* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2.3 and 2.4 Notes
Survey
Document related concepts
Vectors in gene therapy wikipedia , lookup
Citric acid cycle wikipedia , lookup
Lipid signaling wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Genetic code wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Proteolysis wikipedia , lookup
Transcript
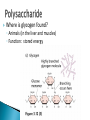
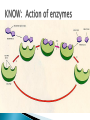
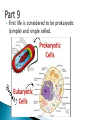
How did you do? Could have used saturated or unsaturated fatty acids for the tails Could only use saturated fatty acids for the tails Macromolecules All life on earth is carbon based. The study of carbon chemistry is called organic chemistry. Carbon has 4 valence electrons, so it forms four single bonds. (it has 4 electrons to lose, gain, or share) These bonds can be single (sharing 1 e-), double (sharing 2 e-), or triple bonds (sharing 3 e-). How many of each type of bond can it make? Single _____, double______, triple_____ How many of each type of bond can it make? Single __4___ double___2__ triple___1__ They can also form straight chains, branched chains, or “rings.” Q: How are these two similar? Q: How are these two different? Isomers are compounds with the same chemical formula, but different structures. For example, C6H12O6 is a sugar. However, it could be either glucose or fructose. They both have the same number of atoms / the same molecular formula, they just are structured differently. Carbon containing molecules that are large and found in living things are called macromolecules or biomolecules. The 4 macromolecules are: carbohydrates, lipids, proteins, and nucleic acids. In order to build one of these macromolecules, MANY molecules need to be linked together. ◦ Many = 100’s to 1,000’s The small sub-units that make up the macromolecules are called monomers. Where the prefix “mono” means one. Linking monomers together in long chains forms polymers. The prefix “poly” means many and “mer” means parts. In order to make a polymer, two separate parts must be bonded together. To make this happen, a water must be removed. This reaction is called a dehydration or condensation reaction. To break apart a polymer, requires the use of water to break the bonds. This is called a hydrolysis reaction. Where the prefix “hydro” means water and “lysis” means split. Carbohydrates AKA sugar are composed of the elements C, H, and O. The simplest sugar is a monosaccharide. Recall monomers come together to form polymers. The monomers of sugars are called “simple sugars.” Two sugar monomers linked together are called disaccharides. Many sugar monomers linked together are called polysaccharides. List as many sugars as you can think of: ____________________________________________ What kind of reaction links a monosaccharide to a monosaccharide? What has to happen? Condensation or Dehydration reaction. Making a bond requires removing water. Examples of monosaccharides are glucose and fructose. How would you describe their appearance here? “Ring” shaped An example of a disaccharide is sucrose. This is formed by linking a glucose to a fructose. To make this happen, a water must be removed by a condensation/dehydration reaction. Sucrose is plain old table sugar. The molecular formula for a disaccharide (example: sucrose) is C12H22O11, due to the loss of a water. Examples of polysaccharides are glycogen, cellulose, and starch. They all are made by linking together MANY monomers. Remember that many = 100’s to 1,000’s Where is cellulose found? ◦Plants, plant cell walls ◦Function: structure Where is starch found? ◦Roots of plants ◦Function: stored energy Where is glycogen found? ◦ Animals (in the liver and muscles) ◦ Function: stored energy Which of the 3 polysaccharides is structural / provides support? Which of the 3 polysaccharides is for storage? How is a tulip bulb able to sprout in the spring and then come back again each year? Explain what your body does after fasting for many hours? Which of the 3 polysaccharides is structural / provides support? cellulose Which of the 3 polysaccharides is for storage? Starch (plants) and glycogen (animals) How is a tulip bulb able to sprout in the spring and then come back again each year? Stores starch in the winter in the form of a bulb to use for energy when it cannot go through photosynthesis, then when it warms it can sprout and resume photosynthesis. Explain what your body does after fasting for many hours? It makes use of the stored glycogen in your liver for energy. Lipids AKA fats are composed of the elements C, H, and O. Lipids are made up of a glycerol backbone and (2 to 3) fatty acid tails. What 2 main elements are the 3 fatty acid tails made up of? List examples of lipids: ____________________________________________ Lipids do not mix with water. The part of a lipid that doesn’t like water are the tails. This can be seen with Italian salad dressing (oil separates out) There are two types of lipids: saturated and unsaturated. What differences do you notice between the two below: Saturated lipids have single bonds and are solid at room temperature. An example of a saturated lipid is: Wax, butter, animal fat, crisco… Unsaturated lipids have double bonds and are liquid at room temperature. An example of an unsaturated lipid is oil. Lipids serve several roles in organisms. A couple key roles are they are located beneath the skin to maintain the body’s temperature/keep it warm and they form a phospholipid bilayer around the outside of all cells. Of the 4 macromolecules, you are mostly proteins. Proteins are composed of the elements C, H, O, N, and sometimes S. Proteins are made up of monomers called amino acids. There are 20 different amino acids (now scientists believe there may be upwards of 23). Examples of amino acids include: ◦ Aspartic acid ◦ Alanine ◦ Methionine The part of the amino acid that differs among the 20 amino acids is the R group. Proteins are formed when amino acids are linked together by dehydration / condensation reactions. The types of bonds that link amino acids together are covalent peptide bonds. Proteins are also called polypeptide chains. ◦ This is because the bond is called a peptide bond. allowing muscle cells to contract transporting oxygen in the blood on hemoglobin providing immunity through antibodies sending out chemical messages through hormones like epinephrine /adrenaline. Proteins also help to speed up chemical reactions like enzymes. Enzymes are a type of protein that speeds up chemical reactions. Another way of saying “speeds up” is catalyze AKA a catalyst. The making and breaking of all molecules in the body is called metabolism. If it weren’t for enzymes molecules would metabolize (be made and broken) too slowly. This would cause waste build up in cells and cells would go hungry and die. Enzymes digest foods more quickly then a chemical reaction would do by itself. What molecule is being broken down more quickly with the aide of an enzyme and water? ____________________. Is this a mono or disaccharide? _______________________________. What are the names of the 2 monosaccharides that are products of this reaction? ___________________ and ___________________. What molecule is being broken down more quickly with the aide of an enzyme and water? sucrose Is this a mono or disaccharide? disaccharide What are the names of the 2 monosaccharides that are products of this reaction? Glucose and fructose What happens to the enzyme after it makes the products? It goes back to the beginning to get another sucrose. The molecule that binds to the enzyme is called the substrate. It binds to a cutout in the enzyme called the active site. For example, the enzyme sucrase only breaks down sucrose. While the enzyme lactase only breaks down lactose. Notice, sugars often end in –ose, while enzymes often end in –ase. When sucrose goes into the active site of the enzyme sucrase, it fits like a lock and key or interlocking puzzle pieces. What does it mean that a person is “lactose intolerant?” What does it mean that a person is “lactose intolerant?” Either: a) Have no lactase enzymes OR b) Their lactase enzymes are “broken” or mutated. If enzymes are exposed to extreme heat or acidity they unravel/lose their shape and no longer can metabolize molecules. This is called denaturation. Why can a high fever lead to death? Why can a high fever lead to death? The high heat can denature (unravel) all of the proteins that we need to survive and function. The elements that make up nucleic acids are C, H, O, N, P The monomers of nucleic acids are called nucleotides. Nucleotides are made up of 3 parts: nitrogen base, phosphate group, and a simple sugar. Nucleotide monomers come together to form either the polymer DNA or RNA. DNA stands for deoxyribonucleic acid RNA stands for ribonucleic acid DNA contains the instructions for making proteins and is located inside the nucleus of a cell. 1. 2. 3. 4. 5. List the elements involved in carbohydrates. What are carbohydrates? What is the monomer of carbohydrates? Give an example of a monomer of carbohydrates. What is the reaction called when you make a larger molecule? What has to happen for this to occur? 6. What is the reaction called when you break down a larger molecule into many smaller molecules? What has to happen for this to occur? 7. What is the chemical formula of a dissacharide? 8. What is an example of a disaccharide? What two monosaccharides form it? 9. What are our three main examples of polysaccharides? 10. Where are these three each found (plants or animals)? 11. What are their main purposes? 12. Why are potatoes so starchy? 1. 2. 3. 4. 5. List the elements involved in lipids. What are lipids? Did we discuss a monomer of lipids? Describe the basic structure of a lipid. What elements are the tails mostly composed of? 6. What are the three main differences in the structure between a saturated and unsaturated lipid? 7. What state are all saturated fats in? Give an example. 8. What state are all unsaturated fats in? Give an example. 9. What roles do lipids play in our bodies. 10. What is the double layer of lipids that surrounds our cells called? 11. Do lipids mix well with water? What part of the lipid does not like water? 1. 2. 3. 4. 5. List the elements involved in proteins. What is the monomer of a protein called? How many of these are known to scientists now? What is the part that is the placeholder or changeable piece that determines what amino acid it is? What is the bond called that holds amino acids together? What is the alternate name that scientists use for proteins? 6. What are the three main differences in the structure between a saturated and unsaturated lipid? 7. What state are all saturated fats in? Give an example. 8. What state are all unsaturated fats in? Give an example. 9. What are five examples of roles that proteins serve in the body? 1. 2. 3. Enzymes are a special type of what macromolecule? What is the purpose of enzymes? What would happen if enzymes didn’t exist? What are the elements that make up enzymes? 4. What does the word metabolism mean? 5. Describe the action of enzyme sucrase. Use all important key terms. 6. What is the difference between the substrate and the active site? 7. What is denaturation? Why is it harmful to the cell or body if it occurs? 8. Explain why some people are lactose intolerant. 1. 2. 3. 4. 5. 6. 7. 8. What are the elements that make it up? What is the monomer of a nucleic acid? What are the parts that make up that monomer? What do these monomers come together to form? What does DNA stand for? What does RNA stand for? What is the purpose of DNA? Where is DNA located? Early earth, water, and biomolecules Now where is life? First life is considered to be prokaryotic (simple) and single celled. These can’t produce oxygen. So how do we know that oxygen started to form? Based on the iron content of the oceans! Question: what color does iron turn when it is exposed to oxygen? (aka rusting) Iron turns red when exposed to oxygen! By looking at the rocks of the ancient oceans we can tell that oxygen started when the iron started rusting! Cyanobacteria is a special type of prokaryote that can run photosynthesis! Oxygen can be formed!!!! “Nobody knows how life got started. Most of the evidence from that time was destroyed by impact and erosion. Science works on the frontier of knowledge and ignorance. We’re not afraid to admit what we don’t know. There’s no shame in that. The only shame is to pretend that we have all the answers. Maybe someone watching this, will be the first to solve the mystery of how life on Earth began.” -Neil Degrasse Tyson