* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Functional groups - Montgomery County Schools
Survey
Document related concepts
Vectors in gene therapy wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Catalytic triad wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Genetic code wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Protein structure prediction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Transcript
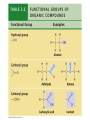
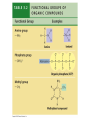
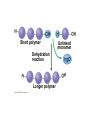
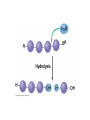
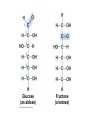
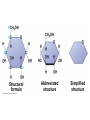
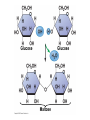
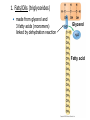
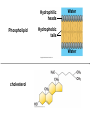
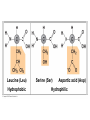
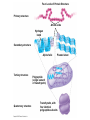
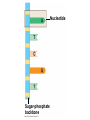
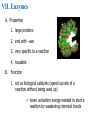
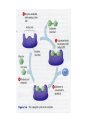
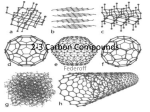
V. Organic Compounds Introduction: Organic compounds = carbon organisms=organic Why carbon? 1. 4 valence e- = 4 covalent bonds 2. can form single, double or triple bonds 3. can bond to other carbon atoms, making long chains, branched molecules or rings. Structural formula Ball-and-stick model Space-filling model Methane The four single bonds of carbon point to the corners of a tetrahedron. Ethane Length. Propane Carbon skeletons vary in length. Butane Isobutane Branching. Skeletons may be unbranched or branched. 1-Butene Double bonds. 2-Butene Skeletons may have double bonds, which can vary in location. Cyclohexane Rings. Benzene Skeletons may be arranged in rings. organic compounds unique properties depend on size & shape of the molecule groups of atoms (functional groups) attached to it compounds containing functional groups are hydrophilic (water-loving) makes them soluble in water Functional groups (pg. 35 formulas) 1) Hydroxyl group 2) Carbonyl group 3) Carboxyl group 4) Amino group 5) Phosphate group * Methyl group (non-polar)*affects shape & function Estradiol Female lion Testosterone Male lion VI. Macromolecules large organic molecules also called polymers made of smaller “building blocks” called monomers Monomers link together to form polymers through dehydration reactions, which remove water Polymers are broken apart by hydrolysis, the addition of water Short polymer Unlinked monomer Short polymer Dehydration reaction Longer polymer Unlinked monomer Hydrolysis A. Carbohydrates – (polysaccharides) 1. monosaccharide (simple sugar) = monomer • glucose, fructose (fruit), galactose (milk) • made of C, H, O (1:2:1) • functional group(s): hydroxyl, carbonyl • used for energy in cells & as raw materials to manufacture other organic molecules Glucose (an aldose) Fructose (a ketose) Structural formula Abbreviated structure Simplified structure 2. disaccharide -two monomers (sugars) joined by dehydration reaction • sucrose (glucose + fructose) = table sugar • maltose (2 glucose) = grain sugar • lactose (glucose + galactose) = milk sugar Glucose Glucose Maltose 3. Polysaccharides – many monomers (sugars) • function in cells as a storage molecule or a structural compound a) Starch- storage molecule in plants made of glucose b) Glycogen- storage molecule in animals made of glucose c) Cellulose- polymer of glucose that forms plant cell walls d) Chitin- used by insects & crustaceans to build an exoskeleton Starch granules in potato tuber cells Glycogen granules in muscle tissue STARCH Glucose monomer GLYCOGEN CELLULOSE Cellulose fibrils in a plant cell wall Hydrogen bonds Cellulose molecules B. Lipids • not true polymers • non-polar, water insoluble (hydrophobic) • made of C, H, few O • hydroxyl & methyl groups • long term energy storage, insulation, cushion/protect organs, prevent water loss, chemical messengers, cell membranes • contain twice as much energy as a polysaccharide 1. Fats/Oils (triglycerides) • made from glycerol and 3 fatty acids (monomers) linked by dehydration reaction Glycerol Fatty acid a. unsaturated fats - have fewer than the maximum number of hydrogen atoms (good) • made of fatty acids that contain double bonds, causing kinks or bends in the carbon chain • usually liquid at room temp • plants, fish Copyright © 2009 Pearson Education, Inc. b. saturated fats – have maximum number of hydrogens, no double bonds (bad) • solid at room temp • animal fat (lard), butter 2. Phospholipids -important part of cell membrane 3. Steroids -lipids made of fused ring structures • cholesterol a steroid that plays a significant role in the structure of the cell membrane & sex hormones 4. Waxes – cuticle coating on plants Hydrophilic heads Phospholipid Water Hydrophobic tails Water cholesterol Carboxyl group Amino acid Amino group Amino acid Peptide bond Dehydration reaction Dipeptide Leucine (Leu) Hydrophobic Serine (Ser) Aspartic acid (Asp) Hydrophilic C. Protein (polypeptide) • amino acids (20) = monomers central C atom & 4 other things: 1) amino group 2) carboxyl group 3) another chemical group represented as “R” 4) H peptide bonds holds amino acids together • made of C, H, O, N Amino group Carboxyl group 1. Functions of Proteins (8) – determined by shape a. Structural – hair, nails, fibers in tendons b. Contractile (movement) - found in muscles c. Signal (regulation) – hormones d. Storage – egg white (albumin) e. Transport – hemoglobin carries O2 in blood f. Defense - antibodies of the immune system g. Receptors - built into cell membranes h. Enzymes – control chemical reactions Four Levels of Protein Structure Primary structure Amino acids Hydrogen bond Secondary structure Alpha helix Tertiary structure Quaternary structure Pleated sheet Polypeptide (single subunit of transthyretin) Transthyretin, with four identical polypeptide subunits D. Nucleic Acids (DNA & RNA) • Nucleic acid = monomer • Made of C, H, O, N, P 3 parts 1) five-carbon sugar (pentose) Nitrogenous base (adenine) ribose in RNA deoxyribose in DNA Phosphate group 2) phosphate group 3) nitrogenous base (1 of 4) Sugar • 4 nitrogenous bases adenine (A) thymine (T) (DNA only) cytosine (C) guanine (G) uracil (U) (RNA only) Nucleotide Sugar-phosphate backbone 1. DNA = 2 strands wrapped around each other forming a double helix • A pairs with T • C pairs with G Functions: compose genes, determine the structure of proteins 2. RNA = single strand Functions: copy & transfer DNA so proteins can be made Copyright © 2009 Pearson Education, Inc. Base pair VII. Enzymes A. Properties 1. large proteins 2. end with –ase 3. very specific to a reaction 4. reusable B. Function 1. act as biological catalysts (speed up rate of a reaction without being used up) • lower activation energy needed to start a reaction by weakening chemical bonds C. Enzyme-Substrate Complex 1. Active site- specific shape on enzyme 2. Substrate- reactant(s) that can attach to active site to react • lock & key design - shape of active site is so precise that only the intended substrate(s) can attach. Also called induced fit. D. Factors Affecting Enzyme Activity (Rate) 1. temperature – too high will denature (unfold) enzyme, too low will slow down rate 2. pH – needs to be around 6-8; other levels can denature enzyme 3. concentration of substrate – if more substrate than enzymes, rate slows down 4. Inhibitors – can stop/slow rate a. competitive – resemble substrate & compete for active site b. non-competitive – attach to enzyme some place other than active site, altering shape of active site; substrate cannot fit