* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hypothalamus - aHuman Project
Psychoneuroimmunology wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Multielectrode array wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Haemodynamic response wikipedia , lookup
Signal transduction wikipedia , lookup
Neural coding wikipedia , lookup
Nervous system network models wikipedia , lookup
Axon guidance wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Causes of transsexuality wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Neuroanatomy wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Optogenetics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
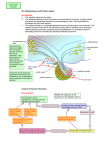
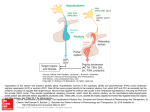
Lecture outline • Structural organisation of hypothalamus Hypothalamus – Localisation + nuclei – Input/output pathways • Physiological function of hypothalamus [email protected] Overview of anatomy anterior chiasmatic Overview of anatomy tuberal posterior medial view Overview of physiological functions coronal view Third ventricle Mamillo-thalamic tract Dorsal hypothalamic area Dorsomedial nucleus Fornix Lateral hyothalamic area Supraoptic nucleus Medial forebrain bundle Amygdala Amygdala Lateral tuberal nucleus Median eminence Arcuate nucleus Optic tract Ventromedial nucleus 1 Overview of physiological functions Overview of physiological functions • Maintenance of milieu interne • Maintenance of milieu interne • Behaviour • Behaviour • Memory • Memory ¾ Regulation of energy metabolism (food intake, metabolic rate, temperature regulation, growth) ¾ Regulation of energy metabolism (food intake, metabolic rate, temperature regulation, growth) ¾ Reproductive function (including milk production, social interactions) ¾ Reproductive function (including milk production, social interactions) ¾ Biological clock, sleep-wake cycles ¾ Biological clock (sleep-wake cycles) ¾ Control of blood flow (cardiac output, blood osmolarity and renal clearance, thirst regulation) ¾ Control of blood flow (cardiac output, blood osmolarity and renal clearance, thirst regulation) Overview of physiological functions Overview of physiological functions • Detection of (changes in) ¾Regulation of the autonomic nervous system ¾ Blood osmolarity ¾ Blood nutrient levels ¾Release of hormones Hypothalamic neurons can release hormones (neuro-endocrine) ¾ Blood hormone levels ¾ Body temperature ÍDirectly and indirectly Overview of connections • Input from ¾ Retina (retinohypothalamic tract – terminates in SCN) ¾ Olfactory receptors (medial forebrain bundle) ¾ Cutaneous receptors ¾ Higher (limbic) system (hippocampal formation: fornix – to mammillary bodies; amygdala: stria terminalis – to medial hypothalamus) ¾ Viscera Overview of connections • Output to ¾ Thalamus (via mammillothalamic tract (Papez circuit: cingulate gyrus – hippocampal formation – mammillary bodies – anterior thalamic nucleus – cingulate gyrus)) (also mammillotegmental tract to midbrain tegmentum) ¾ Amygdala (from medial hypothalamus) ¾ Midbrain PAG (from medial hypothalamus) (aggression, rage, flight) ¾ Frontal and parietal lobes, habenular nucleus, midbrain… ¾ Blood stream (pituitary) 2 Overview of basic functions • Feed-back system Overview of basic functions • Feed-back system • Feed-forward system – Hypothalamus corrects deviations from a given set-point: – Hypothalamus corrects deviations from a given set-point: – Hypothalamus can override feed-back under special conditions • measures current value • compares current value with supposed value • makes adjustments to achieve supposed value • measures current value • compares current value with supposed value • makes adjustments to achieve supposed value • Stress responses • Fever (body T set point is changed to higher T) – helps maintain body homeostasis – helps maintain body homeostasis Overview of basic functions • Feed-back system • Feed-forward system – Hypothalamus corrects deviations from a given set-point: – Hypothalamus can override feed-back under special conditions • measures current value • compares current value with supposed value • makes adjustments to achieve supposed value • Stress responses • Fever (body T set point is changed to higher T) – helps maintain body homeostasis • Anticipation • Look at neuroendocrine functions of hypothalamus • Look at regulation of non-endocrine functions (ANS) of hypothalamus – Hypothalamus adjusts its output to meet future needs • Insulin secretion prior to food intake Neuroendocrine hypothalamus • Hypothalamic neurons can act as neuroendocrine cells • Neurotransmitter = (neuro)hormone is released directly into blood stream • Site of hormone release is pituitary gland • 2 principal pathways for eliciting hormone release: – Via the anterior pituitary (adenohypophysis) • 2-tiers process – Via the posterior pituitary (neurohypophysis) • 1-step process Neuroendocrine hypothalamus • Via anterior pituitary (adenohypophysis) – Hypothalamic parvocellular neurons release releasing or inhibiting hormones into hypothalamo-pituitary portal veins – Hypothalmo-pituitary portal veins carry these hormones to anterior pituitary – Anterior pituitary has cells responding to the different releasing or inhibiting hormones – Responsive cells release or stop releasing hormones in response to binding of hypothalamic releasing or inhibiting hormones into systemic circulation 3 Neuroendocrine hypothalamus Neuroendocrine hypothalamus • Via anterior pituitary (adenohypophysis) – Hypothalamic parvocellular neurons secrete releasing or inhibiting hormones into hypothalamo-pituitary portal veins – Hypothalmo-pituitary portal veins carry these hormones to anterior pituitary – Anterior pituitary has cells responding to the different releasing or inhibiting hormones – Responsive cells release or stop releasing hormones in response to binding of hypothalamic releasing or inhibiting hormones into systemic circulation • Via anterior pituitary Hypothalamus R I Anterior Pituitary Neuroendocrine hypothalamus Hypothalamus R I Anterior Pituitary Neuroendocrine hypothalamus • Via anterior pituitary (adenohypophysis) – Hypothalamic parvocellular neurons secrete releasing or inhibiting hormones into hypothalamo-pituitary portal veins – Hypothalamo-pituitary portal veins carry these hormones to anterior pituitary – Anterior pituitary has cells responding to the different releasing or inhibiting hormones – Responsive cells release or stop releasing hormones in response to binding of hypothalamic releasing or inhibiting hormones into systemic circulation (adenohypophysis) – Hypothalamic parvocellular neurons secrete releasing or inhibiting hormones into hypothalamo-pituitary portal veins – Hypothalamo-pituitary portal veins carry these hormones to anterior pituitary – Anterior pituitary has cells responding to the different releasing or inhibiting hormones – Responsive cells release or stop releasing hormones in response to binding of hypothalamic releasing or inhibiting hormones into systemic circulation • Via anterior pituitary Hypothalamus R I Anterior Pituitary Rel./Inhib. hormone: (adenohypophysis) – Hypothalamic parvocellular neurons secrete releasing or inhibiting hormones into hypothalamo-pituitary portal veins – Hypothalamo-pituitary portal veins carry these hormones to anterior pituitary – Anterior pituitary has cells responding to the different releasing or inhibiting hormones – Responsive cells secrete or stop secreting hormones in response to binding of hypothalamic releasing or inhibiting hormones into systemic circulation Rel./Inhib. hormone: GnRH gonadotrope FSH+LH CRH corticotrope ACTH TRH thyrotrope TSH GHRH somatotrope Sost DA gonads Hypothalamus R Anterior Pituitary Ant. Pit. target cell: GnRH gonadotrope FSH+LH CRH corticotrope ACTH TRH thyrotrope TSH GH GHRH somatotrope GH somatotrope GH Sost somatotrope GH lactotrope prolactin DA lactotrope prolactin thyroid I gonads thyroid 4 Rel./Inhib. hormone: Ant. Pit. target cell: Hormone: target: release from: Rel./Inhib. hormone: GnRH gonadotrope FSH+LH gonads arcuate CRH corticotrope ACTH adrenal gland TRH thyrotrope TSH thyroid GHRH somatotrope GH Sost somatotrope GH many cells (bones) DA lactotrope prolactin mammary glands Neuroendocrine hypothalamus • Via anterior pituitary – Hypothalamic parvocellular neurons release releasing or inhibiting hormones into Hypothalamus hypothalamo-pituitary portal veins – Hypothalmo-pituitary portal veins carry these hormones to anterior pituitary – Anterior pituitary has cells median to the different responding eminence releasing or inhibiting hormones – Responsive cells release or posterior stop releasing hormones in pituitary response to binding of hypothalamic releasing or inhibiting hormones into systemic circulation • Via posterior pituitary (neurohypophysis) – Hypothalamic magnocellular neurons release hormones directly into systemic veins that drain into the systemic circulation – Hypothalamohypophyseal tract (axons of neuroendocrine magnocellular neurons) Neuroendocrine hypothalamus Ant. Pit. target cell: Hormone: target: GnRH gonadotrope FSH+LH gonads paraventricular (PVN) CRH corticotrope ACTH adrenal gland TRH thyrotrope TSH thyroid arcuate GHRH somatotrope GH somatotrope GH many cells (bones) lactotrope prolactin anterior Sost HT arcuate DA mammary glands Neuroendocrine hypothalamus • Via anterior pituitary – Hypothalamic parvocellular neurons release releasing or inhibiting hormones into Hypothalamus hypothalamo-pituitary portal veins – Hypothalmo-pituitary portal veins carry these hormones to anterior pituitary – Anterior pituitary has cells median to the different responding eminence releasing or inhibiting hormones – Responsive cells release or posterior stop releasing hormones in pituitary response to binding of hypothalamic releasing or inhibiting hormones into systemic circulation • Via posterior pituitary (neurohypophysis) – Hypothalamic magnocellular neurons release hormones directly into systemic veins that drain into the systemic circulation – Hypothalamohypophyseal tract (axons of neuroendocrine magnocellular neurons) Neuroendocrine hypothalamus Release from: Hormones released: Hormone targets: Release from: Hormones released: Hormone targets: paraventricular (PVN) + supraoptic (SON) ADH kidneys ADH kidneys oxytocin mammary gland + uterus paraventricular (PVN) + supraoptic (SON) oxytocin mammary gland + uterus bonding (autism?) bonding (autism?) 5 ADH-release • ADH promotes water retention in kidneys • release is modified when blood osmolarity changes by more than ~ 1% from set point (~ 280 mOsm/kg) – Hypotonic conditions inhibit ADH release – Hypertonic conditions stimulate ADH release ADH-release ¾How do cells in supra-optic and paraventricular nuclei know that blood osmolarity has changed? • Osmosensitive neurons • Intrinsically osmosensitive neurons in OVLT, SFO and NTS • ADH releasing neurons are intrinsically osmosensitive ¾Firing rate of ADH-releasing neurons integrates central and peripheral information and their own osmosensitivity ADH-release ¾How do cells in supra-optic and paraventricular nuclei know that blood osmolarity has changed? • Osmosensitive neurons • Intrinsically osmosensitive neurons in OVLT, SFO and NTS that directly project to the supraoptic and paraventricular nuclei Circumventricular organ: brain structure that is devoid of blood brain barrier ¾How can a cell be intrinsically osmosensitive? stretch-activated ¾Change in osmolarity will cause cell swelling or shrinking, resulting in increased or decreased stretch of plasma membrane extracellular matrix cytoskeleton ¾Stretch of plasma membrane can gate ion channels Lumpkin & Caterina Nature 445, 858-865 (2007) ADH-release ¾Which is (are) the candidate ion channel(s) involved in osmosensing? tethered indirectly gated cytoskeleton extracellular matrix mechanosensitive protein ADH-release ¾Which is (are) the candidate ion channel(s) involved in osmosensing? Transient Receptor Potential channels of the Vanilloid family (TRPV channels) (non-selective cation channels) ¾TRPV1 ¾opens in response to hypertonic stimulus ¾TRPV4 ¾opens in response to hypotonic stimulus ¾TRPV1 ¾opens in response to hypertonic stimulus ¾TRPV4 ¾opens in response to hypotonic stimulus 6 TRPV1 ADH-release cell volume (cell shrinking) ¾Which is (are) the candidate ion channel(s) involved in osmosensing? (TRPV1-/-) Transient Receptor Potential channels of the Vanilloid family (TRPV channels) (non-selective cation channels) ¾TRPV1 ¾opens in response to hypertonic stimulus membrane membrane conductance conductance N-terminal variant ¾TRPV4 Sharif Naeini et al. Nat. Neurosci. 9: 9, 93 - 98 (2006) ¾opens in response to hypotonic stimulus indirect effect TRPV1 TRPV1 Impaired ADH release in TRPV1 knock-out mice in response to hypertonic solution Action potential firing rate in response to hypertonic solution (TRPV1-/-) Sharif Naeini et al. Nat. Neurosci. 9: 93 - 98 (2006) Sharif Naeini et al. Nat. Neurosci. 9: 93 - 98 (2006) AVP = ADH TRPV4 TRPV4 channels open in response to cell swelling (indirect effect) in expression systems Liedtke & Friedman PNAS;100:13698-13703 (2003) +/+ = wild type -/- = TRPV4 knock-out TRPV4 knock-out mice drink significantly more when infused with ADH-analogue dDAVP (i.e. when water retention is increased, which should result in decreased water intake) than wildtype mice. ADH-release ¾How does it all come together? • Increased blood osmolarity causes osmosensitive OVLT neurons to shrink • TRPV1 channels open, leading to depolarisation and eventually firing of OVLT neurons (graded response) • OVLT neurons make monosynaptic glutamatergic contacts with supra-optic nuclei neurons • This promotes firing of ADH-releasing neurons and hence ADH release • ADH releasing neurons are intrinsically osmosensitive ¾Firing rate of ADH-releasing neurons depends on central and peripheral inputs as well as their intrinsic osmosensitivity 7 ADH-release ADH-release ¾How does it all come together? ¾How does it all come together? • Increased blood osmolarity causes osmosensitive (OVLT) neurons to shrink • TRPV1 channels open, leading to depolarisation and eventually firing of (OVLT) neurons (graded response) • (OVLT) neurons make monosynaptic glutamatergic contacts with supra-optic nuclei neurons • This promotes firing of ADH-releasing neurons and hence ADH release • ADH releasing neurons are intrinsically osmosensitive ¾Firing rate of ADH-releasing neurons depends on central and peripheral inputs as well as their intrinsic osmosensitivity • Increased blood osmolarity causes osmosensitive (OVLT) neurons to shrink • TRPV1 channels open, leading to depolarisation and eventually firing of (OVLT) neurons (graded response) • (OVLT) neurons make monosynaptic glutamatergic contacts with supra-optic nuclei neurons • This promotes firing of ADH-releasing neurons and hence ADH release • ADH releasing neurons are intrinsically osmosensitive ¾Firing rate of ADH-releasing neurons depends on central and peripheral inputs (baroreceptors!) as well as their intrinsic osmosensitivity Diabetes insipidus Summary of neuroendocrine hypothalamus • Arcuate – Central DI • failure to secrete ADH, resulting in excess urine output and dehydration • following pituitary stalk damage (accident) • Brattleboro rat produces no ADH GnRH (FSH, LH); GHRH (GH); DA (prolactin) ¾ Reproduction; growth • PVN CRH (ACTH); TRH (TSH); ADH; oxytocin ¾ Steroid hormone production, energy metabolism, water retention; social behaviours, reproduction • Ant. HT Sost (GH) ¾ Growth • SON ADH; oxytocin ¾ water retention; social behaviours, reproduction Summary of neuroendocrine hypothalamus • Arcuate Non-endocrine control via the hypothalamus GnRH (FSH, LH); GHRH (GH); DA (prolactin) ¾ reproduction; energy metabolism • PVN CRH (ACTH); TRH (TSH); ADH; oxytocin ¾ behaviours, energy metabolism, water retention (blood flow), reproduction • Ant. HT Sost (GH) ¾ energy metabolism • SON ADH; oxytocin ¾ water retention (blood flow), behaviours, reproduction 8 Non-endocrine control via the hypothalamus Non-endocrine control via the hypothalamus • Food and drink intake • Food and drink intake • Thermoregulation • Thermoregulation • Circadian rhythms • Circadian rhythms ¾Parasympathetic and sympathetic control ¾Parasympathetic and sympathetic control Food intake Non-endocrine control via the hypothalamus • Food and drink intake • Thermoregulation • Circadian rhythms ¾ANS control Food intake • Lateral hypothalamic area – “Feeding centre” (bilateral lesions: aphagia) – Receives olfactory input via medial forebrain bundle • Ventromedial nucleus – “Satiety centre” (bilateral lesions: hyperphagia) – Receptors for glucose and free fatty acids • Arcuate nucleus Food intake • Lateral hypothalamic area – “Feeding centre” (bilateral lesions: aphagia) – Receives olfactory input via medial forebrain bundle • Ventromedial nucleus – “Satiety centre” (bilateral lesions: hyperphagia) – Receptors for glucose and free fatty acids • Arcuate nucleus – Receptors for leptin (adipose tissue) and insulin – Receptors for leptin (adipose tissue) and insulin ¾Separate lecture on food intake ¾Separate lecture on food intake 9 Food intake • Lateral hypothalamic area – “Feeding centre” (bilateral lesions: aphagia) – Receives olfactory input via medial forebrain bundle • Ventromedial nucleus – “Satiety centre” (bilateral lesions: hyperphagia) – Receptors for glucose and free fatty acids • Arcuate nucleus Food intake • Lateral hypothalamic area – “Feeding centre” (bilateral lesions: aphagia) – Receives olfactory input via medial forebrain bundle • Ventromedial nucleus – “Satiety centre” (bilateral lesions: hyperphagia) – Receptors for glucose and free fatty acids • Arcuate nucleus – Receptors for leptin (adipose tissue) and insulin – Receptors for leptin (adipose tissue) and insulin ¾Separate lecture on food intake ¾Separate lecture on food intake Drink intake/Thirst Drink intake/Thirst • Subfornical organ – contains osmosensitive neurons – projects to PVN, SON and POA – stimulation of drinking behaviour (thirst) other cirumventricular organs also contribute ¾More in separate lecture Thermoregulation Thermoregulation • Alert consciousness and normal patterned motor activities only when CNS temperature ~ 36 - 39°C • Hypothalamus can stimulate thermogenesis – shivering, piloerection, skin vasoconstriction – behaviours that increase body temperature (or minimise heat loss) • Hypothalamus can stimulate heat loss – sweating, skin vasodilation – behaviours that promote body temperature cooling • Controlled elevation of body temperature (fever) reduces pathogen viability and boosts immune system function 10 Thermoregulation • Anterior hypothalamus (POA) – Lesions cause hyperthermia – Endogenous temperature sensors (warm-sensitive neurons) • T set-point can be changed by pyrogens, causing elevated core temperature (PGE2 acting on EP3 receptors) • Posterior hypothalamic area – Lesions cause hypothermia – Involved in sympathetic activation • Dilation or contraction of cutaneous circulation and control of sweat glands Thermoregulation • Anterior hypothalamus (POA) – Lesions cause hyperthermia – Endogenous temperature sensors (warm-sensitive neurons) • T set-point can be changed by pyrogens, causing elevated core temperature (PGE2 acting on EP3 receptors) • Posterior hypothalamic area – Lesions cause hypothermia – Involved in sympathetic activation • Dilation or contraction of cutaneous circulation and control of sweat glands receive peripheral temperature information also receive peripheral temperature information (TRPM8, TRPV3, TRPV4) (TRPM8, TRPV3, TRPV4) Thermoregulation • What is the central temperature sensor? Thermoregulation • What is the central temperature sensor? 2 current models: 2 current models: 1. Heat directly opens ion channel that then depolarises neuron – AP firing 1. Heat directly opens ion channel that then depolarises neuron – AP firing 2. Heat indirectly promotes depolarisation of neuron – AP firing 2. Heat indirectly promotes depolarisation of neuron – AP firing Thermoregulation TRPV1 Thermoregulation TRPV1 – peripheral or central administration of TRPV1 agonist capsaicin induces hypothermia – peripheral or central administration of TRPV1 agonist capsaicin induces hypothermia – administration of TRPV1 antagonists induces hyperthermia (increased metabolism and reduced heat loss from body surface) – administration of TRPV1 antagonists induces hyperthermia (increased metabolism and reduced heat loss from body surface) – TRPV1 is expressed in anterior hypothalamus – TRPV1 is expressed in anterior hypothalamus ◄However: TRPV1 knock-out mice have no obvious deficit in body temperature control and TRPV1 channels in anterior hypothalamus are not activated by normal body temperature: TRPV1 is unlikely to be the hypothalamic thermosensor! ◄However: TRPV1 knock-out mice have no obvious deficit in body temperature control and TRPV1 channels in anterior hypothalamus are not activated by normal body temperature: TRPV1 is unlikely to be the hypothalamic thermosensor! ¾ thought to be mediated by visceral TRPV1 channels that are tonically active ¾ thought to be mediated by visceral TRPV1 channels that are tonically active 11 Thermoregulation Thermoregulation TRPV1 – peripheral or central administration of TRPV1 agonist capsaicin induces hypothermia – administration of TRPV1 antagonists induces hyperthermia (increased metabolism and reduced heat loss from body surface) • What is the central temperature sensor? still unclear – TRPV1 is expressed in anterior hypothalamus ◄However: TRPV1 knock-out mice no obvious deficit in body temperature control; TRPV1 channels in anterior hypothalamus not activated by normal body temperature: TRPV1 unlikely to be hypothalamic thermosensor! ¾ thought to be mediated by visceral TRPV1 channels that are tonically active Thermoregulation • Anterior hypothalamus (POA) – Lesions cause hyperthermia – Endogenous temperature sensors (warm-sensitive neurons) • T set-point can be changed by pyrogens, causing elevated core temperature (PGE2 acting on EP3 receptors) • Posterior hypothalamic area – Lesions cause hypothermia – Involved in sympathetic activation • Dilation or contraction of cutaneous circulation and control of sweat glands also receive peripheral temperature information (TRPM8, TRPV3, TRPV4) Thermoregulation • Anterior hypothalamus (POA) – Lesions cause hyperthermia – Endogenous temperature sensors (warm-sensitive neurons) • T set-point can be changed by pyrogens, causing elevated core temperature (PGE2 acting on EP3 receptors) Thermoregulation • Anterior hypothalamus (POA) – Lesions cause hyperthermia – Endogenous temperature sensors (warm-sensitive neurons) • T set-point can be changed by pyrogens, causing elevated core temperature (PGE2 acting on EP3 receptors) • Posterior hypothalamic area – Lesions cause hypothermia • Dilation or contraction of cutaneous circulation and control of sweat glands also receive peripheral temperature information (TRPM8, TRPV3, TRPV4) Thermoregulation • Anterior hypothalamus (POA) – Lesions cause hyperthermia – Endogenous temperature sensors (warm-sensitive neurons) • T set-point can be changed by pyrogens, causing elevated core temperature (PGE2 acting on EP3 receptors) • Posterior hypothalamic area • Posterior hypothalamic area – Lesions cause hypothermia – Lesions cause hypothermia • Dilation or contraction of cutaneous circulation and control of sweat glands also receive peripheral temperature information (TRPM8, TRPV3, TRPV4) • Dilation or contraction of cutaneous circulation and control of sweat glands also receive cutaneous temperature information (TRPA1?, TRPM8, TRPV3, TRPV4) 12 Circadian rhythms Circadian rhythms • Suprachiasmatic nucleus – Sleep-wake cycle, feeding, temperature control, hormone release….. – direct input from lightsensitive ganglion cells in retina (melanopsin) (retinohypothalamic tract) – phototransduction cascade similar to invertebrate one • TRPC channels (originally cloned from drosophila photoreceptors) • Separate lecture Mammillary bodies Mammillary bodies • Role in memory: Korsakoff’s syndrome » alcohol-induced Vitamine B1 deficiency: damage to mammillary bodies (but also thalamus) » Symptoms: anterograde and retrograde amnesia, confabulation • Contain several nuclei with distinct connections • Head direction cells (lateral nuclei) » fire selectively when animal faces specific direction in horizontal plane; navigation • Memory formation (medial nuclei) » connected with hippocampus via fornix and fire at theta frequency (4-8Hz), which elicits long term potentiation in hippocampus 13 Summary non-endocrine hypothalamus Summary non-endocrine hypothalamus • Anterior HT ANS regulation; endogenous T sensor; osmoregulation (energy metabolism, blood flow) • Anterior HT ANS regulation; endogenous T sensor; osmoregulation (energy metabolism, blood flow) • SCN circadian rhythms • SCN biological clock • Arcuate nucleus food intake • Arcuate nucleus food intake • Ventromedial nucleus “satiety” centre • Ventromedial nucleus “satiety” centre • Lateral hypothalamus “hunger” centre • Lateral hypothalamus “hunger” centre • Posterior HT ANS regulation (T sensor) • Posterior HT ANS regulation (T sensor) • Mammillary bodies memory (behaviours?) • Mammillary bodies memory (behaviours?) Aggression and the hypothalamus • Neuronal subpopulations of ventromedial hypothalamus cause aggressive behaviour (Lin et al. (2011) Nature 470:221-226) energy metabolism “sexually experienced” male C57BL/6N mouse under investigation Some cells are active during number of different behaviours whereas others (cell 4) are only active during one particular behaviour “sexually experienced” male C57BL/6N mouse under investigation Sex and the hypothalamus Some cells are active during number of different behaviours whereas others (cell 4) are only active during one particular behaviour Went on to selectively initiate behaviours by activating certain neurons and showed that neurons activated during an attack are inhibited during mating 14 Sexual dimorphism of hypothalamus Sexual dimorphism of hypothalamus Sexually dimorphic nucleus of preoptic area: Interstitial nucleus III Sexually dimorphic nucleus of preoptic area: Interstitial nucleus III and also other hypothalamic regions and also other hypothalamic regions VERY controversial data VERY controversial data / woman / woman 15