handford, L. Segura, R. Shiang, J.J. Wasmuth, S.A. Camper, P. Schofield, and P. O’Connell (1994) A missense mutation in the gene encoding the a1 subunit of the inhibitory glycine receptor in the spasmodic mouse. Nature Genet. 7:131–135 Saul, B., V. Schmieden, C. Kling, C. Mulhardt, P. Gass, J. Kuhse, and C.M. Becker (1994) Point mutation of glycine receptor alpha 1 subunit in the spasmodic mouse affects agonist responses. FEBS Lett. 350:71–76. Shiang, R., S.G. Ryan, Y.-Z. Zhu, A.F. Hahn, P. O’Connell, and J.J. Wasmuth (1993) Mutations in the a1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nature Genet. 5:351–358. 509 Stefanelli, A. (1951) The Mauthnerian apparatus in ichthyopsida; Its nature and function and correlated problem of neurohistogenesis. Q. Rev. Biol. 26:17–34. Streisinger, G., C. Walker, N. Dower, D. Knauber, and F. Singer (1981) Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291:293–296. Westerfield, M., D.W. Liu, C.B. Kimmel, and C. Walker (1990) Pathfinding and synapse formation in a zebrafish mutant lacking functional acetylcholine receptors. Neuron 4:867–874. Wu, Y. (1987) On the present status of cyprinid fish studies in China. In S.O. Kullander and B. Fernholm (ed): Proc. V Congr. Europ. Ichtyol., Stockholm 1985, pp. 43–47. Wullimann, M.F., B. Rupp, and H. Reichert (1996) Neuroanatomy of the zebrafish brain. Basel: Birkhäuser Verlag. Yang, X.-D., H. Korn, and D.S. Faber (1990) Long-term potentiation of electrotonic coupling at mixed synapses. Nature 348:542–545. Zottoli, S.J. (1978) Comparative morphology of the Mauthner cell in fish and amphibians. In D.S. Faber and H. Korn (eds): Neurobiology of the Mauthner Cell. New York: Raven Press, pp. 13–45.