* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 5 Gases
Survey
Document related concepts
Radical (chemistry) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Transcript
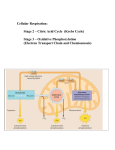
Biology Concepts and Applications | 9e Starr | Evers | Starr Chapter 7 How Cells Release Chemical Energy © Learning2015 2015 © Cengage Cengage Learning Cells break organic molecules apart in small steps (Review) • The bonds of organic molecules hold a lot of energy • Removed electrons are carried by electron carrier molecules. • Redox: one molecule accepts electrons (it’s reduced) from another molecule (it’s oxidized) © Cengage Learning 2015 Electron Transfers (Review) • Electron transfer chain (ETC): array of molecules that accept and give up electrons • The energy of the electrons is released with each step of the sequence by moving (H+) across membrane © Cengage Learning 2015 7.1 How Do Cells Access the Chemical Energy in Sugars? • Cells use the energy stored in molecules, • There are two mechanisms by which organisms break down sugars to make ATP © Cengage Learning 2015 7.3 What Is Glycolysis? • Glycolysis found in ALL cells • Net yield of 2 ATP per glucose • Electrons from glucose are transferred to 2 electron carrier NADH • Produces 2 three-carbon pyruvate molecules © Cengage Learning 2015 What Is Glycolysis? (cont’d.) © Cengage Learning 2015 Aerobic Respiration and Fermentation Compared (cont’d.) • Aerobic respiration follows glycolysis when oxygen is present • Produces more ATP • Main energy-releasing pathway in nearly all eukaryotes and some bacteria © Cengage Learning 2015 7.4 What Happens During the Second Stage of Aerobic Respiration? • Aerobic part occurs inside mitochondria • Breaks down the pyruvate produced during glycolysis © Cengage Learning 2015 What Happens During the Second Stage of Aerobic Respiration? (cont’d.) mitochondrion cytoplasm 2 pyruvate outer membrane inner membrane 2 acetyl–CoA matrix 6 CO2 2 8 2 © Cengage Learning 2015 The breakdown of 2 pyruvate (from glycolysis) to 6 CO2 yields 2 ATP and 10 reduced coenzymes (8 NADH and 2 FADH2). Electrons carried by the coenzymes will power ATP formation in the third stage of aerobic respiration. Acetyl–CoA Formation • Each pyruvate is split into CO2 and a twocarbon acetyl group (Acetyl CoA) • Electrons are removed combine with NADH © Cengage Learning 2015 The Krebs Cycle (a.k.a. Citric acid cycle) • Each acetyl groups are transferred forming citric acid • ATP formed • Three NADH form and 1 FADH2 • Two CO2 released © Cengage Learning 2015 The Krebs Cycle (cont’d.) 2nd stage of aerobic respiration pyruvate (2) © Cengage Learning 2015 carbon dioxide (6) 7.5 What Happens During the Third Stage of Aerobic Respiration? • Last stage occurs on the inner mitochondrial membrane • NADH and FADH2 deliver electrons and H+ to electron transfer chains © Cengage Learning 2015 What Happens During the Third Stage of Aerobic Respiration? (cont’d.) • E.T.C. move H+ actively across the inner membrane • Ion gradient causes the ions to flow through the ATP synthase • Oxygen accepts electrons at the end of mitochondrial electron transfer chains © Cengage Learning 2015 Summary of aerobic respiration: • For each glucose molecule, 4 ATP form in the first- and second-stage reactions • The twelve electron carriers produce 32 additional ATP during the third stage • 36 net ATP are produced in total © Cengage Learning 2015 What Happens During the Third Stage of Aerobic Respiration? (cont’d.) glucose Stage 1 Glycolysis in cytoplasm splits a glucose molecule into 2 pyruvate; 2 NADH and 4 ATP also form. An investment of 2 ATP began the reactions, so the net yield is 2 ATP. 2 Stage 2 Acetyl–CoA formation and the Krebs cycle in the mitochondrial matrix break down the pyruvate to CO2, which leaves the cell. Ten additional coenzymes are reduced. Two ATP form. Stage 3 In electron transfer phosphorylation, the reduced coenzymes give up electrons and hydrogen ions to electron transfer chains in the inner mitochondrial membrane. Energy lost by the electrons as they move through the chains is used to move H+ across the membrane. The resulting gradient causes H+ to flow through ATP synthases, which drives ATP synthesis. © Cengage Learning 2015 4 2 NADH 2 pyruvate 2 NADH 2 NADH 2 acetyl–CoA 2 CO2 4 CO2 6 NADH 2 FADH2 2 32 oxygen H2O 2 (net) 7.6 What Is Fermentation? • Pyruvate is not fully broken down to CO2 • No additional ATP forms • The net yield is two ATP © Cengage Learning 2015 What Is Fermentation? (cont’d.) • Alcoholic fermentation: pathway that produces ATP, CO2, and ethanol • Lactate fermentation: pathway that produces ATP and lactate © Cengage Learning 2015 Alcoholic Fermentation (cont’d.) NADH NAD+ + pyruvate © Cengage Learning 2015 carbon dioxide acetaldehyde ethanol Lactate Fermentation (cont’d.) NADH pyruvate © Cengage Learning 2015 NAD+ lactate Lactate Fermentation (cont’d.) B C © Cengage Learning 2015 7.7 Can the Body Use Any Organic Molecule for Energy? • 36 ATP by fully oxidizing glucose • Other carbohydrates, fats, and proteins can be converted to molecules that enter glycolysis or the Krebs cycle © Cengage Learning 2015 Food 7.7 Can the Body Use Any Organic Molecule for Energy? (cont’d.) a triglyceride (fat) glycerol head Fats fatty acids 2 acetyl–CoA glycerol 3 Complex Carbohydrates Proteins glucose, other simple sugars amino acids 1 4 acetyl–CoA PGAL fatty acid tails NADH pyruvate intermediate of Krebs cycle NADH, FADH2 © Cengage Learning 2015 © Cengage Learning 2015 © Cengage Learning 2015 © Cengage Learning 2015 7.8 Application: Mitochondrial Malfunction • Sometimes when oxygen enters an electron transfer chain, it escapes as a free radical – Free radicals cause damage by oxidizing biological molecules and breaking carbon backbones • Antioxidants in the cytoplasm detoxify free radicals © Cengage Learning 2015 Application: Mitochondrial • A genetic disorder or encounter with a toxin Malfunction (cont’d.) can result in a missing antioxidant or defective electron transfer chain – Free radicals accumulate and destroy first the function of mitochondria, then the cell • This tissue damage is called oxidative stress – Hundreds of incurable disorders are associated with such defects • Cancer, hypertension, Alzheimer’s, and Parkinson’s diseases © Cengage Learning 2015 Application: Mitochondrial Malfunction (cont’d.) © Cengage Learning 2015