* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A cytoarchitectonic and TH-immunohistochemistry
Blood–brain barrier wikipedia , lookup
Neural oscillation wikipedia , lookup
Time perception wikipedia , lookup
Donald O. Hebb wikipedia , lookup
Single-unit recording wikipedia , lookup
Neurolinguistics wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Human brain wikipedia , lookup
Central pattern generator wikipedia , lookup
Neuroinformatics wikipedia , lookup
Neurophilosophy wikipedia , lookup
Development of the nervous system wikipedia , lookup
Selfish brain theory wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Brain morphometry wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neuroeconomics wikipedia , lookup
History of neuroimaging wikipedia , lookup
Brain Rules wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Neuropsychology wikipedia , lookup
Haemodynamic response wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Aging brain wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neuroplasticity wikipedia , lookup
Basal ganglia wikipedia , lookup
Nervous system network models wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Hypothalamus wikipedia , lookup
Synaptic gating wikipedia , lookup
Circumventricular organs wikipedia , lookup
Optogenetics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
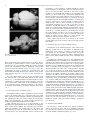
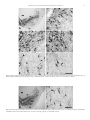
Journal of Chemical Neuroanatomy 55 (2014) 58–66 Contents lists available at ScienceDirect Journal of Chemical Neuroanatomy journal homepage: www.elsevier.com/locate/jchemneu A cytoarchitectonic and TH-immunohistochemistry characterization of the dopamine cell groups in the substantia nigra, ventral tegmental area and retrorubral field in the rock cavy (Kerodon rupestris) José R.L.P. Cavalcanti a,b,*, Joacil G. Soares b, Francisco G. Oliveira b,c, Fausto P. Guzen a,b, André L.B. Pontes d, Twyla B. Sousa b, Jeferson S. Cavalcante d, Expedito S. Nascimento Jrb, Judney C. Cavalcante b, Miriam S.M.O. Costa b a Department of Biomedical Sciences, Laboratory of Experimental Neurology, Health Science Center, University of State of Rio Grande do Norte, Mossoró, RN, Brazil b Department of Morphology, Laboratory of Neuroanatomy, Biosciences Center, Federal University of Rio Grande do Norte, Natal, RN, Brazil c Department of Biological Sciences, Biological Sciences and Health Center, Regional University of Cariri, Crato, CE, Brazil d Department of Physiology, Laboratory of Neurochemical Studies, Biosciences Center, Federal University of Rio Grande do Norte, Natal, RN, Brazil A R T I C L E I N F O A B S T R A C T Article history: Received 10 July 2013 Received in revised form 5 November 2013 Accepted 6 January 2014 Available online 17 January 2014 The 3-hydroxytyramine/dopamine is a monoamine of the catecholamine group and it is a precursor of the noradrenaline and adrenaline synthesis, in which the enzyme tyrosine hydroxylase acts as a ratelimiting enzyme. The dopaminergic nuclei retrorubral field (A8 group), substantia nigra pars compacta (A9 group) and ventral tegmental area (A10 group) are involved in three complex circuitries named mesostriatal, mesocortical and mesolimbic, which are directly related to various behavioral manifestations such as motor control, reward signaling in behavioral learning, motivation and pathological manifestations of Parkinson’s disease and schizophrenia. The aim of this study was to describe the delimitation of A8, A9 and A10 groups and the morphology of their neurons in the brain of the rock cavy (Kerodon rupestris), a typical Brazilian Northeast rodent belonging to the suborder Hystricomorpha, family Caviidae. Coronal and sagittal sections of the rock cavy brains were submitted to Nissl staining and TH immunohistochemistry. The organization of these dopaminergic nuclei in the rock cavy brain is very similar to that found in other animals of the Rodentia order, except for the presence of the tail of the substantia nigra, which is found only in the species under study. The results revealed that, apart some morphological variations, A8, A9 and A10 groups are phylogenetically stable brain structures. ß 2014 Elsevier B.V. All rights reserved. Keywords: Dopamine Retrorubral field Rock cavy Substantia nigra Tyrosine hydroxylase Ventral tegmental area 1. Introduction Abbreviations: 3N, oculomotor nucleus; Aq, cerebral aqueduct; Cli, caudal linear nucleus of the raphe; cp, cerebral peduncle; csc, commissure of the superior colliculus; fr, fasciculus retroflexus; Hb, habenular nucleus; IF, interfascicular nucleus; IP, interpeduncular nucleus; ml, medial lemniscus; MN, mammilary nucleus; ns, nigrostriatal bundle; PAG, periaqueductal gray; PBP, parabrachial pigmented nucleus; pc, posterior commissure; PIF, parainterfascicular nucleus; PN, paranigral nucleus; RLi, rostral linear nucleus; RN, red nucleus; RRF/A8, retrorubral field; rs, rubrospinal tract; SN/A9, substantia nigra pars compacta (nuclear complex); SNCD, substantia nigra dorsal tier; SNCL, substantia nigra lateral cluster; SNCM, substantia nigra medial cluster; SNCV, substantia nigra ventral tier; SNR, substantia nigra reticulate; STh, subthalamic nucleus; SuM, supramammilary nucleus; tSN, tail of the substantia nigra; VTA/A10, ventral tegmental area (nuclear complex); VTAR, ventral tegmental area rostral part; ZI, zona incerta. * Corresponding author at: Department of Biomedical Sciences/Laboratory of Experimental Neurology, Health Science Center, University State of Rio Grande do Norte, 59607-360, Mossoró, RN, Brazil. Tel.: +55 84 33152248; fax: +55 84 33152248. E-mail addresses: [email protected], [email protected] (José R.L.P. Cavalcanti). 0891-0618/$ – see front matter ß 2014 Elsevier B.V. All rights reserved. http://dx.doi.org/10.1016/j.jchemneu.2014.01.002 In the 1950s, 3-hydroxytyramine/dopamine (DA) was described as a neurotransmitter in the central nervous system, in addition to its role as a precursor of the noradrenaline and adrenaline synthesis (Carlsson et al., 1958; Björklund and Dunnett, 2007a). As such, DA is a monoamine included in the catecholamine group, is a major neurotransmitter in the modulation of brain function and plays a crucial role in the adaptation of animal behavior throughout evolution (Smeets and González, 2000; Jones and Pilowski, 2002; Yamamoto and Vernier, 2011). The first detailed description of the distribution of neurons containing catecholamine substances identified 12 neuronal groups, designated A1–A12 in caudorostral direction in the rat encephalon (Dahlström and Fuxe, 1964). Subsequent studies added five more cell groups, A13–A17 (Hökfelt et al., 1984). Of all these, the caudal groups A1–A7 are mainly noradrenergic, J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 A8–A11 cells are mainly dopaminergic, whereas the TH-positive cells in A12, A14 and A15 indicate absence of aromatic amino acid decarboxylase (AADC), the dopamine producing enzyme (Björklund and Dunnett, 2007b). A8, A9 and A10 are developed from the neuromere midbrain, with expansion to prosomere p1 (diencephalon), rostrally, and to isthmus-rhombomere 1 region (I-r1), caudally (Marı́n et al., 2005). These groups are coincident with the retrorubral field (RRF), the substantia nigra (SN), and the ventral tegmental area (VTA), respectively. The neurons of these groups express tyrosine hydroxylase (TH), but not dopamine beta-hydroxylase, which is an enzyme active in the conversion step for noradrenaline/adrenaline. Because of this, the A8, A9 and A10 groups are considered typically dopaminergic groups (Björklund and Dunnett, 2007b). Beside their DA content, these neuronal clusters can be divided on cytoarchitectonic and chemoarchitectonic grounds, as described in the mouse (Fu et al., 2012). It is known that these nuclei are involved in three complex circuitries, named mesostriatal, mesolimbic and mesocortical (François et al., 1999; Smith and Kieval, 2000; Björklund and Dunnett, 2007b) which are involved with motor control, motivation, cognition, reinforcement learning and some neurological/psychiatric disorders, such as Parkinson’s disease and schizophrenia (Chudasama and Robbins, 2004; Nicola et al., 2005; Fields et al., 2007; Cohen et al., 2012). Given the functional and pathological relevance of dopamine we believe it is necessary to expand studies on these neuronal groups in order to reach the greatest number of species. The rock cavy (Kerodon rupestris) is a rodent inhabiting the semiarid Caatinga of the Brazilian Northeast, although it can be also found in the Southeast region as far as the south state of Minas Gerais (Cabrera, 1961). This species reaches adulthood at 200 days, and can reach up to 50 cm in length and 1 kg in body weight (Roberts et al., 1984). According to traditional taxonomy, the rock cavy belongs to the order Rodentia (Carleton and Musser, 2005). According to classifications using the skull shape as a primary characteristic – Anomaluromorpha, Castorimorpha, Hystricomorpha, Myomorpha and Sciuromorpha (Carleton and Musser, 2005), the rock cavy is part of the suborder Hystricomorpha, infraorder Caviomorpha, superfamily Cavioidea, family Caviidae, subfamily Caviinae. Morphological (Silva Neto, 2000) and molecular biology (Rowe and Honeycutt, 2002) studies have connected the genus Kerodon with the genus Hydrochaeris, which includes the capybara (family Hydrochaeridae), and is closely related to the genus Dolichotis of the subfamily Dolichotinae, whose representative in South America is the Patagonian hare (Dolicothis patagonum). The order Rodentia is the most diverse among placental mammals. A new nuclear gene analysis supports the division of this order in ‘‘squirrel-related’’, ‘‘mouse-related’’ clades, as well as the ‘‘Ctenohystrica clade’’, in which the suborder Hystricomorpha is included (Blanga-Kkanfi et al., 2009; Fabre et al., 2012). The rock cavy has a predominantly crepuscular behavior (Sousa and Menezes, 2006) and is adapted to the Brazilian Northeast ecological conditions such as heat, water and food scarcity, especially in periods of severe drought. It inhabits rocky places with numerous crevices where it takes shelter from predators and spends much of its time. Moreover, rock cavies are excellent jumpers and can climb rocks and tree branches where they draw food, consisting mainly of tree bark, unlike other terrestrial caviinaes that eat grass (Carvalho, 1969; Lacher, 1981; Mendes, 1985). For a while this species has been used as an experimental model for studies on the nervous system, for example in research on retinal projections to thalamic nuclei (Nascimento Jr. et al., 2008, 2010a) and circadian centers (Nascimento Jr. et al., 2010b) and the serotonergic system in the brain (Soares et al., 2012). The present study aimed to describe the morphology of A8, A9 and A10 dopamine groups in the rock cavy by TH immunohistochemistry. It provides a foundation for future research on hodological and 59 functional aspects of these neuronal groups in this species, broadening the basis for understanding evolutionary processes associated with the nuclear organization of this neuronal system. 2. Materials and methods Four young adult rock cavies (two males and two females), weighing between 300 and 400 g, from rural municipalities in the state of Rio Grande do Norte, Brazil, were used. Animal capture was authorized by the Brazilian Environmental Agency (IBAMA, license 21440-1). Approval for the experiments was obtained from the local Animal Experimentation Ethics Committee (Protocol 015/2009-addendum) in compliance with National Institute of Health (NIH) guidelines. All efforts were made to minimize the number of animals and their suffering. Individuals were housed for a short adaptation period in 3.00 2.00 2.60 m masonry cages consisting of four wire screen walls, ceramic tile ceilings and natural soil floor, with creeping vegetation and rocks to simulate their natural habitat. The animals were exposed to environmental temperature, air humidity and light, with unlimited access to food and water. Each individual was pre-anesthetized with an intramuscular injection of tramadol chloridrate and xylazine, both 5 mg/kg and maintained with gas isofluoran and 100% oxygen. Upon deep anesthesia, they were perfused through a cannula positioned in the ascending aorta, and connected to a peristaltic pump (Cole-Parmer). After cutting the right auricula, 300 ml of 0.9% saline solution in 0.1 M phosphate buffer, pH 7.4, containing heparin 5000 IU/ml (Parinex, Hipolabor, Sabará, MG, Brazil, 2 ml/1000 ml of saline solution) were injected for approximately 5 min. Next, 700 ml of a 4% paraformaldehyde, 2% picric acid and 0.05% glutaraldehyde fixative solution in 0.1 M phosphate buffer, pH 7.4 (Zamboni and De Martino, 1967) was administered. A flow rate of 70 ml/min was applied for half the solution and 17.5 ml/min for the other half, totaling 30 min for the entire procedure. After perfusion, two animals were placed in the stereotaxic frame and the incisor bar was adjusted until the lambda and bregma were at the same height. The skull bones were removed to expose the dorsal surface of the encephalon, which was sectioned into 3 blocks by means of two coronal sections: one at the bregma level and the other at the lambda level. Finally, the encephalon was removed from the skull, stored in 30% sucrose solution in 0.1 M phosphate buffer, pH 7.4, for 24–48 h, and then sectioned by dry ice freezing in a sliding microtome, obtaining coronal sections of 30 mm. The brains of the other two animals were sectioned at the sagittal plane. In both cases, the sections were collected sequentially into 6 compartments, each containing one of every 6 sections, thereby representing a serial sequence with a distance of 180 mm between the sections. Sections from one series were immediately mounted on gelatin coated glass slides and Nissl stained with thionin, to visualize the cytoarchitectonic delimitation of neuronal groups. Sections from another series were submitted to immunohistochemistry to reveal TH. All the immunohistochemical procedures were performed at room temperature. Free-floating sections, previously submitted to pre-treatment with sodium borohydride and hydrogen peroxide (H2O2), were placed in contact with the mouse anti-TH antibody (Sigma, 1:10,000) and 2% normal goat serum in 0.4% Triton X-100 for 18 h, in a rotator. This was followed by incubation in the secondary antibody, consisting of 1:1000 biotinylated donkey anti-mouse (Jackson Immunoresearch Labs.) under gentle shaking in a rotator, for 90 min. In order to visualize the reaction, the sections underwent 90-min incubation in an avidin–biotin–HRP complex (Vector Elite ABC kit), followed by the final reaction in a medium containing H2O2 as substrate and 3,30 diaminobenzidine tetrahydrochloride as chromogen. H2O2 was offered indirectly, by mixing oxidase glucose and b-D-glucose into the solution, causing a reaction in which the former acting on the latter releases H2O2 (Itoh et al., 1979). The sections were thoroughly washed with a 0.1 M phosphate buffer, pH 7.4, at the beginning, between each step and at the end. Sections were mounted on previously gelatinized glass slides, which, after drying at room temperature, were rapidly submerged in a solution of 0.05% osmium tetroxide to enhance the visibility of the reaction product. The sections were dehydrated in a battery of gradually increasing alcohols, cleared, and then coverslipped in an Entellan1 mounting medium. With respect to staining specificity, a number of sections were submitted to immunohistochemical reactions omitting the primary or secondary antibodies. In these cases, no TH-immunoreactivity was obtained. TH-immunostained coronal sections of the rock cavy brain were analyzed using an optical microscope (Olympus BX41) under bright field illumination. Digital images were obtained from representative sections using a digital video camera (Nikon DXM1200) coupled to the microscope. The digitized images were converted to a gray scale, corrected minimally for brightness and contrast, and mounted using Adobe Photoshop CS5 software (Adobe Systems, Mountain View, CA, USA). Diagrams were obtained from images of Nissl-stained coronal and sagittal sections with Adobe Illustrator CS5 software (Adobe Systems, Mountain View, CA, USA). 3. Results In this study, TH immunohistochemistry was used to delimit dopaminergic neuronal groups A8, A9 and A10 in the rock cavy 60 J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 retroflexus (Fig. 2 C-G). VTAR (Fig. 3A and B) and PBP (Fig. 4A and B) TH-IR neurons are multipolar, rounded or triangular shaped, and do not show dendritic organization. The IF was located medially to the VTAR/PBP and dorsally to the interpeduncular nucleus (Fig. 2C–F). This group is formed by multipolar and bipolar, fusiform and rounded shaped neurons, which do not show a dendritic organization pattern (Fig. 4A and D). The PIF is located laterally to the IF and medially to PBP (Fig. 2C and D). This cluster is formed by a moderate density of TH-IR rounded, multipolar type neurons, whose dendrites are arranged in a dorsoventral orientation (Fig. 3A and C). The PN is located ventrally to the PBP and medially to the SNCM (Fig. 2D–F). This cluster is formed by TH-IR multipolar and triangular neurons, which do not show a dendritic organization pattern (Fig. 4A and C). The RLi (Fig. 3A and D) and CLi (Fig. 7A and D) were located in the brain midline, dorsally to the IF and ventrally to the periaqueductal gray (Fig. 2C–G). They are formed by a moderate density of TH-IR neurons, which are ovoid, bipolar and multipolar, with dorsoventral dendritic orientation. Sparse TH-IR neurons were seen spread in the ventral periaqueductal gray in the territory of the dorsal raphe nucleus (not shown). 3.2. Substantia nigra pars compacta (SNC/A9 complex) Fig. 1. Photographs of the dorsal (A) and ventral (B) aspects of the brain of the rock cavy. Scale bar = 6 mm. brain. TH-immunoreactive (TH-IR) neurons are shown in photomicrographs of immunostained coronal sections taken from several levels of a representative animal. The location of TH-IR neurons was determined according to apparently corresponding sections from the rat brain atlas (Paxinos and Watson, 2007). The nomenclature we used to describe the cytoarchitectonic and immunohistochemically defined groups conforms closely to that adopted for the rat (Paxinos and Watson, 2007) and mouse (Fu et al., 2012). The rostrocaudal length of the rock cavy encephalon, from the olfactory bulb to the bulb-spinal transition, was around 3.6 cm (Fig. 1). TH-immunostained and Nissl-stained sections contributed to establish the anatomical boundaries, cytoarchitecture and possible subdivisions of the dopaminergic groups in the territory from the caudal diencephalon to the isthmus. To facilitate understanding, illustrative diagrams were made (Fig. 2). 3.1. Ventral tegmental area (VTA/A10 complex) The VTA/A10 complex could be subdivided in seven neuronal clusters: the interfascicular (IF), rostral linear (Rli) and caudal linear (Cli) nuclei, situated along the midline, the paranigral (PN) and parainterfascicular (PIF) nuclei, forming an intermediate group, and dorsolaterally, the rostral ventral tegmental area (VTAR) and parabrachial pigmented nucleus (PBP). VTAR and PBP were seen to be located laterally to the mamillary and supramamillary nuclei, medially to the substantia nigra medial cluster (SNCM) and substantia nigra reticulata (SNR) and ventrally to the medial lemniscus, red nucleus and nigro-striatal projections. At caudal levels, the PBP is located dorso-laterally to the interpeduncular nucleus and fasciculus Identification of the subunits along the entire extent of the SNC was possible based on the density of distribution and morphology of its neurons. It could be seen that the SNC was divided into the following: substantia nigra, dorsal tier (SNCD); substantia nigra, ventral tier (SNCV); substantia nigra, lateral cluster (SNCL), SNCM; and tail of the substantia nigra (tSN). The SNCM was identified laterally to the mammillary and supramammillary nuclei, medially to the subthalamic nucleus, ventrally to the medial lemniscus, zona incerta, red nucleus and nigro-striatal projections and dorsally to the cerebral peduncle and SNR (Fig. 2C–E). SNCM neurons are bipolar or multipolar, and ovoid or triangular shaped. Dendrites of rostral neurons are arranged parallel to the edges of the cerebral peduncle and SNR (Fig. 5A and B). However, at caudal levels, this pattern was replaced by a random arborization (not shown). SNCD was found laterally to the SNCM, ventrally to the medial lemniscus and dorsally to the SNR (Fig. 2B–G). SNCD neurons are bipolar and multipolar, with ovoid and triangular formats and their dendritic organization have predominantly medial-lateral orientation (Fig. 5A and C). SNCL was found laterally to the SNCD, dorsally to the SNR, and, at caudal levels, ventrally to the RRF/A8 (Fig. 2C– F). SNCL neurons are multipolar, ovoid or piriform shaped and devoid of any dendritic organizational pattern (Fig. 5A and D). At caudal level, a low density TH-IR neuronal group was found as a vertical dorsal protrusion into the SNCD and SNCL, which was called the tSN (Fig. 2D and E). The tSN contains fusiform and ovoid shaped neurons, whose dendrites are arranged in a dorsoventral orientation (Fig. 5A and E, and Fig. 6A and B). TH-IR neurons were also found ventrally to SNCD and dorsally to cerebral peduncle, inserted into the SNR, forming the SNCV division (Fig. 2D–G). This group has low neuronal density, its neurons are ovoid and multipolar shaped, with dorsoventral dendritic orientation (Fig. 5A and F). 3.3. Retrorubral field (RRF/A8) The A8 group is formed by numerous, sparsely distributed neurons located in the lower half of the midbrain tegmentum (Fig. 2F and G). These neurons are ovoid or fusiform shaped, and do not show dendritic organization pattern (Fig. 7A–C). J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 61 Fig. 2. Drawings of sagittal (A) and coronal (B-G) sections through the rock cavy brainstem depicting the location of the midbrain TH-immunoreactive neuronal groups (gray shaded areas). Numbers on the right indicate distance from the bregma. See list for abbreviations. 4. Discussion 4.1. Technical and morphological considerations The present investigation provides the first detailed description of the distribution of catecholaminergic/dopaminergic neurons in the A8, A9 and A10 in the brain of a Brazilian Northeast rodent, the rock cavy, based on TH immunohistochemistry and Nissl staining. TH is an enzyme common to the synthesis of all the catecholamines, by which TH immunohistochemistry can reveal dopaminergic, noradrenergic and adrenergic neurons. Evidence from physiological, pharmacological, clinical and molecular biology studies agree that TH-IR neurons in the midbrain are DA producers, making TH a reliable dopamine marker in that region (Grimm et al., 2004; Prakash and Wurst, 2006; Margolis et al., 2006). In the rat (German and Manaye, 1993; McRitchie et al., 1996; Paxinos and Watson, 2007), mouse (Fu et al., 2012), human (McRitchie et al., 1996), and baboon (Papio ursinus, McRitchie et al., 1998), A10 neurons are distributed throughout a heterogeneous 62 J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 Fig. 3. Photomicrographs of TH-immunostained brainstem coronal sections illustrating (A) VTA/A10 complex. The boxed regions are shown in higher magnification (B–D): (B) VTAR, (C) PIF, and (D) RLi. See list for abbreviations. Level of section: 4.32 mm p.b. Scale bar: A = 1 mm and B–D = 100 mm. Fig. 4. Photomicrographs of TH-immunostained brainstem coronal sections illustrating (A) VTA/A10 complex. The boxed regions are shown in higher magnification (B–D): (B) PBP, (C) PN and (D) IF. See list for abbreviations. Level of section: 5.22 mm p.b. Scale bar: A = 1 mm and B–D = 100 mm. J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 63 Fig. 5. Photomicrographs of TH-immunostained brainstem coronal sections illustrating (A) SN/A9 complex. The boxed regions are shown in higher magnification (B–F): (B) SNCM, (C) SNCD, (D) SNCL, (E) tSN and (F) SNCV. See list for abbreviations. Level of section: 5.04 mm p.b. Scale bar: A = 1 mm and B–F = 100 mm. Fig. 6. Photomicrographs of TH-immunostained brainstem coronal sections illustrating the SN/A9 complex (A). The boxed region corresponds to tSN and is shown in higher magnification in B. See list for abbreviations. Level of section: 5.22 mm p.b. Scale bar: A = 1 mm and B = 100 mm. 64 J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 Fig. 7. Photomicrographs of TH-immunostained brainstem coronal sections illustrating (A) RRF/A8 and VTA/A10 clusters. The boxed regions are shown in higher magnification (B–D): (B) and (C) RRF and (D) CLi. See list for abbreviations. Level of section: 6.12 mm p.b. Scale bar: A = 1 mm and B–D = 100 mm. complex of nuclei, where seven components can be distinguished. In the midline, the interfascicular nucleus (IF), located in the rostral pole of the interpeduncular nucleus, and immediately above, the raphe caudal linear nucleus (CLi), which is replaced by the raphe rostral linear nucleus (RLi) rostrally. Forming a medial group, there are the paranigral nucleus (PN) and the parainterfascicular nucleus (PIF). The more lateral clusters are the rostral ventral tegmental area (VTAR) and the parabrachial pigmented nucleus (PBP). Regarding the A9 complex, the following divisions can be distinguished: substantia nigra compacta, dorsal tier (SNCD), substantia nigra compacta, ventral tier (SNCV), substantia nigra compacta, lateral cluster (SNCL), and substantia nigra compacta, medial cluster (SNCM). The A8 dopamine cell cluster, contained in the caudal midbrain reticular formation, corresponding to the retrorubral field (RRF), does not permit any subdivisions. Catecholamine groups were studied in several rodent African species, such as Highveld gerbil (Tatera brantsii, Moon et al., 2007) and African pigmy mouse (Mus minutoides, Kruger et al., 2012), both belonging to Muridae family, a mouse-related clade. African rodents of the Ctenohystrica clade, suborder Hystricomorpha were also studied for catecholamine groups: the Highveld mole-rat (Cryptomys hottentotus, Da Silva et al., 2006; Bhagwandin et al., 2008) and Cape dune mole-rat (Bathyergus suillus, Bhagwandin et al., 2008), both of family Bathyergidae, as well as greater canerat (Thryonomys swinderianus, family Thryonomyidae, Dwarika et al., 2008) and the Cape porcupine (Hystrix africaeaustralis, family Hystricidae, Limacher et al., 2008). There is no register of such studies of the Hystricomorpha Caviidae rodent species, beyond the rock cavy (present study). Other African non-rodent species have been used in the study of catecholamine groups, such as the rock hyrax (Procavia capensis, Gravett et al., 2009), rock elephant shrew (Elephantulus myurus, Pieters et al., 2010), giraffe (Giraffa camelopardalis, Bux et al., 2010), among others. According to all those studies, the A10 is divisible into A10, which would correspond to the VTAR, PBP, PN and PIF; A10c (central), which would correspond to the PF; and A10d (dorsal), which would correspond to the CLi and RLi, and even the A10dc (dorsal caudal), the latter being a dopamine neuronal cluster spread in the periaqueductal gray in the territory of the dorsal raphe nucleus. Those neurons are also considered a component of A10 group by Smeets and González (2000). Although we have seen sparse TH-IR neurons in that location in the rock cavy brain, however, we did not consider them a component of A10, in compliance with the terminology adopted in Paxinos and Watson (2007). In the abovementioned species, the A9 complex could be subdivided in: A9pc, which would correspond to SNCD; A9l, which would correspond to SNCL; A9m, which would correspond to SNCM; and A9v, which would correspond to SNCV. The A8 dopamine cell cluster, contained in the caudal midbrain reticular formation, corresponding to the retrorubral field (RRF), does not permit any subdivisions. Among mammals, the Megachiropteria species, such as the rousette flying fox (Rousettus aegyptiacus, Maseko et al., 2007); the straw-coloured fruit bat (Eidolon helvum) and the Wahlberg’s epauletted fruit Bat (Epomophorus wahlbergi) (Dell et al., 2010) have been studied with respect to the catecolamine groups, revealing all subdivisions found for the most mammals. However, among microbats, such as the long-fingered bat (Miniopterus schreibersii, Maseko and Manger, 2007) and Cardioderma cor, Chaerophon pumilus, Coleura afra, Hipposideros commersoni and Triaenops persicus (Kruger et al., 2010) it was noted a trend to absence of some subdivisions. Our proposal of subdivision of the A8, A9 and A10 dopaminergic groups agrees with what has been described for other species, although some discrepancies can be attributed to different terminologies. The exception found refers to the presence of the tSN as a component of A9 complex, described in our material in the rock cavy, which, to date, has been described only in the Göttingen minipig (Nielsen et al., 2009). One could guess that the tSN is part J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 of the A9l or even an extension of the RRF. However, the cytoarchitectonic aspects of the tSN, such as its dorsoventral dendritic orientation, allow us to distinguish it as a distinct subdivision of A9. 4.2. Evolutionary considerations Based in previous studies in other animals, mainly referring to those on the Rodentia order, it has been possible to deduce that, although noticing differences relative to phenotypic characteristics, the nuclear complexity of the dopamine centers in the brain seems be different among the different orders, but not within the same order. Thus, it has been suggested that the phenotypic variations, lifestyle, and evolutionary characteristics in the Rodentia order do not lead to a significant variation in the brain nuclei (Manger, 2005). The results revealed that the rock cavy A8, A9 and A10 dopaminergic nuclei are, in general, similar to what has already been described in other species of mammals, suggesting that these nuclei are phylogenetically stable brain structures in the species. An exception exists with respect to the presence of the tSN, which, besides in the rock cavy brain (present study) has been described only in a non-rodent species, the Göttingen minipig (Susscrofa domesticus), a laboratory-developed ungulate derived from the domestic pig (Nielsen et al., 2009). In this point it is worth emphasizing that, although the dopaminergic groups have been studied in many rodent species, to date, the rock cavy (present study) is the first species of the Ctenohystrica clade, Hystricomorpha suborder, among the Caviomorpha superfamily to be studied in this particular way. To our knowledge, there is no register of studies involving other species, such as agouti, capybara or paca, animals of the suborder Hystricomorpha, characteristic of South America. This raises the need for more detailed studies about these neuronal clusters, with particular reference to tSN. We recommend an expansion of the comparative analysis of brain samples from animals of the same and different orders, suborders and families, or even different classes of vertebrates, in order to test the consistency of such nuclear division. This work reported on the organization of the A8, A9 and A10 dopaminergic neurons of the rock cavy brain and discussed this in light of the features of other rodent or non-rodent mammalian species. Further studies are needed to clarify some variations, as well as to expand our understanding about the many different functions of this system. Acknowledgments This study was financially supported by the National Council for Scientific and Technological Development (CNPq), Coordination for High Level Staff Improvement (CAPES) and Research and Projects Financing (FINEP), Brazil. References Bhagwandin, A., Fuxe, K., Bennett, N.C., Manger, P.R., 2008. Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brains of two species of African mole-rats. J. Chem. Neuroanat. 35, 371–387. Björklund, A., Dunnett, S., 2007a. Fifty years of dopamine research. Trends Neurosci. 30, 185–187. Björklund, A., Dunnett, S., 2007b. Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202. Blanga-Kkanfi, S., Miranda, H., Penn, O., Pupko, T., DeBry, R.W., Huchon, D., 2009. Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 9, 71–82. Bux, F., Bhagwandin, A., Fuxe, K., Manger, P.R., 2010. Organization of cholinergic, putative catecholaminergic and serotonergic nuclei in the diencephalon, midbrain and pons of sub-adult male giraffes. J. Chem. Neuroanat. 39, 189–203. 65 Cabrera, A., 1961. Catálogo de los mamı́feros de America del Sur. Rev. Mus. Argentino Cien. Nat. 4, 1–732. Carleton, M.D., Musser, G.G., 2005. Order Rodentia In: Wilson, D.E., Reeder, D.M. (Eds.), Mammal Species of the World: A Taxonomic and Geographic Reference. John Hopkins University Press, Baltimore, MD, pp. 745–752. Carlsson, A., Lindqvist, M., Magnusson, T., Waldeck, B., 1958. On the presence of 3hydroxytyramine in brain. Science 127, 471. Carvalho, J.C.M., 1969. Notas de viagem de um zoólogo à região das caatingas e áreas limı́trofes. Imprensa universitária do Ceará, Fortaleza. Chudasama, Y., Robbins, T.W., 2004. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology (Berl) 174, 86–98. Cohen, J.Y., Haesler, S., Vong, L., Lowell, B.B., Uchida, N., 2012. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88. Dahlström, A., Fuxe, K., 1964. Evidence for the existence of monoamine containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. 232, 1–55. Da Silva, J.N., Fuxe, K., Manger, P.R., 2006. Nuclear parcellation of certain immunohistochemically identifiable neuronal systems in the midbrain and pons of the Highveld molerat (Cryptomys hottentotus). J. Chem. Neuroanat. 31, 37–50. Dell, L.A., Kruger, J.L., Bhagwandin, A., Jillani, N.E., Pettigrew, J.D., Manger, P.R., 2010. Nuclear organization of cholinergic, putative catecholaminergic and serotonergic systems in the brains of two megachiropteran species. J. Chem. Neuroanat. 40, 177–195. Dwarika, S., Maseko, B.C., Ihunwo, A.O., Fuxe, K., Manger, P.R., 2008. Distribution and morphology of putative catecholaminergic and serotoninergic neurons in the brain of the greater canerat, Thryonomys swinderianus. J. Chem. Neuroanat. 35, 108–122. Fabre, P.-H., Hautier, L., Dimitrov, D., Douzery, E.J.P., 2012. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol. Biol. 12, 88–106. Fields, H.L., Hjelmstad, G.O., Margolis, E.B., Nicola, S.M., 2007. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci. 30, 289–316. François, C., Yelnik, D., Tandé, D., Agid, Y., Hirsh, E.C., 1999. Dopaminergic cell group A8 in the monkey: anatomical organization and projections to the striatum. J. Comp. Neurol. 414, 334–347. Fu, Y., Yuan, Y., Halliday, G., Rusznák, Z., Watson, C., Paxinos, G., 2012. A cytoarchitetonic and chemoarchitetonic analysis of the dopamine cell groups in the susbstantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct. Funct. 217, 591–612. German, D.C., Manaye, K.F., 1993. Midbrain dopaminergic neurons (Nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J. Comp. Neurol. 331, 297–309. Gravett, N., Bhagwandin, A., Fuxe, K., Manger, P.R., 2009. Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brains of the rock hyrax, Procavia capensis. J. Chem. Neuroanat. 38, 57–74. Grimm, J., Mueller, A., Hefti, F., Rosenthal, A., 2004. Molecular basis for catecholaminergic neuron diversity. Proc. Natl. Acad. Sci. U.S.A. 38, 11389–13896. Hökfelt, T., Matensson, R., Björklund, A., Kleinau, S., Goldstein, M., 1984. Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund, A., Hökfelt, T. (Eds.), Classical Transmitters in the CNS, part I. Handbook of Chemical Neuroanatomy, vol. 2. Elsevier, Amsterdam, pp. 227– 379. Itoh, K., Konish, A., Nomura, S., Mizuno, N., Nakamura, Y., Sugimoto, T., 1979. Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res. 175, 341–346. Jones, H.M., Pilowski, L.S., 2002. Dopamine and antipsychotic drug action revisited. Br. J. Psychiatry 181, 271–275. Kruger, J.L., Dell, L.A., Bhagwandin, A., Jillani, N.E., Pettigrew, J.D., Manger, P.R., 2010. Nuclear organization of cholinergic, putative catecholaminergic and serotonergic systems in the brains of five microchiropteran species. J. Chem. Neuroanat. 40, 210–222. Kruger, J.L., Patzke, N., Fuxe, K., Bennett, N.C., Manger, P.R., 2012. Nuclear organization of cholinergic, putative catecholaminergic, serotonergic and orexinergic systems in the brain of the African pygmy mouse (Mus minutoides): organizational complexity is preserved in small brains. J. Chem. Neuroanat. 44, 45–56. Lacher Jr., T.E., 1981. The comparative social behavior of Kerodon rupestris and Galea spixii and the evolution of behavior in the cavidiae. Bull. Carnegie Mus. Nat. Hist. 17, 5–71. Limacher, A.M., Bhagwandin, A., Fuxe, K., Manger, P.R., 2008. Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brain of the Cape porcupine (Hystrix africaeaustralis): increased brain size does not lead to increased organizational complexity. J. Chem. Neuroanat. 36, 33–52. Manger, P.R., 2005. Stablishing order at the systems level in mammalian brain evolution. Brain Res. Bull. 66, 282–289. Marı́n, F., Herrero, M.T., Vyas, S., Puelles, L., 2005. Ontogeny of tyrosine hydroxylase mRNA expression in mid- and forebrain: neuromeric pattern and novel positive regions. Dev. Dyn. 234, 709–717. Margolis, E.B., Lock, H., Hjelmstad, G.O., Fields, H.L., 2006. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol. 577, 907–924. 66 J.R.L.P. Cavalcanti et al. / Journal of Chemical Neuroanatomy 55 (2014) 58–66 Maseko, B.C., Bourne, J.A., Manger, P.R., 2007. Distribution and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brain of the Egyptian rousette flying fox (Rousettus aegyptiacus). J. Chem. Neuroanat. 34, 108–127. Maseko, B.C., Manger, P.R., 2007. Distribution and morphology of cholinergic, catecholaminergic and serotonergic neurons in the brain of Schreiber’s longfingered bat, Miniopterus schreibersii. J. Chem. Neuroanat. 34, 80–94. McRitchie, D.A., Cartwright, H., Pond, S.M., Van der Schyf, C.J., Castagnoli Jr., N., Van der Nest, D.G., Halliday, G.M., 1998. The midbrain dopaminergic cell groups in the baboon Papio ursinus. Brain Res. Bull. 47, 611–623. McRitchie, D.A., Hardman, C.D., Halliday, G.M., 1996. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J. Comp. Neurol. 364, 121–150. Mendes, B.V., 1985. Alternativas tecnológicas para a agropecuária do semi-árido. Nobel, São Paulo, pp. 171. Moon, D.J., Maseko, B.C., Ihunwo, A.O., Fuxe, K., Manger, P.R., 2007. Distribution and morphology of catecholaminergic and serotonergic neurons in the brain of the highveld gerbil, Tatera braintsii. J. Chem. Neuroanat. 34, 134–144. Nascimento Jr., E.S., Duarte, R.B., Silva, S.F., Engelberth, R.C.G.J., Toledo, C.A.B., Cavalcante, J.S., Costa, M.S.M.O., 2008. Retinal projections to the thalamic paraventricular nucleus in the rock cavy (Kerodon rupestris). Brain Res. 1241, 56–61. Nascimento Jr., E.S., Cavalcante, J.S., Cavalcante, J.C., Costa, M.S.M.O., 2010a. Retinal afferents to the thalamic mediodorsal nucleus in the rock cavy (Kerodon rupestris). Neurosci. Lett. 475, 38–43. Nascimento Jr., E.S., Sousa, A.P., Duarte, R.B., Magalhães, M.A., Silva, S.F., Cavalcante, J.C., Cavalcante, J.S., Costa, M.S.M.O., 2010b. The suprachiasmatic nucleus and intergeniculate leaflet in the rock cavy (Kerodon rupestris): retinal projections and immunohistochemical characterization. Brain Res. 1320, 34–46. Nicola, S.M., Taha, S.A., Kim, S.W., Fields, H.L., 2005. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience 135, 1025–1033. Nielsen, M.S., Sørensen, J.C., Bjarkam, C.R., 2009. The substantia nigra pars compacta of Göttingen minipig: an anatomical and stereological study. Brain Struct. Funct. 213, 481–488. Paxinos, G., Watson, C., 2007. The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego. Pieters, R.P., Gravett, N., Fuxe, K., Manger, P.R., 2010. Nuclear organization of cholinergic, putative catecholaminergic and serotonergic nuclei in the brain of the eastern rock elephant shrew, Elephantulus myurus. J. Chem. Neuroanat. 39, 175–188. Prakash, N., Wurst, W., 2006. Development of dopaminergic neurons in the mammalian brain. Cell. Mol. Life Sci. 63, 187–206. Roberts, M.E., Maliniak, E., Deal, M., 1984. The reproductive biology of the rock cavy, Kerodon rupestris in captivity: a study of reproductive adaptation in a tropic specialist. Mammalia 48, 253–266. Rowe, D.L., Honeycutt, R.L., 2002. Phylogenetic relationships, ecological correlates, and molecular evolution within the Cavioidea (Mammalia, Rodentia). Mol. Biol. Evol. 19, 263–277. Silva Neto, E.J., 2000. Morphology of the regions ethmoidalis and orbitotemporalis in Galea musteloides Meyen 1832 and Kerodon rupestris (Wied-Neuwied 1820) (Rodentia: Caviidae) with comments on the phylogenetic systematics of the Caviidae. J. Zool. Syst. Evol. Res. 38, 219–229. Smeets, W.J.A.J., González, A., 2000. Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res. Rev. 33, 308–379. Smith, Y., Kieval, J.Z., 2000. Anatomy of the dopamine in the basal ganglia. Trends Neurosci. 23 (Suppl. 10) S28–S33. Soares, J.G., Cavalcanti, J.R.L.P., Oliveira, F.G., Pontes, A.L.B., Sousa, T.B., Freitas, L.M., Cavalcante, J.S., Nascimento Jr., E.S., Cavalcante, J.C., Costa, M.S.M.O., 2012. Nuclear organization of the serotonergic system in the brain of the rock cavy (Kerodon rupestris). J. Chem. Neuroanat. 43, 112–119. Sousa, R.A., Menezes, A.A.L., 2006. Circadian rhythms of motor activity of the Brazilian rock cavy (Kerodon rupestris) under artificial photoperiod. Biol. Rhythm Res. 3, 443–450. Yamamoto, K., Vernier, P., 2011. The evolution of dopamine systems in chordates. Front. Neuroanat. 5, 1–21. Zamboni, L., De Martino, L., 1967. Buffered picric acid formaldehyde: a new rapid fixative for electron microscopy. J. Cell Biol. 35, 148A.