* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download THE CHEMICAL BASICS OF LIFE
Survey
Document related concepts
Two-hybrid screening wikipedia , lookup
Western blot wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Drug discovery wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Genetic code wikipedia , lookup
Peptide synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Phosphorylation wikipedia , lookup
Blood sugar level wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Transcript
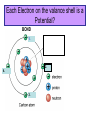
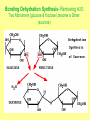
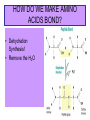
1. ORGANIC MOLECULES COMPOUNDS THAT CONTAIN CARBON CO2 IS THE EXCEPTION • THERE ARE MILLIONS OF ORGANIC COMPOUND COMBINATION • WHY? (HINT: IMAGINE A CARBON ATOM AND THINK OF BONDING) http://www.npr.org/templates/story/story.php?storyId=9943298 Each Electron on the valance shell is a Potential? BOND 1. 2. 4. 3. IV. COMPOUNDS OF LIFE http://www.pbs.org/wgbh/nova/education/body/bodyneeds.html?elq=6b604da68d704d3a9445086e87b767b9&elqCampaignId=431 • THERE ARE FOUR GROUPS OF ORGANIC COMPOUNDS FOUND IN LIVING THINGS: – CARBOHYDRATES – LIPIDS – PROTEINS – NUCLEIC ACID – Serving Sizes WHY EAT CARBS? OUR BODY REQUIRES IT FOR ENERGY 1. CARBOHYDRATES • COMPOSED OF CARBON, HYDROGEN, AND OXYGEN (1:2:1 RATIO) • C6H12O6 –GLUCOSE MONOSACCHARIDE • 3 TYPES -SUGAR, STARCH, AND FIBER • SIMPLE FORM KNOWN AS MONOSACCHARIDE • COMPLEX FORM KNOWN AS POLYSACCHARIDE MONOSACCHARIDES GLUCOSE FRUCTOSE GALACTOSE B. MAKING LARGER SUGARS BY POLYMERIZATION Link for dehydration synthesis & hydrolysis DEHYDRATION SYNTHESIS (REMOVE H2O) CREATES Disaccharides Monomers Dimers Glucose + fructose = sucrose + water Glucose + galactose = lactose + water Glucose + glucose = maltose + water 2 MONOSACCHARIDES = 1 DISACCHARIDE Bonding Dehydration Synthesis- Removing H2O Two Monomers (glucose & fructose) become a Dimer (sucrose) Breaking down molecules bonds HYDROLYSIS add H2O example a dimer(sucrose) becomes 2 monomers glucose & fructose • ADDING WATER BREAKS UP POLYMERS C. POLYSACCHARIDES ARE BIG CARBS. • GIANT POLYMERS OF THOUSANDS OF LINKED MONOSACCHARIDES -glucose • A STORAGE PRODUCT WHICH CAN BE BROKEN DOWN INTO MONOSACCHARIDES. TYPES OF PLANT POLYSACCHARIDES • 1. STARCH: A Polysaccharide (big sugar) STORED IN PLANTS FOR ENERGY USE PLANT POLYSACCHARIDE • 2. CELLULOSE: GIVE PLANTS STRENGTH & STRUCTURE • This is also known as Fiber! • Plant cell walls structure of veggies Tree Bark ANIMAL POLYSACCHARIDE • GLYCOGEN: STORED IN MUSCLES AND LIVER OF ANIMALS • Too much can build up to become FAT or can be BROKEN DOWN into GLUCOSE for energy GLYCOGEN IS STORED IN THE LIVER & MUSCLES 3. POLYMERIZATION- A Process of building molecules • BUILDING LARGE COMPOUNDS USING SMALLER COMPOUNDS • MER-MEANS PART • A SINGLE COMPOUND IS A MONOMER • Dimer two monomers • THREE OR MORE COMPOUNDS TOGETHER ARE POLYMERS • MANY POLYMERS ARE KNOWN AS MACROMOLECULES PROTEINS • POLYMERS OF AMINO ACIDS LINKED BY PEPTIDE BONDS • Contain -3 parts – 1. AN R GROUP (any compound or Element) – 2. An Amino (contains Nitrogen) – 3. A Carboxyl (COOH/COO) EXAMPLES OF AMINO ACIDS There are just 20 types Pink Boxes show the R groups PROTEINS HAVE PEPTIDE BONDS Animation of Peptide Bond Formation • TWO bonded AMINO ACIDS is a DIPEPTIDE • MANY bonded AMINO ACIDS is a POLYPEPTIDE • A Polypeptide is a PROTEIN HOW DO WE MAKE AMINO ACIDS BOND? • Dehydration Synthesis! • Remove the H2O FUNCTIONS OF PROTEINS Let’s watch the video • ENZYMES (speed up) CHEMICAL REACTIONS – Reduces the energy needed for the reaction • STRUCTURE-MUSCLES/TISSUES IN BODY • CELL SIGNALING –Tells HORMONES to go from the glands to target organs • TRANSPORT Channels to move items into/out of Cell Membranes • DEFENSE –Immune system, White blood cells in the blood How Enzymes fit to only its specific Substrate http://bcs.whfreeman.com/thelifewire/content/chp06/0602001.html Active Site SPECIAL TYPES OF PROTEINS ENZYMES • A PROTEIN THAT INCREASES THE RATE OF CHEMICAL REACTIONS • Each ENZYME has its own SUBSTRATE (molecule) that it fits to • They fit like a LOCK AND KEY…OR PUZZLE PIECES • Where they meet is called THE ACTIVE SITE What can prevent an Enzyme from binding to its substrate? • Heat • pH • Changing the active site so binding substrate does not happen Enzymes lower the amount of energy needed in a reaction. http://bcs.whfreeman.com/thelifewire/content/chp06/0602001.html Active sites