* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
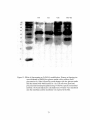
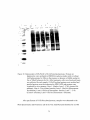
Download Glucosamine-Induced Insulin Resistance in Primary Rat
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biochemistry wikipedia , lookup
Lipid signaling wikipedia , lookup
Gene expression wikipedia , lookup
Signal transduction wikipedia , lookup
Gene regulatory network wikipedia , lookup
Biochemical cascade wikipedia , lookup
Proteolysis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Expression vector wikipedia , lookup
Western Michigan University ScholarWorks at WMU Dissertations Graduate College 1-2011 Glucosamine-Induced Insulin Resistance in Primary Rat Hepatocytes and the Role of Selenium as an Insulin Mimetic Sandhya N. Adiyodi Veetil Western Michigan University Follow this and additional works at: http://scholarworks.wmich.edu/dissertations Part of the Biochemistry, Biophysics, and Structural Biology Commons, and the Chemistry Commons Recommended Citation Adiyodi Veetil, Sandhya N., "Glucosamine-Induced Insulin Resistance in Primary Rat Hepatocytes and the Role of Selenium as an Insulin Mimetic" (2011). Dissertations. Paper 475. This Dissertation-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Dissertations by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. GLUCOSAMINE INDUCED INSULIN RESISTANCE IN PRIMARY RAT HEPATOCYTES AND THE ROLE OF SELENIUM AS AN INSULIN MIMETIC by Sandhya N Adiyodi Veetil A Dissertation Submitted to the Faculty of The Graduate College in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Department of Chemistry Advisor: Susan R. Stapleton, Ph.D. Western Michigan University Kalamazoo, Michigan June 2011 GLUCOSAMINE INDUCED INSULIN RESISTANCE IN PRIMARY RAT HEPATOCYTES AND THE ROLE OF SELENIUM AS AN INSULIN MIMETIC Sandhya N Adiyodi Veetil, Ph.D. Western Michigan University, 2011 Type 2 diabetes is mediated by insulin resistance, the inability of insulin to elicit a normal biological response in insulin responsive tissues. Several cellular models have been utilized to determine the mechanism of induction of insulin resistance but questions remain unanswered. One model, implicates the products of the Hexosamine Biosynthetic Pathway (HBP) in the induction of insulin resistance under hyperglycemia. The major end product of HBP, UDP-GlcNAc, is the substrate for O-GlcNAc transferase, an enzyme that catalyzes the O-GlcNAcylation of numerous proteins. This modification may play a role in induction of insulin resistance and thus needs to be evaluated in different cell types. Therefore, we set out to test whether or not insulin resistance could be established in primary rat hepatocytes. To accomplish this we used a HBP precursor, glucosamine and first assessed whether or not insulin resistance was established by evaluating insulin's effect on a key signaling protein, Akt. Results indicate that the insulin induced phosphorylation of Akt was decreased in the presence of glucosamine when compared to the control, suggesting an insulin resistant state. Signal protein activation is very important for the insulin regulation of gene expression. If the activation of signal proteins is altered, then the effect on gene expression should also be altered. Results show that under glucosamine treatment, insulin was no longer able to control the gene expression of a number of key enzymes in major metabolic pathways. Additionally an increase in O-GlcNAc modified proteins under glucosamine compared to the control was observed and a number of these proteins were identified through LC-MS. Lastly, the effect of selenium, an insulin mimetic agent, was examined in this model system. The effects of selenium on the phosphorylation of the insulin signaling protein, Akt and on the expression of the key enzymes of the major metabolic pathways both under normal and insulin resistant conditions were examined. Results show that selenium is a potent insulin mimetic not only under the normal/control condition, but also under the insulin resistant condition as well. UMI Number: 3467829 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMT Dissertation Publishing UMI 3467829 Copyright 2011 by ProQuest LLC. All rights reserved. This edition of the work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Copyright by Sandhya N Adiyodi Veetil 2011 ACKNOWLEDGEMENTS I would like to express my sincere gratitude to my advisor, Dr. Susan R. Stapleton. I am grateful for her guidance, understanding, patience and continuous support throughout my graduate training. I would also like to thank my committee members Drs Michael Barcelona, David Reinhold and Pamela Hoppe for their patience, encouragement and guidance. I would also like to thank present and previous members of the Stapleton's lab. You are all such amazing people and I have been fortunate to have the privilege to work with you. Finally, and most importantly I would like to give my deepest thanks to my husband Raghu for his support, patience and understanding. I could not have achieved my degree without you. Finally, I would like to thank my sister and my father for their support and encouragement to whom I dedicate this thesis. Sandhya N Adiyodi Veetil 11 TABLE OF CONTENTS ACKNOWLEDGMENTS ii LIST OF TABLES vii LIST OF FIGURES viii CHAPTER 1. INTRODUCTION 1 Glucose homeostasis 1 Glucose metabolic pathways of the liver 5 Insulin signaling pathway 10 PI 3-kinase -Akt pathway 11 Insulin resistance and type 2 diabetes 14 Prevalence of diabetes 15 Molecular mechanism of insulin resistance 18 Decreased expression of insulin signaling components 18 Increase in the ratio of regulatory subunit/catalytic subunit ofPB-kinase 19 Serine phosphorylation of IRS 20 Suppresors of cytokine signaling (SOCS) 21 Phosphatase 22 O-GlcNAcylation of serine/threonine residues 23 Hexosamine biosynthetic pathway (HBP) 26 Role of hexosamine biosynthetic pathway in insulin resistance 27 iii Table of Contents- Continued CHAPTER Selenium-insulin mimetic agent 31 Significance of study 34 Objectives of study 35 2. MATERIALS AND METHODS 37 Primary rat hepatocytes isolation and maintenance 37 Cell treatment and processing 38 Protein isolation and western blot analysis 41 Protein isolation 41 Determination of protein content 42 Immunoprecipitation 42 Western blot analysis 43 LDH Assay 44 RNA Isolation 44 Determination of RNA content and quality assessment 45 Reverse transcriptase polymerase chain reaction (RT PCR) 46 Isolation and purification of O-GlcNAcylated proteins 47 Wheat germ agglutinin lectin affinity chromatography 47 SDS-PAGE (sodium dodecyl polyacrylamide gel electrophoresis) 48 Identification of O-GlcNAc proteins by LC-MS analysis 48 iv Table of Contents- Continued CHAPTER Statistical Analysis 48 3. RESULTS 49 Effect of glucosamine on insulin induced Akt phosphorylation 50 Effect of glucosamine on insulin induced G6PDH gene expression 51 Effect of glucosamine on insulin induced fatty acid synthase (FAS) 55 Effect of glucosamine on insulin inhibition of PEPCK gene expression 56 Effect of glucosamine on insulin regulation of Foxa2 59 Effect of selenium on Akt phosphorylation 61 Effect of selenium on G6PDH gene expression 63 Effect of selenium on FAS gene expression 64 Effect of selenium on PEPCK gene expression 65 Effect of selenium on Foxa2 gene expression 68 Lactate dehydrogenase assay 70 Effect of glucosamine on O-GlcNAc modification 71 Identification of O-GlcNAc modified proteins by LC-MS 74 Functions of the identified O-GlcNAc modified proteins 86 v Table of Contents- Continued CHAPTER 4. DISCUSSION 89 APPENDIX 112 IACUC approval form for animal studies BIBLIOGRAPHY 112 113 VI LIST OF TABLES 1. Treatment conditions to establish an insulin resistance model in primary rat hepatocytes 40 2. Treatment conditions to study the effect of selenium, an insulin mimetic agent 41 3. Primers for RT-PCR 47 4. O-GlcNAc modified proteins found uniquely in the glucosamine treatment as compared to control (no addition) 78 5. O-GlcNAc modified proteins found uniquely in glucosamine plus insulin treatment compared to insulin 82 6. O-GlcNAc modified proteins found in the glucosamine plus selenium treatment compared to selenium 83 7. O-GlcNAc modified proteins found in both glucosamine and glucosamine plus insulin treatment 85 8. O-GlcNAc modified proteins found uniquely in glucosamine plus insulin treatment but not present in glucosamine plus selenium treatment 85 vn LIST OF FIGURES 1. Summary of insulin actions in different tissues 4 2. Estimates of the global prevalence of diabetes in the year 2000 and projections for 2030 17 3. Hexosamine Biosynthetic Pathway 27 4. Effect of glucosamine on insulin induced Akt phosphorylation 53 5. Effect of glucosamine on G6PDH gene expression 54 6. Effect of glucosamine on FAS gene expression 56 7. Effect of glucosamine on PEPCK gene expression 58 8. Effect of glucosamine on Foxa2 gene expression 60 9. Effect of glucosamine on selenium induced Akt Phosphorylation 62 10. Effect of selenium on G6PDH gene expression 64 11. Effect of selenium on FAS gene expression 65 12. Effect of selenium on PEPCK gene transcription 67 13. Effect of selenium on Foxa2 gene transcription 69 14. Effect of glucosamine on lactate dehydrogenase release into the media 71 15. Effect of glucosamine on O-GlcNAc modification 73 16. SDS-PAGE of O-GlcNAcylated proteins 75 viii CHAPTER 1 INTRODUCTION Glucose homeostasis Glucose is an essential fuel for the body and is a regulated metabolite. The concentration of glucose in the peripheral circulation is highly regulated such that over a 24-h period, the concentration is 5.5-6 mmol/L with a maximum of approximate 9 mmol/L postprandially (after meal) and a minimum of approximate 3 mmol/L in a state of fast (Gerich 1993). In the fasting state, circulating glucose is derived from two processes: 1) glycogenolysis which is the release of glucose from stored glycogen, and 2) gluconeogenesis which is the formation of glucose from metabolites in the body such as amino acids, glycerol, lactate, pyruvate and intermediate metabolites of the tricarboxylic acid (TCA) cycle. In the fed state, circulating glucose is derived from dietary glucose and from absorbed gluconeogenic substrates, i.e. amino acids, fructose and galactose, as well as from endogenous gluconeogenic substrates. Blood glucose level is controlled by a system, which involves several body organs and tissues. The maintenance of glucose in a low range is important for two major reasons. The first is the requirement of the brain for glucose as a fuel. In a fed state, the brain uses glucose almost exclusively, and the rate of utilization is estimated to be ~ 117- 1 142g/day (Felig, Marliss et al. 1969; Gottstein 1979). The second reason for regulating glucose concentration is that glucose is toxic. The amount of glucose in the bloodstream is tightly regulated by actions of insulin and glucagon during fed and fasting states (Flakoll PJ 2000). Insulin, a hormone secreted by the P- cells of the islets of Langerhans in the pancreas, plays an important role in maintaining glucose homeostasis and is secreted in response to elevated blood glucose. Glucagon, a hormone secreted by the a- cells of the pancreas is another major player in glucose homeostasis and is secreted in response to a fall in blood glucose. During the fed state the increase in circulating insulin level promotes glucose disposal mainly by skeletal muscle and adipose tissues, while inhibiting glycogenolysis and gluconeogenesis in the liver (Flakoll PJ 2000). However, a drop in insulin levels during the fasting state results in increased gluconeogenesis, glycogenolysis, and lipolysis in order to maintain the plasma glucose concentration. Besides glucose homeostasis, insulin also is involved in the anabolism of protein through a number of signaling cascades that promote ion and amino acid transport and protein synthesis in skeletal muscle, liver, and adipose tissue (Flakoll PJ 2000). In addition, insulin promotes de novo synthesis of fatty acids in liver and adipose tissues (Flakoll PJ 2000). A summary of the variety of insulin actions in different tissues is presented in Figure 1. Hepatic glucose production is one of the factors responsible for glucose homeostasis. It is a major determinant of fasting glucose level (Weyer, Bogardus et al. 1999). Insulin inhibits glucose production by both direct (activation of insulin signaling in hepatocytes) and indirect actions (activation of insulin receptors in extra hepatic sites). 2 The direct action takes place in the liver itself, by responding to sinusoidal insulin levels. In contrast, the indirect insulin action occurs in peripheral tissues such as the pancreas, skeletal muscle, adipose tissue, and brain, which accordingly influence the direct actions of insulin in the liver. Examples of indirect insulin action include the inhibition of glucagon secretion from the pancreas that consequently decreases hepatic glucose production (Ishihara, Maechler et al. 2003) caused by a decrease in the flow of free fatty acids from adipose tissue (Sindelar, Chu et al. 1997) and gluconeogenesis precursors from skeletal muscles (Sindelar, Balcom et al. 1996) in response to insulin induced inhibition of lipolysis and proteolysis as well as a decrease in hepatic gluconeogenesis. Furthermore, it has been shown that insulin signaling in a discreet area of the hypothalamus plays a role in regulation of insulin action in liver (Obici, Feng et al. 2002), showing the importance of the brain in regulating hepatic glucose output. In this study, a transient defect in hypothalamic insulin signaling was generated by infusing an antisense oligodeoxynucleotide designed to blunt the expression of the insulin receptor (IR) protein in rat hypothalmic area. This selective decrease in hypothalamic insulin receptors resulted in increased hepatic glucose production even in the presence of equal plasma insulin concentrations. In skeletal muscle, insulin increases glucose utilization and enhances the perfusion of muscle by increasing vasodilation in skeletal muscle under euglycemia (Baron 1994). After glucose is taken up into skeletal muscle, it is largely oxidized and the rest is stored in the form of glycogen. In addition, the utilization rate of glucose in skeletal muscle can be extremely high during hyperglycemia (Mandarino, Consoli et al. 3 1993). Glucose uptake in skeletal muscle determines the postprandial glucose level (DeFronzo, Jacot et al. 1981). If the actions of insulin are impaired, the earliest manifestation of this will be a reduction of glucose uptake in skeletal muscle (Zierath and Wallberg-Henriksson 2002). tijfc,* Muscle Suppression of gluconeogenesis -Elevation of glycogen and fat synthesis -Elevation of glucose uptake -Elevation of glycogen and protein synthesis -Inhibition of lipolysis -Elevation of glucose uptake -Elevation of fat synthesis Glucose homeostasis Figure 1: Summary of insulin actions in different tissues. In adipose tissue, the actions of insulin are responsible for promotion of glucose uptake and suppression of lipolysis. In fact, adipose tissue serves as a depository of an alternative source of energy during glucose deprivation. When glucose availability is limited and the insulin level is low (Avruch (2001), breakdown of fat in the form of triglyceride will result and serve as an alternative source of energy. In obesity, suppression of lipolysis by insulin becomes less sensitive compared to normal individuals. Oversupply of free fatty acids to the liver eventually will cause hepatic insulin resistance, elevation of glucose production and hypertriglyceridemia (Dimitriadis GD 2000). In summary, insulin regulates glucose homeostasis by integration of its function in liver, skeletal muscle, and adipose tissues. Insulin controls hepatic glucose production by direct effects of the hormone. Insulin is also responsible for the glucose uptake in skeletal muscle as well as adipose tissue. In addition, insulin exerts an indirect effect by inhibiting lipolysis in adipose tissues to further control glucose metabolism. Glucose metabolic pathways of the liver Glucose first enters the liver via the glucose transporter, GLUT2, which allows for passive movement of glucose across the cell membrane. GLUT2 transporters are facilitative glucose transporters with a high km for glucose (17-20mM) (Gould and Holman 1993). They have high transport capacity which increases as a direct function of extracellular glucose concentration and are not saturated under most physiological conditions. Thus, glucose transport is never rate limiting for the entry of glucose into the liver (Rencurel and Girard 1998). GLUT2 transporters are constitutively present on the cell membrane and are not regulated by insulin (Gould and Holmanl993). Once glucose has entered the cell, it is phosphorylated by glucokinase (GK) to glucose-6- phosphate (G6P). The expression level of glucokinase is regulated by insulin (Weinhouse 1976; 5 Iynedjian, Gjinovci et al. 1988).The resulting G6P can enter glycolysis or the pentose phosphate pathway, resulting in various metabolic intermediates, reducing equivalents. Glycogen can be synthesized via the glycogenesis pathway or oxidized to provide energy or substrate for macromolecule synthesis via the glycolysis pathway. Glycogen synthesis predominates in the fed state. The formation of glycogen in the liver can occur via a direct pathway, in which G6P is produced through the phosphorylation of glucose obtained from the circulation, or an indirect pathway, in which G6P is formed from gluconeogenic precursors (Kuwajima, Golden et al. 1986). Once G6P is formed through either pathway, it is converted to glucose 1-phosphate by phosphoglucomutase and to UDP-glucose by UDP-glucose phosphorylase. UDP-glucose serves as the substrates for glycogen synthase, which catalyzes the addition of glucose residues to the glycogen particle via the formation of a-1, 4-glycosidic bonds. A branching enzyme then links blocks of glucose residues through a-l,6-glycosidic linkages to form the highly branched structure of mature glycogen (Kuwajima, Golden et al. 1986). The rise in insulin in the fed state stimulates phosphorylation of glycogen synthase kinase 3 (GSK-3) via a branch of the insulin signaling pathway that includes PI3-Kinase and Akt-1 (protein kinase B). Phosphorylation of GSK-3 reduces its kinase activity, thereby resulting in reduced phosphorylation of glycogen synthase and an increase in its enzymatic activity (Frame, Cohen etal. 2001). In glycolysis, one molecule of glucose is converted into two molecules of pyruvate via a series of 10 enzymatic reactions, yielding energy in the form of ATP. Seven of the ten steps in this pathway are catalyzed by enzymes with equilibrium 6 constants that allow the forward (glycolytic) or the reverse (gluconeogenic) direction of the reaction to proceed depending on physiologic changes in the relative concentrations of substrates and products. The three other enzymatic steps of the pathway are catalyzed by glucokinase (GK) (glucose phosphorylation step), phosphofructoinase (PFK) (conversion of fructose-6-phosphate to fructose-1, 6-bisphosphate), and pyruvate kinase (conversion of phosphoenolpyruvate to pyruvate), and are considered to be irresversible because of the large release of free energy associated with these reactions. Modulation of the concentrations and activities of GK, PFK and pyruvate kinase are the primary mechanisms for control of glycolytic rate. Hepatic glucose output is mainly from breakdown of glycogen (glycogenolysis) or de novo synthesis from lactate, pyruvate, or amino acids through the gluconeogenesis pathway (Pilkis and Granner 1992; Moore, Hsieh et al. 1999; Nordlie, Foster et al. 1999). Glycogenolysis is the process by which the liver produces glucose from its own glycogen reserves. This process provides a glucose supply during short periods of fasting. In prolonged fasting, hepatic glycogen stores are reduced and glucose is formed from noncarbohydrate precursors such as lactate, pyruvate, glycerol and a-ketoacids. This process is called gluconeogenesis. Gluconeogenesis is basically a reversal of the glycolytic pathway except for three irreversible steps in glycolysis that are bypassed by enzymes unique to gluconeogenic pathway. The first of these bypasses involves a series of cytosolic and mitochondrial reactions that catalyze the conversion of pyruvate to phosphoenolpyruvate. In the mitochondria, excess acetyl coenzyme A (CoA) in the presence of adequate energy activates pyruvate carboxylase and then converts pyruvate to 7 oxaloacetate. Oxaloacetate is reduced to malate for entry into the cytosol, malate is reoxidized to oxaloacetate and oxaloacetate is then decarboxylated by cytosolic phosphoenolpyruvate carboxylase (PEPCK) to form phosphoenolpyruvate (Rognstad 1979). The gluconeogenic and glycolytic pathway are reciprocally regulated in order to prevent pointless cycling of substrates that are common to both pathways. Enzymes in the gluconeogenic and glycolytic pathways can be regulated in the short term by allosteric and covalent modifications and in the long term by changes in gene expression. A unique characteristic of the PEPCK enzyme is that it is exclusively regulated at the level of gene transcription, rather than by allosteric or covalent modifications, which is unlike the majority of the other enzymes involved in these pathways (Hanson and Reshef 1997). Insulin decreases gluconeogenesis directly by inhibiting PEPCK gene transcription. Insulin also decreases PEPCK gene expression indirectly by inhibiting glucagon gene expression and by increasing cAMP degradation, thus accelerating the degradation of PEPCK mRNA. When blood glucose levels decrease, insulin levels drop and glucagon levels rise. This activates a series of reactions that are mediated through the intracellular second messenger, cAMP, with a net effect being an increase in hepatic gluconeogenesis and glycogenolysis. As mentioned before, following conversion into glucose-6-phosphate, glucose can be channeled to the pentose phosphate pathway rather than glycolysis. The pentose phosphate pathway (PPP) is a pathway that generates NADPH and synthesizes pentose (5-carbon sugars) (Au, Gover et al. 2000). There are two major phases in the PPP, the 8 oxidative phase and the non-oxidative phase. During the oxidative phase reducing equivalents are generated in the form of nicotinamide adenine dinucleotide phosphate (NADPH) for reductive biosynthesis reactions within the cell. The synthesis of 5-carbon sugars, which are essential for DNA and RNA synthesis, occur during the non-oxidative phase. Glucose-6-phosphate dehydrogenase (G6PDH) is the first and the key enzyme of the PPP. It catalyzes the oxidation of G6P into 5-glucono-l,5-lactone-6-phosphate, while reducing one NADP+ molecule to NADPH by transferring an electron from one molecule to another. Being the key rate determining step of the PPP, G6PD is responsible for maintaining adequate levels of NADPH within the cell (Au, Gover et al. 2000). The liver has a role not only in controlling glucose homeostasis (described above), but also in lipid homeostasis. In addition to being able to store ingested carbohydrates as glycogen, the liver can use these carbohydrates to synthesize lipids de novo through the lipogenesis pathway. Glucose flux through glycolysis is crucial for providing carbons from glucose for lipid synthesis. Lipid metabolism involves the synthesis of fatty acids, which is a condensation reaction involving the 2 carbon units of acetyl-CoA as the subunit of a fatty acid chain. Key enzymes in this process are acetyl-CoA carboxylase (ACC), which catalyzes the formation of malonyl-CoA from acetyl-CoA, and fatty acid synthase (FAS), which catalyzes successive rounds of the condensation reaction. Lipogenesis is regulated according to the nutritional state. 9 Insulin signaling pathway The biological effects of insulin are initiated by activation of its receptor on the surface of the cell. Insulin receptors (IR) are present in all the vertebrate tissues, although the concentration varies from as few as 40 to more than 200,000 receptors per cell (White 1997). This receptor consists of 2a and 2p subunits, which are held together by disulphide bonds and noncovalent interactions (Cheatham and Kahn 1995; Ottensmeyer, Beniac et al. 2000). Binding of insulin to the a -subunit of the insulin receptor induces a conformational change in the p-subunits. Subsequent autophosphorylation of specific tyrosine residues leads to increased intrinsic tyrosine kinase activity of the receptor. This results in subsequent recruitment of docking proteins such as insulin receptor substrates IRSl and IRS2, which are tyrosine phosphorylated by the insulin receptor and coupled to several important signaling pathways. Stimulation of the insulin receptor results in the activation of three major pathways: 1) the mitogenactivated protein (MAP) kinase cascade, which mediates the mitogenic, growth and cell differentiation effects; 2) the phosphatidylinositol 3-kinase (PBK)-Akt pathway, which is mainly involved in the control of metabolic actions by insulin (glucose, lipid, protein metabolism) and has been extensively studied (Saltiel and Kahn 2001; Lizcano and Alessi 2002); and 3) the CAP/Cbl/TclO pathway, which controls the membrane translocation of the glucose transporter (GLUT4). The protein of interest is Akt, it will be discussed in detail in the PI 3-Kinase-Akt pathway, and the other two pathways are beyond the scope of the study. 10 PI 3-kinase -Akt pathway Activation of insulin receptors leads to the recruitment of insulin receptor substrate 1(IRS1) and its tyrosine phosphorylation. Some of the tyrosine phosphorylation sites are recognized by the src homology-2 domains (SH2) of p85, the regulatory subunit of PI 3kinase. P85 contains a src homology -3 domain (SH3) and two SH2 domains; between the SH2 domains lies a binding site for pi 10, the PI3-kinase catalytic subunit. Recruitment of this p85/pl 10 activates the enzyme and brings it to the membrane, where its substrate phosphatidylinositol 4 phosphate (PI3 4P) lies and subsequently generates PI 3, 4-bisphosphate (PI 3,4P2) and PI 3, 4, 5-triphosphate (PI 3, 4 P3) in the cell membrane. The pleckstrin homology domain (PH domain) of Akt is highly specific to PI 3, 4-bisphosphate (PI 3, 4P2) (Klippel, Kavanaugh et al. 1997). The binding of PH domain to PI 3, 4P2 recruits Akt to the plasma membrane where it encounters the phospholipid -associated protein kinases, PDK1 (Cohen, Alessi et al. 1997). PDK1 contains a PH domain that binds to phospholipids products of the PI3 kinase, which mediates its association with membranes where it catalyzes phosphorylation of Thr 309 of Akt (Meier and Hemmings 1999). The phosphorylation of Thr 309 partially activates Akt, but full activation occurs after the phosphorylation of Ser 474 by a second enzyme called PDK-2 (Meier and Hemmings 1999). Akt, a serine threonine kinase is a 57 kDa protein, also known as protein kinase B (PKB) or RAC -PK (protein kinase related to protein kinase A and C). It is known to exist in three isoforms a, P and y (Akt-1, Akt-2 and Akt-3) (Vanhaesebroeck and Alessi 2000). Each isoform possesses an amino terminal pleckstrin homology (PH) domain , a 11 kinase domain and a carboxy-terminal regulatory domain (Coffer, Jin et al. 1998). The pleckstrin homology (PH) domain consists of the first 100 amino acids at the amino terminal and is responsible for binding phospholipids. The regulatory domain consists of the last 70 amino acids at the carboxy terminal tail. The kinase domain consists of 258 amino acids. The phosphorylation of Akt occurs at two specific regulatory sites, one localized in the kinase domain (Thr 309) and the other in the C-terminal regulatory domain (Ser 474). All three Akt isoforms are ubiquitously expressed in mammals, although the level of expression varies among tissues (Jones, Jakubowicz et al. 1991; Altomare, Guo et al. 1995; Brodbeck, Cron et al. 1999). Aktl is the predominant isoform in most tissues. The highest expression of Akt2 is observed in the insulin responsive tissues like skeletal muscle, heart, liver and kidney, suggesting that this isoform is important for insulin signaling (Altomare, Guo et al. 1995; Kim, Peroni et al. 2000; Vuguin, Raab et al. 2004). Higher levels of Akt 3 were detected in testis and brain and low levels in the adult pancreas, heart and kidney (Brodbeck, Cron et al. 1999; Nakatani, Sakaue et al. 1999). Akt mediates many metabolic actions. Akt is an important mediator of the biological functions of insulin. The initial evidence for the involvement of Akt in insulin signaling came from the work carried out by Cross et al (1995), which demonstrated that Akt is responsible for the inactivation of glycogen synthase kinase 3 (GSK-3) and thus may regulate glycogen synthesis in insulin-sensitive tissues. Since then, Akt has been linked to a number of insulin and insulin-like growth factor-1 (IGF-l)-induced responses, including glucose uptake (Kohn, Summers et al. 1996), protein synthesis (Hajduch, 12 Alessi et al. 1998; Ueki, Yamamoto-Honda et al. 1998), preadipose cell differentiation (Magun, Burgering et al. 1996), the regulation of gene expression (Cichy, Uddin et al. 1998; Liao, Barthel et al. 1998; Wang and Sul 1998), and cell survival (Dudek, Datta et al. 1997; Kulik, Klippel et al. 1997; Chen, Su et al. 1998). In response to growth factors, Akt signaling regulates nutrient uptake and metabolism through a variety of downstream targets. One of the most important physiological roles of Akt is to stimulate glucose uptake in response to insulin. Akt2 has been found to associate with glucose transporter 4 (Glut 4)- containing vesicles upon insulin stimulation of adipocytes (Calera,_Martinez et al. 1998) and Akt activation leads to Glut 4 translocation to the plasma membrane. The exact molecular mechanisms by which Akt stimulates Glut 4 translocation to the plasma membrane are still being investigated. It has been found that Rab-GAP AS 160 (also known as TBC1 domain family member 4; TBC1D4) is a direct target of Akt involved in this process (Sano, Kane et al. 2003; Eguez, Lee et al. 2005). Akt activation can also alter glucose and lipid metabolism. Upon entry into the cell, glucose is converted into glucose-6-phosphate by hexokinases. Akt has been shown to stimulate the association of hexokinase isoforms with the mitochondria (Racek, Holecek et al.), where they phosphorylate glucose, but the direct target of Akt responsible is currently unknown. Glucose -6-phosphate can be stored by conversion to glycogen or catabolized to produce energy through glycolysis, and Akt signaling can regulate both these processes. In muscle and liver, Akt phosphorylates and inhibits GSK-3 which inturn 13 prevents GSK3 from phosphorylating and inhibiting its substrate glycogen synthase, thereby stimulating glycogen synthesis. Akt activation also increases the rate of glycolysis (Elstrom, Bauer et al. 2004). Akt's ability to enhance the rate of glycolysis is due, at least in part, to its ability to promote the expression of glycolytic enzymes through HIFa (Semenza, Roth et al. 1994; Majumder, Febbo et al. 2004; Lum, Bui et al. 2007) Akt-mediated phosphorylation and inhibition of FOXOl also contribute to glucose homeostasis, as FOXOl promotes hepatic glucose production and regulates the differentiation of cells involved in metabolic control (Accili 2004). In hepatocytes, Akt can also inhibit gluconeogenesis and fatty acid oxidation through direct phosphorylation of serine 570 on PGC-la (Li et. al. 2007), which is a coactivator that can regulate genes with FOXOl and other transcription factors. Akt signaling regulates lipid metabolism through phosphorylation and inhibition of GSK3. GSK3 has been shown to promote degradation of the sterol regulatory element-binding proteins (SREBPs), which are transcription factors that turn on the expression of genes involved in cholesterol and fatty acid biosynthesis (Sundqvist, Bengoechea-Alonso et al. 2005). Therefore, Akt-mediated inhibition of GSK3 promotes SREBP stability and enhances lipid production. Insulin resistance and type 2 diabetes Insulin resistance is the failure to respond to normal concentrations of circulating insulin. Clinically, insulin resistance, accordihg to the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK 2005), is indicated by a plasma glucose concentration between 100 and 125 mg/dL (milligrams of glucose per deciliter of blood) following a 12-hour fast. In the 1930s, Himsworth first differentiated patients with 14 diabetes mellitus into "insulin sensitive" and "insulin insensitive" based on the ability of subcutaneous insulin administration to dispose of an oral glucose load. The former is now classified a type 1 diabetes mellitus and the latter "insulin insensitive" classified as type 2 diabetes mellitus. With the development of the radioimmunoassay technique in 1960, Yalow and Berson demonstrated that patients with adult-onset diabetes (Type 2 diabetes) had higher circulating insulin levels than non-diabetic subjects. It was thus concluded that "the tissues of the maturity onset diabetic do not respond to insulin as well as the tissues of the nondiabetic subjects respond to insulin" Impaired insulin action occurs when target tissues are unable to respond to the normal circulating concentration of insulin. In response, P- cells in the pancreas need to secrete increased amount of insulin to maintain normal blood glucose level. However, overtime, functional defects in insulin secretion prevent the P- cells from maintaining high rates of insulin secretion, and hyperglycemia and eventually type 2 diabetes develop (DeFronzo, Bonadonna et al. 1992). Insulin resistance affects glucose disposal in muscle and fat and has an effect on insulin suppression of hepatic glucose output (DeFronzo 1988; DeFronzo, Bonadonna et al. 1992; Reaven 1995). Prevalence of diabetes It is estimated that diabetes affects more than 220 million people worldwide according to world health organization (WHO). Recent WHO calculations indicate that worldwide almost 3 million deaths per year are attributed to diabetes. The number of people with diabetes is increasing due to population growth, aging, urbanization, and increasing prevalence of obesity and physical inactivity. Estimates of the global 15 prevalence of diabetes in the year 2000 (as used in the World Health Organization [WHO] Global Burden of Disease Study) and projections for 2030 are shown in Figure 2. Type 1 diabetes is referred to as insulin- dependent diabetes mellitus (IDDM) or juvenile- onset diabetes. It develops when the body's immune system destroys pancreatic beta cells, which are responsible for making the hormone insulin that regulates blood glucose. Children and young adults are usually affected by this form of diabetes. In adults, type 1 diabetes accounts for 5% to 10% of all diagnosed cases of diabetes. Risk factors for type 1 diabetes may be autoimmune, genetic, or environmental. Type 2 diabetes is referred to as non-insulin-dependent diabetes mellitus (NIDDM) or adult onset diabetes. In adults, type 2 diabetes accounts for about 90% to 95% of all diagnosed cases of diabetes. As discussed before, it usually begins as insulin resistance, a disorder in which the cells do not use insulin properly. As the need for insulin rises, the pancreas gradually loses its ability to produce it. Type 2 diabetes is associated with older age, obesity, a family history of diabetes, history of gestational diabetes, impaired glucose metabolism, physical inactivity, and race/ethnicity. According to the Center for Disease Control and Prevention (CDC), African Americans, Hispanic/Latino Americans, American Indians, and some Asian Americans and Native Hawaiians or other Pacific Islanders are at particularly high risk for type 2 diabetes and its complications. Type 2 diabetes in children and adolescents, although still rare, is being diagnosed more frequently among American Indians, African Americans, Hispanic/Latino Americans, and Asians/Pacific Islanders. 16 Prevalence of diabetes twspt «^'< f IP: < 0 M \%2*~*>m i bate**. t The tc^j to country, m numbers of people wrth diabetes* am fcrarjfenc* s? * * * * * ( * J-tej s « e * £ - * * f&n fmfta cht&a USA Indonesia Japan Pakistan Russia 8raifJ Italy Bangladesh y§af tenktnq 1 a 3 Country / 2000 2030 Mopk with tiistem ImBom) India China united stat« of America 3U 79.4 20,8 17 J 42J 30.3 '**.„. ure 2: Estimates of the global prevalence of diabetes in the year 2000 and projections for 2030. (http://www0whoint/diabetes/actionnow/en/mapdiabpreVopdf) 17 Molecular mechanism of insulin resistance The molecular mechanisms that cause insulin resistance are not yet entirely clear and several factors have been proposed to contribute to the pathogenesis of this metabolic effect. Multiple mechanisms of how insulin resistance occurs have been documented. A single factor can cause insulin resistance by several mechanisms. On the other hand, multiple factors may share the same molecular target. Understanding the molecular mechanisms of insulin resistance precisely will explain and help predict the pathological consequences. Some of the molecular mechanisms of insulin resistance are: Decreased expression of insulin signaling components Any abnormality in the insulin receptor or its signaling cascade may lead to insulin resistance. In normal physiology, insulin binds to its tyrosine kinase receptor, initiates a cascade of intracellular signal pathways, and eventually facilitates glucose transport, glycogen and lipid synthesis, and appropriate gene expression (Le Roith and Zick 2001). Mutations in the insulin receptor (IR) gene could result in impaired receptor biosynthesis, decreased affinity of insulin binding (Kolterman, Gray et al. 1981; Sinha, Pories et al. 1987) or impaired tyrosine kinase activity (Caro, Sinha et al. 1987; Maegawa, Shigeta et al. 1991). Decreased expressions of IR and IRS have been reported in a number of studies of insulin resistant animal models (Biddinger, Miyazaki et al. 2006). For example, the expression of IR and IRS-1 was decreased significantly by 32 and 60%, respectively, in the genetically obese Spontaneously Hypertensive Koletsky rat (SHROB) (Friedman, Ishizuka et al. 1997). This effect seems to be reversible, because an antihyperglycemic agent restored the expression of IR and IRS-1 in this model (Haxhiu, 18 Dreshaj et al. 1999). In addition, the ob/ob mice (a model of insulin resistance of obesity and non-insulin-dependent diabetes mellitus) exhibit a significant decrease in IR, IRS-1, and IRS-2 expression in skeletal muscle and in IRS-1 and IRS-2 expression in liver (Kerouz, Horsch et al. 1997).This diminished receptor function will impact downstream events. The majority of insulin resistance is still believed to be associated with postreceptor mechanism. Several lines of evidences from human and animal studies suggest defects in insulin signaling pathways in target tissues. Reduced expression and/or diminished phosphorylation of early insulin signaling molecules have been observed in insulin target tissues of obese and Type 2 diabetic patients. For example, Goodyear et al reported that impaired insulin stimulated glucose uptake in human skeletal muscle of obese individuals was associated with decreased IRS-1 phosphorylation and decreased IRS-1 associated PI3-kinase activity. (Goodyear, Giorgino et al. 1995; Bjornholm, Kawano et al. 1997; Meshkani, Taghikhani et al. 2006). Increase in the ratio of the regulatory subunit/catalytic subunit of PI3-kinase As mentioned before, PI3-kinase has two subunits: p85a (regulatory subunit) and pi 10 (catalytic subunit). Elevation of the p85a regulatory subunit of PI3-kinase has been reported in some insulin resistant states. For example, a significant increase in expression of the p85a monomer leads to insulin resistance in the mouse model of insulin resistance during pregnancy (Barbour, Shao et al. 2004). p85a competes with the p85/pll0 heterodimer for binding to the IRS-1 protein. This competition impairs binding between IRS-1 and PI3-kinase, subsequently decreasing activation of PI3-kinase (Barbour, Shao et al. 2004). Moreover, increased p85/pl 10 expression correlates with decreased insulin 19 sensitivity in skeletal muscle of insulin resistant obese and type 2 diabetic subjects (Dey, Mukherjee et al. 2005). In another study carried out in obese Zucker rats (OZR), the regulatory subunit of PI3-kinase (p85a) was increased significantly (Anai et al. 1998) and some of the alternatively spliced isoforms of the regulatory subunits of PI3-kinase also were elevated in liver of ob/ob mice compared to lean controls (Kerouz, Horsch et al. 1997). Targeted disruption of the gene encoding the murine p85 a subunit (Pik3rl-/-) results in hypoglycemia and enhanced insulin sensitivity, which is due to increased glucose transport in skeletal muscle and adipocytes. Insulin stimulated PI3-kinase activity associated with IRSs was mediated through full-length p85 alpha in wild-type mice, but through the p50 alpha alternative splice form of the same gene in Pik3rl-/-mice. This isoform was associated with an increase in insulin-induced generation of phosphatidylinositol (3, 4, 5) triphosphate (Ptdlns (3, 4, 5) P3) in Pik3rl-/- adipocytes and facilitates Glut4 translocation to the plasma membrane (Terauchi, Tsuji et al. 1999). Serine phosphorylation of IRS Accumulating data have highlighted the critical role of serine phosphorylation of IRS in insulin resistance. Yet, a mechanism of how serine phosphorylation could decrease IRS-1 function has been difficult to formulate, in part because of a large number of potential serine phosphorylation sites on IRS-1 (Aguirre, Werner et al. 2002). One model is that serine phosphorylation induces a conformational change in the functional domain of IRS-1. For instance, serine 307 of IRS-1 (serine 312 in human IRS-1) is located at the end of the phosphotyrosine-binding (PTB) domain, which is involved in the binding of IRS-1 to the IR. Serine 307 is phosphorylated by several different kinases, 20 depending upon the cellular conditions; this modification reduces the interaction between IRS-1 and IR and could be one important mechanism for regulating the early stages of insulin signaling. In addition, several studies have shown that phosphorylation of serine residues could induce IRS-1 breakdown through the proteasome degradation pathway (Pederson, Kramer et al. 2001). Many serine/threonine kinases have been proposed to phosphorylate various serine residues of IRS-1, such as extracellular signal-regulated kinase (ERK) (Bouzakri, Roques et al. 2003), c-Jun NH2-terminal kinase (JNK) (Aguirre, Uchida et al. 2000), inhibitor KB kinase (IKK) (Gao, Hwang et al. 2002) and the mammalian target of rapamycin (mTOR) (Carlson, White et al. 2004). For example, full body reduction of JNKl resulted in a decrease in weight gain on a high fat diet and in a reduction of insulin resistance with a decrease in IRSl serine phosphorylation (Hirosumi, Tuncman et al. 2002). Hepatic reduction of JNKl activity improves insulin sensitivity and hepatic steatosis. On the other hand muscle JNKl does not contribute to insulin resistance (Singh, Wang et al. 2009). High levels of JNKl activity in adipose tissue may promote insulin resistance through the deregulation of adipocytokines (cell signaling proteins released by adipocytes) (Sabio, Das et al. 2008). Similarly, ERK1 deficient mice are protected against diet induced obesity and insulin resistance because of decreased adipogenesis (Bost, Aouadi et al. 2005). Suppresors of cytokine signaling (SOCS) Another mechanism that may be involved in defective insulin signaling pathway could be induction of inhibitory factors such as suppressors of cytokine signaling (SOCS1, 3). SOCS proteins block insulin signaling via competition with IRS-1 for association 21 with the insulin receptor and by augmentation of proteosomal degradation of IRS-1 (Kile, Schulman et al. 2002). The expression of SOCS3 is enhanced by various inflammatory cytokines and hormones, including IL-6 and leptin (Emanuelli, Peraldi et al. 2001; Rieusset, Bouzakri et al. 2004; Shi, Tzameli et al. 2004). The levels of SOCS 1 and SOCS6 have also been shown to be enhanced during cytokine-mediated inhibition of insulin signaling (Mooney, Senn et al. 2001; Rui, Yuan et al. 2002). Overexpression of SOCS1 or SOCS3 inhibited insulin-induced glycogen synthesis in myotubes and glucose uptake in adipocytes (Ueki, Kondo et al. 2004), while hepatocyte-specific SOCS3 deletion improved insulin sensitivity in the liver (Torisu, Sato et al. 2007). SOCS proteins inhibit insulin-induced signaling either by binding to the IR or reducing the receptor's ability to phosphorylate IRS on tyrosine residues for activation, or by targeting IRS proteins for proteosomal degradation (Rui, Yuan et al. 2002). Phosphatase The insulin signaling pathway is initiated by the tyrosyl phosphorylation of the insulin receptor (IR), which is reversible. The IR dephosphorylation takes place rapidly in intact cells even with the continued presence of insulin (Kole, Kole et al. 1998). Because a critical regulatory step in insulin signal transduction is the dephosphorylation of signaling molecules by protein tyrosine phosphatase (PTPs), it is plausible that enhanced activity of one or more protein tyrosine phosphatase could lead to insulin resistance. Support for the role of PTPs in the regulation of insulin action comes from transgenic and gene knockout studies. The Khan group and Elchebly group generated PTP-1 null mice and found that these mice have increased insulin sensitivity, manifested by enhanced 22 insulin stimulated phosphorylation of IR and IRS-1 in muscle and liver (Elchebly, Payette et al. 1999; Klaman, Boss et al. 2000). In addition, insulin-stimulated whole body glucose disposal is enhanced in protein tyrosine phosphatase-IB (PTPIB) deficient mice (Klaman, Boss et al. 2000). Surprisingly, this effect is tissue-specific: insulin-stimulated glucose uptake is elevated in skeletal muscle but not in adipose tissue. These data suggest that overexpression of PTPIB in insulin-target tissues in vivo may contribute to insulin resistance. Consistent with this hypothesis, Zabolotny et al. demonstrated that selective overexpression of PTPIB in skeletal muscle impairs insulin-stimulated PI3K activity and causes mild insulin resistance in vivo (Zabolotny, Haj et al. 2004). Recent studies have indicated that liver-specific deletion of PTPIB improves insulin resistance and attenuates diet-induced endoplasmic reticulum stress (Delibegovic, Zimmer et al. 2009). Similarly, Haj et al. showed that liver-specific re-expression of PTPIB in PTPIB deficient mice leads to marked attenuation of their enhanced insulin sensitivity (Arias-Salgado, Haj et al. 2005). O-GlcNAcylation of serine/threonine residues Traditionally protein glycosylation refers to the covalent attachment of complex oligosaccharides to proteins that are in intralumenal compartments or cellular membranes, or destined for secretion. A novel form of glycosylation surfaced when proteins in the nucleus and cytosol were found to be modified with a single P-Nacetylglucosamine monosaccharide moiety through an O-P glycosidic attachment (OGlcNAc) (Torres and Hart 1984) to serine and threonine side chains of the polypeptide backbone (Holt and Hart 1986; Holt, Snow et al. 1987). The addition of O-GlcNAc to 23 proteins is catalyzed by uridine diphospho-N-acetylglucosamine:polypeptide p-Nacetylglucosaminyltransferase O-GlcNAc transferase (OGT), which uses uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) as the direct sugar donor (Haltiwanger, Blomberg et al. 1992). The removal of O-GlcNAc from proteins is catalyzed by P-N-aetylglucosaminidase (O-GlcNAcase) (Dong and Hart 1994; Gao, Wells et al. 2001). Both are found in the cytosol and nucleus. The O-GlcNAc is attached and removed multiple times in the life of a polypeptide, at different rates and at different sites in response to cellular signals. While there has been no consensus motif identified for O-GlcNAc attachment, many sites appear to be identical to those used by serine/threonine kinases. A reciprocal relationship as well as adjacent occupancy between phosphorylation and O-GlcNAcylation has been observed for some proteins, and both mechanisms are touted as the way O-GlcNAcylation can interfere with phosphorylation. Interplay between O-GlcNAcylation and phosphorylation has been postulated to occur through several different mechanisms: [1] direct competition for site occupancy at a single hydroxyl group, [2] competition via steric hindrance by reciprocal modification at proximal sites in a polypeptide, and [3] regulation of O-GlcNAc enzymes by phosphorylation and conversely regulation of kinases or phosphatases by OGlcNAcylation. Studies of several proteins are consistent with all of these mechanisms. For example, Thr-58 of c-Myc (Chou, Hart et al. 1995; Kamemura, Hayes et al. 2002) and Ser-16 of estrogen receptor-P (Cheng and Hart 2001) reciprocally carry either the OGlcNAc or the O-phosphate modification at the same hydroxyl moiety. Furthermore, as in the case of the tumor suppressor protein p53, an O-GlcNAc modification adjacent to a 24 known regulatory phosphorylation site can affect phosphorylation of that residue and subsequent protein function (Shaw, Freeman et al. 1996). A significant amount of research has been directed towards understanding the role that O-GlcNAc plays in the development of type II diabetes (Buse 2006). However, the exact role that this modification may play in this multifaceted, complex physiological disease is still unclear. O-GlcNAc has been shown to be present on or added to transcription factors involved in insulin regulation (Gao, Miyazaki et al. 2003; Andrali, Qian et al. 2007; Vanderford, Andrali et al. 2007). For example, in Min6 cells, elevated glucose concentration leads to an increase in O-GlcNAc modification of the transcription factor, pancreatic/duodenal homeobox-1 protein (PDX-1), and this hyperglycosylation correlates with an increase in DNA binding activity of PDX-1 (Gao, Miyazaki et al. 2003). Other studies, such as Andrali et al, presented data that showed increased OGlcNAcylation leading to relocalization of the P-cell specific transcription factor NeuroDl (Andrali, Qian et al. 2007). Additionally, proteins that are normally activated downstream of the insulin signaling pathway have been shown to be modified by OGlcNAc, such as IRS-1 and Akt (Park, Ryu et al. 2005; Ball, Berkaw et al. 2006). So, OGlcNAc modifications may directly affect the ability of a cell to respond to insulin as an extracellular stimulus. Lastly, proteins known to be involved in membrane fusion events that allow for the relocalization of the Glucose Transporter Type 4 (GLUT4) following insulin stimulation have been modified by O-GlcNAc (Chen, Liu et al. 2003). 25 Hexosamine biosynthetic pathway (HBP) Glucose upon entering the cell fluxes through various metabolic pathways. A small portion of incoming glucose can enter a nutrient-sensing pathway called hexosamine biosynthetic pathway (HBP). Amino sugars are physiologically synthesized through the hexosamine biosynthetic pathway. This pathway results in the synthesis of UDP-N-acetyl-D-glucosamine (UDP-GlcNAc), a precursor for all amino sugars used for synthesis of proteoglycans, glycoproteins and glycolipids. This pathway involves the transamidation and N-acetylation of glucose followed by the activation of the Nacetylated amino sugar. The activated acetylated sugar is then used in the synthesis of glycoproteins, proteoglycans and glycolipids. Glucose is converted into glucosesphosphate by hexokinase. Phosphoglucose isomerase then catalyzes the reversible isomerization of glucose-6-phosphate to fructose-6-phosphate (Fru-6-P). The HBP (Figure 3) begins with conversion of fructose-6-phosphate to form glucosamine-6-phosphate (GlucN-6-P). Glutamine serves as donor of the aminogroup. The reaction is catalyzed by the rate-limiting enzyme glutamine: fructose-6-phosphateamidotransferase (GFAT). Acetyltransferase catalyzes the N-acetylation of glucosamine6-phosphate into N-acetyl-D-glucosamine-6-phosphate. N-acetyl-D-glucosamine-6phosphate isomerizes into N-acetyl-D-glucosamine 1-phosphate, which upon addition of Uridine triphosphate (UTP) is converted into UDP-N-acetyl-D-glucosamine (UDPGlcNAc). UDP-GlcNAc, the end point of the HBP is the donor for a common form of intracellular o-glycosylation of nuclear and cytoplasmic proteins, O-linked P- Nacetylglucosamine (O-GlcNAc). 26 Glucose Glucosamine * Glc-6-P (Glucose-6-phosphate) GFAT ^ Fruc-6-P 1 ^GIcN-i 6-P (Fructose-6-phosphate) ^UDP-GlcNAc (UDP-N-AcetylGlucosamine) i i i Glycolysis O-GlcNAc-modified cytosolic and nuclear proteins TCA Cycle GFAT-Glutamine Fructose-6-phosphate Amidotransferase Figure 3: Hexosamine Biosynthetic Pathway Role of the hexosamine biosynthetic pathway in insulin resistance The adverse effect of glucose that is mediated through the HBP was first demonstrated by Marshall et al (1991) who showed that the induction of insulin resistance requires three components: insulin, glucose, and glutamine. When any one of these components is omitted, little or no densensitization is observed. This was explained by the fact that formation of hexosamine products requires a supply of both glucose (in the form of F-6-P) and glutamine (as a cofactor for GFAT in the transfer of an amide group to fructose-6-P). The primary role of insulin in this proposal is to facilitate the uptake of glucose by increasing the number of cell surface glucose transporters. They 27 used two approaches to test their hypothesis that enhanced flux through the HBP culminates in insulin resistance. They first demonstrated that glucose-induced desensitization could be prevented by glutamine analogs that irreversibly inactivate glutamine-requiring enzymes such as GFAT. Second, they showed that glucosamine, in addition to glucose, could induce cellular insulin resistance. Glucosamine was shown to enter adipocytes through the glucose transport system and directly enter the hexosamine pathway distal to GFAT (through the formation of GlcN-6-P). In 1993, Robinson et al. reported that glucosamine induces insulin resistance in isolated rat skeletal muscles, revealing that this glucose sensing pathway is operative in other tissues (Robinson, Sens et al. 1993). Further evidence of the involvement of HBP in insulin resistance came from work carried out by Rossetti et al (1995), Baron et al (1995.1998), and Giarccari et al (1995), who found that infusion of glucosamine for 4-6 hours under euglycemic conditions resulted in marked insulin resistance. Additional studies by Baron et al showed that glucosamine induces insulin resistance in vivo by causing a defect in insulin-responsive glucose transporter (GLUT 4) translocation from the cell interior to the cell surface. Further work carried out by McClain et al (1996) confirmed the involvement of HBP in insulin resistance. He studied transgenic mice overexpressing GFAT in the adipocytes. In response to excess nutrient flux as indicated by an increase in hexosamine flux, the adipocytes became more insulin resistant while increasing GLUT4 expression, glucose uptake, and fat synthesis, resulting in increased adiposity, and adipocytes size. Subsequent transgenic studies carried out by McClain et al extended the glucose sensing 28 and regulatory role of the HBP to the liver and P-cells of the pancreas. In liver overexpression of the rate limiting enzymes GFAT led to glucose intolerance with a significant decrease in glucose disposal rates (Veerababu, Tang et al. 2000). Similarly, overexpression of GFAT in pancreatic P-cell resulted in hyperinsulinemia and eventual insulin resistance as the transgenic mice aged (Veerababu, Tang et al. 2000). Later work carried out in both in vitro and in vivo supports the model where increased UDP-GlcNAc, a results of increased flux through the HBP, raises O-GlcNAc levels, and this result in insulin resistance (Buse, Robinson et al. 2002; McClain 2002; Vosseller, Wells et al. 2002). Blocking the removal of O-GlcNAc by inhibiting OGlcNAcase, using 0-(2-acetamido-2-deoxy-D-glucopyranosylidene) amino-Nphenylcarbamate (PUGNAc), results in decreased glucose uptake in response to insulin in 3T3-L1 adipocytes (Vosseller, Wells et al. 2002). Treatment of rat primary adipocytes with PUGNAc also reduced insulin-stimulated glucose uptake and glucose transporter 4 (GLUT4) translocation to the plasma membrane (Park, Ryu et al. 2005). In this model system, reduced phosphorylation and increased O-GlcNAc modification of Akt were thought to be the possible mechanisms by which O-GlcNAc altered insulin signaling in the adipocytes (Park, Ryu et al. 2005). In skeletal muscle, infusion of glucosamine in Wistar rats induces insulin resistance of skeletal muscle through decreased phosphorylation of insulin receptor substrate-1 (IRS-1) and its association with phosphatidylinositol 3-kinase (PI3-K) (Patti, Virkamaki et al. 1999) and in turn results in impaired translocation of GLUT4 to the plasma membrane (Baron, Zhu et al. 1995). Similarly, exposure of glucosamine also 29 inhibits insulin-stimulated glucose transport and glycogen synthesis in isolated rat skeletal muscles (Robinson, Sens et al. 1993). Similar to what was observed in adipocytes, treating isolated skeletal muscle with PUGNAc-impaired insulin stimulated glucose transport (Arias, Kim et al. 2004). However, contrary to the adipocytes studies, Akt phosphorylation in the skeletal muscle was not significantly altered (Arias, Kim et al. 2004). Other studies have also shown that glucosamine infusion in rats can also impair insulin-stimulated glucose disposal through decreased IRS-1 association with PI3K without affecting Akt activity (Kim, Nikoulina et al. 1999). The effect of HBP on insulin signaling in liver was first demonstrated with the overexpression of the rate-limiting enzyme GFAT under the control of the PEPCK promoter. Those transgenic mice gained weight with age and developed glucose intolerance with a significant decrease in glucose disposal rates (Veerababu, Tang et al. 2000). It was recently demonstrated that adeno viral-mediated overexpression of OGT in mouse liver attenuated the ability of insulin to suppress hepatic glucose production. The mechanism of this phenomenon involves decreased Akt phosphorylation and Akt activity, which in increased expression of gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase). It was proposed that this regulation was facilitated by OGT itself binding to phosphoinositide (3, 4, 5) phosphate (PIP3), resulting in OGT translocation to the plasma membrane where it colocalized with Akt (Yang, Ongusaha et al. 2008). Taken together, these data suggest crucial and diverse roles for HBP in mediating the adverse effects of hyperglycemia. 30 Selenium- an insulin mimetic agent Insulin regulates many biological processes and pathways important to potently understand but also find viable ways to either mimic effects or overcome with lack of or no response. Thus, various agents have been studied that have been shown to stimulate metabolic functions of insulin. Several investigators are studying trace elements that have insulin- like properties. Selenium is one of the trace elements that has been shown to have antidiabetic effects. Selenium is an integral component of several enzymes, such as glutathione peroxidase, iodothyronine deiodinase, thioredoxin reductase, selenoprotein P and W. In addition to its role as an antidiabetic agent, it is also involved in prevention of cancer (Combs, Clark et al. 2001) and cardiovascular disease (Rayman 2002). Osamu Ezaki first showed the effect of selenate as a potent insulin-like agent. In this study, incubation of rat adipocytes with selenium stimulated glucose transport activity in a dose dependent manner, and this increase in glucose transport activity was due to the translocation of two types of glucose transporters (GLUT-1 and GLUT-2) to the membrane surface (Ezaki 1990). Maximal transport activity (lOOuM for 30 min, assessed from the rate of 3-0-14Cmethyl-D-glucose uptake) was equivalent to InM insulin for 30 min incubation. Stimulation of glucose transport was observed 2 min after addition of selenite and reached a steady state within 10 min (Ezaki 1990). Further studies carried out on rat soleus muscle by Furnsinn C et al. also showed marked a increase in glucose uptake when muscle was exposed to selenate (Furnsinn, Englisch et al. 1996). Expanding on the work carried out by Ezaki, McNeill et al. showed that selenium acted as an insulinmimetic in vivo. Treating streptozotocin-induced diabetic animals with selenium 31 improved both food and water intake level to both control levels. Weight gain of the animals improved, and within 2 weeks of treatment plasma glucose levels had improved significantly and remained low throughout a seven week experiment (McNeill, Delgatty et al. 1991). Similar studies carried out by Berg et al. in rats and Ghosh et al. in mice also showed a blood glucose lowering effect of selenium (Ghosh, Mukherjee et al. 1994; Berg, Wu et al. 1995) in diabetic animals. In addition to influencing glucose uptake, selenium was shown to regulate the activity and expression of various enzymes involved in the processes of glycolysis, gluconeogenesis, fatty acid synthesis and the pentose phosphate pathway. Oral administration of selenate to diabetic animals partly reversed abnormal liver expression of both glycogenic and gluconeogenic enzymes (Becker, Reul et al. 1996). Regulation by insulin-mimetics of the expression of lipogenic enzymes was found to be similar to that of insulin. Treatment of the diabetic animals or rat hepatocytes in culture with selenate restored the expression of both FAS and G6PDH, demonstrating that selenate was capable of stimulating lipogenesis in the liver (Ghosh, Mukherjee et al. 1994; Berg, Wu etal. 1995). One of the main causes of mortality in diabetes is myocardial disease. A depressed level of overall lipid metabolism in diabetics is believed to be a contributing factor to a higher risk of heart disease and stroke. Selenate was shown to lower plasma lipid levels. Studies carried out by Battell, M.L et al. in streptozotocin induced diabetic rats treated with selenate, found that the plasma lipid levels, triglycerides, cholesterol and free fatty acid levels were improved compared to untreated diabetic rats (Battell, Delgatty 32 et al. 1998). Further studies, showed that sodium selenate has cardioprotective effects. In those studies, the effects of intra peritoneal administration of sodium selenite for 4 week on the heart structure in streptozotocin-induced diabetic rats were evaluated. Examination by electron and light microscopy revealed that selenite administration corrected the changes in the diabetic heart. The structure of selenium-treated diabetic hearts appeared very similar to the structure of control rat hearts (Ayaz, Can et al. 2002). The enhanced phosphorylation of diverse cellular proteins is believed to be responsible for an elevated translocation of glucose transporters, an increased glucose uptake and modified expression of metabolic enzymes. Like insulin, selenium treatment triggers a variety of phosphorylation events targeting proteins of the insulin signaling pathway. For example, treatment of NIH3T3 HIR 3.5 cells with selenate stimulated phosphorylation of the insulin receptor (Pillay and Makgoba 1992). In a later study, incubation of either primary rat hepatocytes or 3T3-LI adipocytes in culture with selenate caused not only a concentration and time dependent increase in phosphorylation of the P subunit of the insulin receptor, but also increased phosphorylation of IRS-1 as determined through immunoprecipitation studies (Stapleton, Garlock et al. 1997). This increase in phosphorylation of IRS-1 was observed within lhr of selenium treatment, whereas only few minutes of incubation is required for increased phosphorylation by insulin, suggesting that more time is required for selenate to enter the cell and mediate insulinregulated processes through a post-insulin receptor kinase mechanism. These results are supported by the fact that although selenate is capable of increasing phosphorylation of 33 the insulin receptor, this does not appear to directly increase insulin receptor tyrosine kinase activity (Ezaki 1990) (McNeill, Delgatty et al. 1991). Further downstream proteins of signal transduction pathway, triggered by selenium have been identified over the years. For example, our laboratory has shown that selenate increases PI 3-kinase activity in rat hepatocytes in culture. One protein that has been identified to lie downstream of PI3-kinase is p70 S6 kinase. S6 kinase can play a critical role in initiation of protein synthesis. Selenate has been shown to stimulate not only S6 kinase phosphorylation but also kinase activation. In addition to the IRS family and the PI 3-kinase pathway, selenate have been shown to activate mitogen activated protein kinase (MAPK) (Stapleton, Garlock et al. 1997; Hei, Farahbakhshian et al. 1998). In both hepatocytes in culture and 3T3-L1 adipocytes, selenate caused a marked increase in not only the phosphorylation of MAPK in a both time and concentration dependent manner but also its activity as measured by an 'in-gel' kinase assay (Stapleton, Garlock et al. 1997). Significance of study The molecular mechanisms that cause insulin resistance are not yet entirely clear. There is evidence that suggests that high glucose induced insulin resistance may be mediated by products of the HBP. But, most of the research is focused on two insulin sensitive tissues i.e. adipocytes and skeletal muscle and less attention has focused on the liver. The liver plays a key role in coordinating the whole body metabolism including carbohydrate, lipid and protein metabolism. It is important to understand the mechanism 34 of insulin resistance as mediated by products of the HBP in all the insulin sensitive tissues. Over the years, there is growing evidence that have shown that selenium, an insulin mimetic agent, can mediate a number of insulin-like actions both in vivo and in vitro models of type 1 diabetes. However, fewer studies have established its effectiveness on models of type 2 diabetes or insulin resistance. Understanding the mechanism of action of selenium, as insulin mimetic in insulin resistant models will aid in using it as a therapeutic agent in management of type 2 diabetes. Objectives of study The goal of this project was to investigate the effect of glucosamine on a key insulin signaling protein and key metabolic enzymes of the pentose phosphate pathway, fatty acid biosynthesis pathway and gluconeogenesis. Here we hypothesize that increased flux through the hexosamine biosynthetic pathway can generate insulin resistance through increased glycosylation and that selenium, an insulin mimetic, can overcome the insulin resistance state. The goals of this study were to: 1) To assess the effect of glucosamine on an insulin induced key signaling protein. 2) To assess the effect of glucosamine on the insulin induced gene expression of key metabolic enzymes. 35 3) To test the effect of selenium on the activation of key insulin signaling pathway in glucosamine induced insulin resistance. 4) To test the effect of selenium induced gene expression of key metabolic enzymes in glucosamine induced insulin resistance. 5) To examine whether the treatment of glucosamine increases O-glycosylation of proteins. 6) To identify O-GlcNAc modified proteins. 36 CHAPTER 2 MATERIALS AND METHODS Primary rat hepatocytes isolation and maintenance Male Sprague-Dawley rats (Charles River, Kalamazoo) weighing 180-200g were housed in a facility approved by the WMU Institutional Animal Care and Use Committee (IACUC). Rats were maintained on Lab diet 5001 standard rodent chow and drinking water ad libitum. Animals were fasted for approximately 48 hrs prior to hepatocyte isolation and anesthetized with an intra-peritoneal injection of pentobarbital (4550mg/Kg). Primary hepatocytes were isolated using collagenase-hyalurodinase perfusion and digestion method (Stapleton, Stevens et al. 1993). At first the liver was perfused via the portal vein with a perfusion solution containing 0.148M NaCl, 0.01M HEPES, 0.017M fructose, 0.049mM EGTA, 0.5% phenol red, and 6unit/ml heparin with pH-7.2. Then a digestion solution containing lOOug/ml collagenase D, 93unit/ml hyaluronidase, 160unit/ml trypsin inhibitor, and 0.2% BSA was passed through the portal vein. The liver was then excised and forced through three layers of gauze over a beaker using the digestion solution, and then centrifiiged in 50ml tubes at 4°C for 3 minutes at 50xg.The supernatant was aspirated and the cell pellet was suspended, washed and centrifiiged twice with cold Waymouth's MB 752/1 (Sigma -Aldrich Corporation, St., MO) medium containing 0.5% Bovine Serum Albumin (BSA). The cell pellet obtained after the final 37 washing was gently resuspended in the medium and an aliquot was used to determine cell concentration and cell viability using trypan blue exclusion method with a hemocytometer. Cells with greater than 85% viability were plated to 90% confluency on sterilized 60mm collagen (rat tail) coated plates (Falcon-30002). The cells were incubated in 4ml Waymouth's MB 752/1 medium containing 5% BSA (Sigma, St Louis, MO) and supplemented with gentamicin (10|ag/mL) (Sigma) under a humidified atmosphere of 5% CO2 and 95%) air at 37?C for 4 hrs. After a 4hr attachment period, the media was aspirated and cells were washed once with 1ml BSA free Waymouth's medium and then incubated with 4ml of fresh medium without BSA for an overnight incubation. Cell treatment and processing To assess the effect of glucosamine on an insulin induced key signal protein and to investigate the effect of glucosamine on O-GlcNAc modification by western blot, primary hepatocytes were incubated in DMEM low glucose media (5mM) (Gibco, Grand Island, NY) with or without ImM glucosamine (Sigma) for 18hrs followed by media change with low glucose media and then treated with 80nM (Sigma) insulin for 1 hr (Table 1). To test the effect of selenium induced activation of a key insulin signaling protein in glucosamine induced insulin resistance, primary hepatocytes were incubated in DMEM low glucose media (5mM) with or without ImM glucosamine for 18hrs followed by media change with low glucose media and then treated with selenium (500|LIM) for 3hr (Table2). To investigate the effect of glucosamine on the 38 insulin or selenium induction of the gene expression of key metabolic enzymes, cells were incubated in DMEM low glucose media with or without glucosamine for 18hrs along with or without insulin at 44nM concentration at various time periods (1 or 18hr). To test the effect of selenium, the cells were incubated in DMEM low glucose media with or without glucosamine for 18hrs along with or without 500|uM selenium at various time periods (1, 3 or 6 hrs). To study PEPCK and Foxa2 gene expression, the cells were treated with 500|nM cAMP (Sigma) along with 44nM insulin for lhr or 500|uM selenium for 1, 3 or 6hrs. After the cells were treated using the various conditions, they were processed by first removing the media and then washing twice with 1ml of cold phosphate buffered saline (PBS) before isolating either protein or mRNA. 39 Table 1: Treatment conditions to establish an insulin resistance model in primary rat hepatocytes Assay Signaling protein Treatment Glucosamine Insulin Western blot Akt* 18hr lhr NA Number of animals (n) 4 qRT-PCR Metabolic enzymes** Glucose 6 phosphate dehydrogenase Fatty acid synthase Phosphoenolpyruvat e carboxylase Foxa2 18hr 18hr NA 4 18hr 18hr 18hr lhr NA lhr 5 8 18hr 18hr lhr lhr lhr 5 4 Mass Spectrometry *= Insulin treatment after 18hr glucosamine treatment **= Insulin treatment along with 18hr glucosamine treatment 40 cAMP Table 2: Treatment conditions to study the effect of selenium, an insulin mimetic agent Assay Signaling protein Treatment Glucosamine Western blot Akt* 18hr 3hr qRT-PCR Metabolic enzymes** Glucose 6 phosphate dehydrogenase Fatty acid synthase 18hr 1,3 and 6hrs 1,3 and 6hrs 1,3 and 6hrs 1,3 and 6hrs 3hr Phosphoenolpyruvat e carboxylase Forkhead box A2 (Foxa2) Mass Spectrometry 18hr 18hr 18hr 18hr Selenium cAMP NA Number of animals (n) 4 NA 4 NA 4 lhr 5 lhr 5 lhr 4 *= Selenium treatment after 18hr glucosamine treatment **= Selenium treatment along with 18hr glucosamine treatment Protein isolation and western blot analysis Protein isolation For isolating proteins from cells, a IX reporter lysis buffer (Promega-cat# E3971) (500(il for each 60mm dish) was added and incubated at room temperature for 10 minutes. After the incubation, the cells were then scraped from the plates and the dish containing the cell suspension was tipped at an angle and allowed to sit for an additional 10 minutes. The cell lysate was then collected in eppendorf tubes and centrifiiged at 10,000 rpm for 5 minutes at 4°C. The supernatants were then transferred to a new 41 eppendorf tube for protein assay, western blot protein analysis or affinity column chromatography. Determination of protein content Total protein content of the sample was measured using the Micro BCA Protein Assay Kit (Pierce). For the standard, a bovine serum albumin (Sigma) stock was prepared at lmg/ml by dissolving BSA in water. In a micro titer plate, standard solution of BSA from 2-20|il (2-20|^g) was used and the final volume of the standard was adjusted and diluted to 110|nl with H2O. For the wells containing sample, lOjal of the sample was diluted to 110|il with H2O and each sample was run in duplicate. Working reagent (WR) was prepared freshly by mixing BCA reagent A (MA), 50 parts and BCA reagent B, 1 part. Each sample had 150JLX1 of WR added to it, and then the plate was incubated at 37°C for 30 minutes. Samples were measured spectrometrically at 562nm. Immunoprecipitation A total of 300|ig of protein was used for immunoprecipitation. To investigate the phosphorylation of Akt, two microliters of the Phospho-AKT (Ser 473) antibody (Cell Signaling) was added and to investigate the O-GlcNAc modified proteins, two microliters of the anti-O-P-GlcNAc specific monoclonal antibody (CTD 110.6) (Pierce) was added and incubated overnight at 4°C on a shaker. In the morning a 50% agarose beads (Upstate) slurry was prepared by washing it 3 times with PBS and collecting the pellet after each wash. An equal amount of PBS was added to the pellet and the 50% slurry was prepared. For each sample, 30(il of slurry was added and then incubated for 4hrs at 4°c on a shaker. Samples were then centrifiiged at 5,000-6,000 rpm for 30 seconds and the pellet 42 was collected. The pellet was then washed an additional 3 times with PBS. To each of the samples, 25|ul of 3X sodium dodecyl sulfate (SDS) sample buffer (Cell Signaling) and ImM DTT (final concentration) was added. The samples were again centrifiiged at 14,000rpm for 2 minutes to break agarose beads. The supernatant was collected and heated for 5minutes at 90°C. After this sample were cooled on ice and stored at -20°C for further use. Western blot analysis Twenty microliters of immunoprecipitated sample was subjected to electrophoresis in 10% Tris- Glycine gels at lOOv for 90 minutes with a IX running buffer (25mM Trizma base, 192mM glycine, 0.1 % SDS). Five microliters of protein marker (Cell Signaling) was used and 2ul of sample buffer was loaded in the empty lanes. Proteins were then transferred to a PVDF membrane (Immobilin, Millipore, and CA). After blocking with 5% non-fat dry milk, the blots were incubated overnight at 4°C with diluted antibodies (1:1000) against Phospho-AKT (cell signaling) and (1:1000) against O-P-GlcNAc. Blots were washed three times with 0.1% Tween-20 in Tris-buffered saline (0.02M Tris base and 0.14 M NaCl in water, pH -7.6) and then blots were incubated with diluted (1:2000) horseradish peroxidase-conjugated anti rabbit IgG, and diluted (1:1000) anti-biotin antibody, for 1 hr at room temperature. The membranes were washes again as above, and the bands were detected by chemiluminescence (HRP-Western Detection Kit, Cell Signaling Technology, Inc). The membrane was then exposed to Kodak X-OMAT autoradiography film and exposure time was varied depending on the intensity. Blots were quantified using scanning densitometry (NIH Image). 43 LDH assay Lactate dehydrogenase, an intracellular enzyme released into the extracellular environment when the cell membrane is compromised (Rae 1977) was assayed using a spectrophotometric method (Wroblewski 1955). After treatment with glucosamine for 18hrs, media was removed from the control as well as treated plates and assayed for lactate dehydrogenase leakage. For the assay, a stock solution of 0.1 M phosphate buffer, 0.02mM pyruvate and 14mM NADH was prepared. The final concentration in a 1ml assay was 83mM phosphate buffer, 0.67mM pyruvate and 0.23mM NADH. Two hundred microliters of the media from each plate was used for the assay. The reaction velocity was determined in a spectrometer by measuring a decrease in absorbance at 340nm resulting from the oxidation of NADH. Protein concentration in the media was measured using the BCA Protein Assay kit and units of enzyme activity were then calculated. RNA isolation Total RNA was isolated using TRIzol reagent (TRIzol reagent, Invitrogen). Cells were lysed using 1ml of TRIzol/60mm dish. The cell suspension was incubated at room temperature for 5minutes to dissociate nucleoprotein complexes. Chloroform (0.2ml per lml TRIzol) was then added to the cell suspension and vigorously shaken for 15 seconds which was then followed by incubation for 3 minutes at room temperature and then centrifiiged for 15 minutes (13400 xg @ 4°C). Following centrifugation, the mixture separates into lower red phenol chloroform phase, an interphase, and a colorless upper aqueous phase that contains the RNA. The upper aqueous phase was transferred to a fresh 44 eppendorf tube. The volume of aqueous phase is about 60%> of the volume of TRIzol used for cell processing. RNA is then precipitated from the aqueous phase with isopropyl alcohol (0.5ml per 1.0 ml TRIzol reagent used). After incubation at room temperature for 10 minutes, samples were centrifiiged for 10 minutes (13400 xg @4°C). The RNA precipitate forms a gel like pellet on the side and bottom of eppendorf tube. After removing the supernatant, RNA pellet was then washed with 75% ethanol (1ml per 1ml TRIzol/60mm dish used).The RNA pellet was again centrifiiged (3300 xg @ 4°C) for 5 minutes. The gel like RNA pellet at the bottom was dissolved in diethyl pyrocarbonate (DEPC) treated water. RNA samples were then analyzed for both concentration and quality using spectrophotometer. Determination of RNA content and quality assessment For quantification of the RNA isolated, spectrophotometer readings at 260 nm and 280 nm were taken. The readings at 260 nm give the concentration of nucleic acid in the sample. The readings at 280 nm give the amount of protein in the sample. The isolated RNA is of high quality has an OD 260/OD 280 value between 1.8 to 2.0. For quantification of sample, 2ul of sample was diluted with 998ul DEPC water. The RNA concentration read was then calculated by using the following formula: OD 260* 40ng/ul * dilution factor. 1 O.D (optical density) at 260nm for RNA = 40ng/|nl of RNA 45 Reverse transcriptase polymerase chain reaction (RT PCR) Two step real time quantitative PCR (qRT PCR) was carried out to evaluate the changes in gene expression. All the reagents, kits and target gene probes were purchased from Applied Biosystem (ABI). First 150ng total RNA was reverse transcribed into cDNA on a thermocycler (Eppendorf Master Cycler gradient) using a Taqman Reverse Transcription Reagent kit containing Taqman buffer, magnesium chloride, random hexamers, dNTPs, RNase inhibitor and reverse transcriptase (product # N8080234, ABI). The quality of cDNA was checked by agarose gel electrophoresis before the second step of PCR amplification. The second step was performed on the Step One Plus Real time PCR system (Applied Biosystems). Real time PCR reaction was carried out in a 96-well plate containing 12.5ul of 2X Taqman Universal PCR Master Mix, 1.25ul of 20X Taqman Gene expression Assays-containing specific PCR primers and FAM Tm dyelabeled probes, cDNA obtained from the first strand synthesis reaction and water to a final reaction volume of 25|il. After an initial incubation step for 2 minutes at 50°C and denaturation for 10 minutes at 95°C, qRT-PCR was carried out using 40 cycles of PCR (95°C for 15s. 60°C for 60s), p actin was used as reference gene. Primer/probe set for the genes studied was commercially obtained from Applied Biosystems (Table 3). 46 Table 3: Primers for RT-PCR Assay ID Gene Glucose 6 phosphate dehydrogenase Fatty acid synthase Rn00569117_ml Phosphoenolpyruvate carboxy kinase Rn01529009_gl Forkhead box A2 (Foxa2) Rn00562517_ml Isolation and purification of O-GlcNAcvlated proteins Wheat germ agglutinin lectin affinity chromatography To Isolate and purify O-GlcNAcylated proteins from whole cell lysate, we used glycoprotein isolation kit (Pierce). The sample containing 1 to 1.5 mg of total protein was diluted (4:1) with 5x Binding/wash buffer solution. Then Wheat germ agglutinin (McGarry, Kuwajima et al.) column was prepared by transferring 200JLL1 of 50% resin slurry to the column provided. The column was centrifiiged for 1 minutes at 1000 x g, to discard the storage buffer and to pack the column tightly. The column was washed twice with 200JLX1 IX Binding/Wash buffer by centrifuging the column for 1 minute at 1000 x g. This step was performed to equilibrate the column with binding buffer. Then samples were added and the column was incubated for 10 minutes at room temperature with end -over-end mixing using a rotator. After incubation, the column was washed again thrice with 400jil IX Binding/Wash Buffer, to remove any unbounded 47 proteins. Then 200|LI1 of elution buffer was added to the column and incubated for 20 minutes at room temperature with end-over-end mixing using a rotator. After centrifugation for 1 minute at 1000 x g, the eluate was collected in the collection tubes and stored on ice for immediate use or kept at -80°C for later use. SDS-PAGE (sodium dodecyl polyacrylamide gel electrophoresis) A total of 25|ng of eluted proteins from the wheat germ affinity chromatography was subjected to electrophoresis in 10% Tris-Glycine gels at lOOv for 90 minutes with a IX running buffer (Trizma base, glycine, SDS). Before subjecting the samples to electrophoresis, 3X SDS sample buffer (cell signaling) was added and the samples were heated for 5minutes at 90°C. Five microliters of prestained protein marker (Cell Signaling) was used as a standard. After the run was complete, proteins were visualized by silver staining. Identification of O-GlcNAc proteins by LC-MS analysis After purification, protein samples were submitted to the Mass Spectrometry and Proteomics Lab at the Van Andel Research Institute, directed by Greg Cavey. Statistical Analysis The results were expressed as the mean ±SEM of N number of animals used for each experiment. The comparisons within groups were performed with one way analysis of variance (ANOVA) followed by Student-Newman-Keuls test. Statistical significance was tested at p<0.05. 48 CHAPTER 3 RESULTS The first direct evidence supporting a link between flux through the hexosamine biosynthetic pathway (HBP) and the development of insulin resistance came from in vitro studies done in rat adipocytes (Marshall, Bacote et al. 1991). Since then, multiple in vitro and in vivo studies have shown that chronic elevated flux through the HBP may represent one mechanism by which hyperglycemia can lead to insulin resistance. There are three routinely used experimental approaches to evaluate the roles of hexosamine flux in the development of insulin resistance: 1) exposure to chronic hyperglycemia 2) exposure to glucosamine and 3) perturbing GFAT activity. We have used glucosamine to induce insulin resistance in primary rat hepatocytes. One of the molecular mechanisms responsible for the insulin resistant condition in type 2 diabetes is caused by alterations at one or several levels of the insulin-signaling cascade in the insulin sensitive tissues, skeletal muscles, adipose and liver. Previous studies in various other cell models have shown the involvement of the PI3-kinase pathway in insulin resistance is mediated by the increased flux in HBP (Kim, Nikoulina et al. 1999; Patti, Virkamaki et al. 1999). In adipocytes and pancreatic P cells, insulin resistance is mediated by the impaired activation of Akt caused by reduced insulin 49 stimulated phosphorylation of Akt (Vosseller, Wells et al. 2002; Parker, Lund et al. 2003; DAlessandris, Andreozzi et al. 2004; Park, Ryu et al. 2005). However, contrary to the adipocytes and pancreatic P cells studies, Akt phosphorylation in the skeletal muscle was not significantly altered (Arias, Kim et al. 2004). These contradictory results in these insulin sensitive tissues led us to further investigate the phosphorylation of Akt in primary rat hepatocytes. Hence, this was the first target for the investigation. Effect of glucosamine on insulin induced Akt phosphorylation To study the effect of glucosamine on insulin induced Akt phosphorylation, primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs, followed by media change with low glucose media and then treatment with 80nM insulin for 1 hr. The conditions of ImM glucosamine and 18hrs of incubation were established after treating the cells with varying concentrations over different lengths of time. After treatment, cells were lysed and the phosphorylated Akt was immunoprecipitated using phospho-Akt (Ser 473) antibodies and western analysis performed. The film was quantified with densitometry using NIH software. The results show insulin treatment increased the phosphorylation of Akt 7 fold as compared with the basal level in control, whereas the insulin stimulated phosphorylation of Akt was drastically decreased 4 fold in the presence of glucosamine when compared to insulin treated phosphorylation of Akt in the control (Figure 4). Glucosamine by itself did not induce phosphorylation of Akt. These results indicate that one of the mechanisms through 50 which glucosamine interferes with insulin signaling is through inhibiting insulin stimulated Akt phosphorylation at Ser-307 in primary rat hepatocytes. As mentioned before, insulin, after binding to its receptor, regulates many cellular processes and the expression of several genes through the activation of various signaling proteins and pathways. If the activation of the signaling proteins is affected, then gene expression should also be altered. Insulin regulates the transcription of the genes for several metabolic enzymes: Glycogen synthase (Sutherland, Leighton et al. 1993), phosphoenolpyruvate carboxylase (PEPCK) (Forest, O'Brien et al. 1990), fatty acid synthase (FAS) (Moustaid, Beyer et al. 1994) and glucose 6 phosphate dehydrogenase (G6PDH) are rate limiting enzymes in glycogen synthesis, gluconeogenesis, fatty acid biosynthesis and pentose phosphate pathway respectively, and have been the target of studies with regard to action of insulin. Therefore, our next objective was to examine the effect of glucosamine on the insulin regulation of the expression of key metabolic enzymes. Effect of glucosamine on insulin induced G6PDH gene expression G6PDH is a lipogenic enzyme that catalyses the first reaction in the pentose phosphate pathway, a metabolic pathway that supplies reducing energy to cells by maintaining the level of the co-enzyme nicotinaminde adenine dinucleotide phosphate (NADPH), which is required by fatty acid synthase for de novo lipogenesis. Early studies in our lab using diabetic animals showed that G6PDH expression was regulated by insulin (Berg, Wu et al. 1995). To understand the mechanism by which insulin and/or carbohydrate regulates the expression of this gene, the DNA sequence was cloned for the 51 promoter region of the gene (Rank, Harris et al. 1994). Using this promoter sequence cloned into a reporter gene, as well as by measuring endogenous mRNA levels, we showed that G6PDH expression was regulated transcriptionally by insulin (Wagle, Jivraj et al. 1998). To investigate the effect of glucosamine on the insulin induced expression of G6PDH, primary rat hepatocytes were incubated in DMEM low glucose media with or without glucosamine for 18hrs along with or without 44nM insulin for 18hrs. The cells were then processed and the G6PDH mRNA was measured using qRT PCR. These results show that insulin significantly increased the gene expression of G6PDH by 2 fold when compared to control and these results are comparable to our previous reports using northern analysis (Figure 5). Insulin stimulated expression of G6PDH under glucosamine treatment decreased significantly compared to insulin stimulated G6PDH gene expression in the control. In fact the levels measured were comparable to basal control (Figure 5), suggesting that insulin was no longer able to regulate G6PDH expression. This decrease in the insulin induced gene expression of G6PDH under glucosamine treatment is significant metabolically since the pentose phosphate pathway is responsible for most of the NADPH production and NADPH is needed not only for fatty acid synthesis but other reductive biosynthetic processes such as nucleic acid synthesis. 52 NA B Ins 9.00 8.00 Gin +lns Gin ^r - ~~ < 7,00 + . ~ ~ 3 6.00 £ 5.00 | 4 A ^0 & £ 3.00 A J 2 00 5 2 ioo 0.00 + ! I § NA ins ! i • '— Gin Gin* Ins Figure 4: Effect of glucosamine on insulin induced Akt phosphorylation. A. Representative western blot showing phospho-Akt (Ser-473). B. Quantitation of Phospho-Akt blots. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs followed by media change with low glucose media and then treated with insulin at 80nM for 1 hr. The cells were lysed and processed and immunoprecipitated using Phospho-AKT (ser 473) antibodies. Western blot was run with samples and blots were quantified. Data represent the mean +/- S.E.M (n=4), "n" is the number of animals used. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). tUf = Significant increase compared to NA. /V= Significant decrease compared to insulin induced Akt phosphorylation in control. NANo addition, Ins- Insulin, Gin- Glucosamine, Gln+Ins- Glucosamine +Insulin. 53 2.50 • T 1 2.00 1.50 <U JU. A3 Q. E o i T> 1.00 u <u QuO C A T I cz JO 0.50 ^ 0.00 I I NA Ins - i Gin Gln+lns Figure 5: Effect of glucosamine on G6PDH gene expression. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs along with or without insulin at 44nM for 18hrs. The cells were then processed and the G6PDH mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=4), "n" is the number of animals used. Amount of G6PDH mRNA was normalized to control probe, P-actin. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05).). All the data in the figures are presented as fold changes compared to the control (NA).*= Significant increase compared to NA. A = Significant decrease compared to insulin induced G6PDH. NA- No addition, Ins- Insulin, GlnGlucosamine, Gln+lns- Glucosamine +Insulin. 54 Effect of glucosamine on insulin induced fatty acid synthase (FAS) FAS synthesizes long-chain fatty acids by using acetyl coenzyme A (CoA) as a primer, malonyl-CoA as a 2-carbon donor, and NADPH as a reducing equivalent. Insulin is considered as a positive regulator of fatty acid synthesis by increasing fatty acid synthase (FAS) mRNA transcription (Moustaid, Beyer et al. 1994). The effect of insulin on FAS transcription is mediated by the activation of the phosphoinositide (PI) 3kinase/Akt pathway (Wang and Sul 1998). These results show that insulin significantly increased the expression of FAS by 1.6 fold (Figure 6) when compared to control, which is comparable to the published reports (Fukuda, Katsurada et al. 1992). On the other hand, insulin stimulated gene expression of FAS under glucosamine treatment decreased significantly compared to insulin stimulated FAS gene expression in control. This indicates that glucosamine inhibits insulin stimulated FAS gene expression. 55 24 < 2: 2.1 o TJ <X) u. re Q. b o u 0) w> c u TJ O u_ 1.8 1 * T 1 1.5 i * .. _L. 1.2 ± 0.9 0.6 0.3 1 0 NA 1 " Ins 1 » Gin 1 1 Gln+lns Figure 6: Effect of glucosamine on FAS gene expression. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs along with or without 44nM insulin for 18hrs. The cells were then processed and the FAS mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=5), "n" is the number of animals used. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). All the data in the figure are presented as fold changes compared to the control (NA). *k= Significant increase compared to NA. A = Significant decrease compared to insulin induced FAS gene expression. NA- No addition, Ins- Insulin, GlnGlucosamine, Gln+lns- Glucosamine +Insulin. Effect of glucosamine on insulin inhibition of PEPCK gene expression Phosphoenolpyruvate carboxylase catalyzes the conversion of oxaloacetate to phosphoenolpyruvate, which is the rate limiting step in gluconeogenesis. Gluconeogenesis plays a key role in diabetes as it is the process by which the liver makes 56 glucose. The hepatic PEPCK gene is regulated at the transcriptional level by a variety of hormones (Forest, O'Brien et al. 1990; Hall, Scott et al. 1992) including glucocorticoids (GC), retinoic acid (RA), thyroid hormone, and glucagon (acting through cyclic AMP) which increase the rate of synthesis of the enzyme (Sasaki, Cripe et al. 1984; Lucas, O'Brien et al. 1991; Park, Jerden et al. 1995; Scott, Stromstedt et al. 1998). Insulin exerts an opposite, dominant negative effect (O'Brien, Lucas et al. 1990) primarily through inhibiting the initiation of hepatic PEPCK gene transcription but also reducing the rate of transcript elongation. In H4IIE cells, the inhibitory effects of insulin are dominant, since it prevents cAMP and glucocorticoid mediated increases to PEPCK gene transcription. In this experiment, cAMP was used to induce the gene expression of PEPCK. It was found that the addition of 500uM of cAMP for 1 hr in the control group caused an increase in PEPCK gene expression by 27 fold (Figure 7), which was comparable to previous studies (Beale, Andreone et al. 1984; Imai, Miner et al. 1993). Following treatment of insulin after cAMP a 25% decrease was observed in the cAMP induced gene expression. In cells treated with glucosamine, cAMP still caused an increase in the expression of PEPCK by 23 fold, results comparable to those observed without glucosamine. As expected, the inhibition of cAMP induced PEPCK expression by insulin was not observed in the glucosamine treated cells (Figure 7). 57 35 ++ * < 30 z o "2 25 A (0 r¥ a I 20 o a> S 15 s: o 1 10 IX. 1I1 & N<r J f a? &* r^-i # <^ J o^ J &« Figure 7: Effect of glucosamine on PEPCK gene expression. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs in the presence or absence of 44nM insulin and 500uM cAMP for lhr. The cells were then processed and the PEPCK mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=8), "n" is the number of animals used. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). * = Significant increase compared to NA. A = Significant decrease compared to insulin, it it = no significance. NA- No addition, Ins- Insulin, Gin- Glucosamine, Gln+lns- Glucosamine +Insulin, cAMP- Cyclic adenosine monophosphate. 58 Effect of glucosamine on insulin regulation of Foxa2 The transcriptional regulation of the gene encoding PEPCK involves complex interactions of a variety of transcription factors and other proteins. The PEPCK promoter contains an insulin response sequence (IRS). This sequence has been functionally characterized, and the transcription factors binding to this region have been studied. The PEPCK promoter has hepatic nuclear factor-3 (HNF-3 also known as Foxa) binding sites, and members of the Foxa family are involved in the glucocorticoid-stimulated expression of PEPCK. Furthermore, in the PEPCK promoter, an HNF-3 binding site is located in close proximity to the IRS with a partial overlap of both structures. Therefore, HNF-3 has also been discussed as a transcription factor that may mediate insulin's effect on the PEPCK promoter. So our next objective was to examine whether or not the insulin mediated reduction in PEPCK expression could be attributed to reduced levels of Foxa2 (HNF-3P) and if so what effect it would have. An approximately 40% decrease in Foxa2 expression was observed following treatment of insulin after cAMP. Glucosamine did not affect Foxa2 expression or alter the effect of cAMP (Figure 8). With glucosamine treatment, there was no significant difference between cAMP induced Foxa2 gene expression with or without insulin. This result shows that under glucosamine treatment insulin no longer was able to inhibit the induction of cAMP induced gene expression of Foxa2. 59 •• ^2.5 v~ (S Q. o D) §1.5 O s 1 U S0.5 ^ ^ / &* & / V Figure 8: Effect of glucosamine on Foxa2 gene expression. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs along with or without 500uM for lhr followed by insulin at 44nM at for lhr. The cells were then processed and the Foxa2 mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=5), "n" is the number of animals used. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). it = Significant increase compared to NA. A = Significant decrease compared to insulin. ^ ^ = no significance. 60 Effect of selenium on Akt phosphorylation Selenium, an essential trace element, has been shown to mimic some of insulin's actions both in vivo and in vitro. To determine whether selenium can act as an antidiabetic agent in this model system, the effect of selenium on Akt phosphorylation was first investigated. If selenium truly acts as a mimetic, then an increase in phosphorylation of Akt with selenium should occur. Our results show that selenium treatment increased the phosphorylation of Akt by 13 fold as compared to the basal level in control (Figure 9). Selenium induced phosphorylation of Akt is unaffected in the presence of glucosamine unlike what was observed with the quenching effect of glucosamine on the insulin induced phosphorylation of Akt. This suggests that selenium can act as a potent insulin - mimetic not only under normal/ control conditions but also under insulin resistant condition as well. NA Se Gin 61 Gln+Se B Figure 9. Effect of glucosamine on selenium induced Akt phosphorylation. A. Representative western blot showing phospho-Akt (Ser-473). B. Quantitation of phospho-Akt blots. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs followed by media change with low glucose media and then treated with selenium at 500uM for 3 hr. The cells were lysed and processed and immunoprecipitated using Phospho-AKT (Ser 473) antibodies. Western blot was run with samples and blots were quantified. Data represent the mean +/S.E.M (n=4), "n" is the number of animals used.. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). ic = Significant increase compared to NA. itit= no significant difference between selenium and glucosamine + selenium. 62 The next objective of the study was to investigate whether or not similar selenium effect could be found on the expression of key metabolic enzymes in glucosamine induced insulin resistance. Our lab had shown that like insulin selenium can affect the expression of two key lipogenic enzymes, G6PDH and FAS. So we chose these two metabolic enzymes as our first targets for study. Effect of selenium on G6PDH gene expression Our results show that selenium induces the gene expression of G6PDH by approximate 1.5 fold at 6hr respectively. Unlike our findings with insulin in the presence of glucosamine, selenium induction of G6PDH was similar in the presence or absence of glucosamine (Figure 10). 63 < 1.80 z 1.60 o 4-i •o 9> Q. 1.40 1.20 I * • * JL I T- •• E 1.00 o o 0.80 0.60 C ** •• HEn.... 1 rn -±- ri-| i X I 03 O 0.40 73 O LL 0.20 0.00 1 NA Se-1hr Se-3hr Se-6hr Gin 1 Gln+Se1hr Gln+Se3hr Gin+Se6hr Figure 10: Effect of selenium on G6PDH gene expression. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs along with or without Selenium at 500uM concentration at various time period ( 1, 3, 6 hrs). The cells were then processed and the G6PDH mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=4), "n" is the number of animals used.. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). All the data in the figures are presented as fold changes compared to the control (NA). it = significant increase compared to NA.^Ht = No significant induction of G6PDH in the presence or absence of glucosamine. NA- No addition, Se-Selenium, Gln-Glucosamine, Gln+seGlucosamine +Se Effect of selenium on FAS gene expression The results show that selenium induces the gene expression of FAS by 1.32 fold at 6hr (Figure 11). There was no significant difference between selenium induced FAS gene expression under glucosamine treatment compared to the selenium induced FAS gene expression under control. Similar to the findings with G6PDH, the expression of FAS is still responsive to selenium even when glucosamine is present. 64 Figure 11: Effect of selenium on FAS gene expression. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs along with or without Selenium at 500uM concentration at various time period ( 1, 3, 6 hrs). The cells were then processed and the G6PDH mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=4) "n" is the number of animals used. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). All the data in the figures are presented as fold changes compared to the control (NA). it = significant increase compared to NA.^H^r = No significant difference in selenium induction of FAS in the presence or absence of glucosamine. C-NA- No addition, C-Se- Control selenium, GlnNA -Glucosamine No addition, Gln-Se. Effect of selenium on PEPCK gene expression As mentioned before, gluconeogenesis plays a key role in diabetes as it is the process by which the liver makes glucose. We therefore examined expression of PEPCK, the key enzyme of this pathway. In these studies, cAMP was used to induce the gene 65 expression of PEPCK. It was found that addition of 500uM of cAMP for 1 hr in control group caused an increase in PEPCK gene expression by 27 fold (Figure 12). Selenium treatment decreased cAMP induced gene expression of PEPCK significantly and expression was near basal level by 6hr. Again, like with FAS and G6PDH, selenium had similar effects on the expression of PEPCK in the presence of glucosamine suggesting its actions are not sensitive to glucosamine. 66 35 00 30 00 I • 25 00 < z o g 20 00 l re c £ o o |» <c i> o o "* * * 15 00 10 00 • HE- 1 fl J 5 00 n ^ 0 00 , r-i , r-i , r~1 , I a? ^ c? cf>* • r*i , n Jf J?Jf frJ& J?J* <b« 8 -$ ^ ^ ,/ *T & ^ ' c? 4? r& , ri , r*i , 1 of ^ c? C& <3* C** ? Figure 12: Effect of selenium on PEPCK gene transcription. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs along with or without selenium for various time period (1, 3, 6 hrs) at 500uM followed with 500uM cAMP for lhr. The cells were then processed and the PEPCK mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=5), "n" is the number of animals used. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). * = significant increase compared to NA. irk = No significant difference in cAMP induced selenium induction of PEPCK in the presence or absence of glucosamine. • = significant decrease compared to cAMP induced PEPCK gene expression. NA- No addition, Ins- Insulin, Gin- Glucosamine, cAMPcyclic AMP. 67 Effect of selenium on Foxa2 gene expression Our result shows that the addition of 500uM of cAMP for 1 hr in control group caused an increase in Foxa2 gene expression by approximately 2.3 fold. Selenium treatment decreased cAMP induced gene expression of Foxa2 significantly compared to cAMP induced gene expression of Foxa2. While under glucosamine treatment, cAMP did increase the expression of Foxa2 by 2.5 fold. Selenium treatment under insulin resistant state could inhibit the induction of cAMP induced gene expression of Foxa2. Again, like with FAS, G6PDH, PEPCK, selenium had similar effects on the expression of Foxa2 in the presence of glucosamine suggesting its actions are not sensitive to glucosamine (Figure: 13). 68 3 00 • •• 2.50- 2.00 • 1.50 • £ 1.00 • **l * 0.50 - A A J3L 0.00 • ^ ^ cp ^ cf> ^ & j£ <T ** *P J? <f Ji? ^ ft f*l ^ (^ J? ^ ^ c? ^ Ji? ^ c? r^ c? r& ,j£ JUL ^ ^ xO c? cf> <f <? & 0* t? C$> /J&' 4 ~ & £~ & «v Figure 13: Effect of selenium on Foxa2 gene transcription. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs along with or without selenium for various time period (1, 3, 6 hrs) at 500uM followed with 500uM cAMP for lhr. The cells were then processed and the Foxa2 mRNA was measured using qRT PCR. Data represent the mean +/- S.E.M (n=5). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Student -Newman Keuls test (PO.05). i* = significant increase compared to NA. irk= No significant difference in cAMP induced selenium induction of PEPCK in the presence or absence of glucosamine. 0 = significant decrease compared to cAMP induced PEPCK gene expression. NA- No addition, Se-Selenium, Gin- Glucosamine, cAMP- cyclic AMP. 69 Lactate dehydrogenase assay Lactate dehydrogenase is a cytosolic enzyme present within all mammalian cells. The normal plasma membrane is impermeable to LDH, but damage to the cell membrane results in a change in the membrane permeability and subsequent leakage of LDH into the extracellular fluid (Rae 1977). In vitro release of LDH from cells provides an accurate measure of cell membrane integrity and cell viability. As a result, the release of lactate dehydrogenase has proved to be a reliable test for cytotoxicity. In order to assess whether or not there was any effect of glucosamine on cell viability, primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs. The release of LDH into the culture supernatant correlates with the amount of cell death and membrane damage, providing an accurate measure of the cellular toxicity induced. We used the direct spectrophotometric assay (Wroblewski and Ladue 1955) which is based upon the ability of LDH to catalyze the reaction: Lactate + NAD > Pyruvate + NADH Changes in optical absorbance, measured at 340nm, reflect changes in the concentration of NADH and hence the level of LDH in the sample. The result shows that there is no significant difference in glucosamine treated cells versus control which suggests the cells are not compromised with glucosamine treatment (Figure 14). 70 zuu / lou 2 CO p C -£= CD 05 O C O O 100 ^ /-/ ^^" -^ - ^ W^Wi 1 W!$m CD •o 303 Wmlm • ^ WlmiM p^.n OU n- i Control jmRrmm Glucosamine Figure 14 Effect of glucosamine on lactate dehydrogenase release into the media Results are expressed as percent of LDH activity in the media from control Data represent the mean ±SEM (n=3), Statistical significance was tested at pO05 Effect of glucosamine on O-GlcNAc modification Protein modification through post-translational events like phosphorylation, acylation or glycosylation can dramatically alter a proteins activity Since glucosamine increases the flux through the HBP and O-GlcNAc is a product, a possible consequence is increased O-GlcNAc modified proteins We first examined O-GlcNAc modified 71 proteins were increased using western blot. For this, primary rat hepatocytes were incubated in DMEM low glucose media with or without imM glucosamine for i8hrs in the presence or absence of 44nM insulin for lhr. After treatment, cells were lysed and lysate was collected for each sample. The sample was then immunoprecipitated using O-GlcNAc specific monoclonal antibody 110.6 and subjected to electrophoresis. Proteins were transferred onto the membrane and the membrane was exposed to the film. The results indicate that the glucosamine and glucosamine plus insulin treatments showed an increase in proteins that are O-GlcNAcylated compared to control (Figure 15). Since more proteins are modified, it is attractive to speculate that this modification of key proteins is linked to the induction of insulin resistance. 72 NA Ins Gin Gln+lns 200 140 100 W 60 SO 40 30 20 ure 15: Effect of glucosamine on O-GlcNAc modification. Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs followed by media change with low glucose media and then treated with 44nM insulin for 1 hr. The cells were lysed and processed and immunoprecipitated using O-GlcNAc specific monoclonal antibody 110.6 and subjected to electrophoresis. Proteins were transferred onto the membrane and the membrane was exposed to the film. 73 Identification of O-GlcNAc modified proteins by LC-MS We observed O-GlcNAc modified proteins were increased in the presence of glucosamine using western blot analysis. The next goal was to identify the OGlcNAcylate- modified proteins. For this study primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs in the presence or absence of 44nM insulin for lhr or 500uM selenium for 3hr. O-GlcNAcylated proteins were isolated and purified using Wheat Germ Agglutinin (McGarry, Kuwajima et al.) column chromatography. Purified proteins were then subjected to SDS PAGE and proteins were visualized by silver staining (Figure 16). 74 ^fggg Figure 16 Representative SDS-P AGE of O-GlcNAcylated proteins Primary rat hepatocytes were incubated in DMEM low glucose media with or without ImM glucosamine for 18hrs in the presence or absence of 44nM insulin for lhr or 500uM selenium for 3hr After treatments, cells were lysed and lysate was collected for each sample O-GlcNAcylated proteins were isolated and purified proteins were then subjected to SDS PAGE and proteins were visualized by silver staining Lane 1 -Marker, Lane 3- C-NA (control-No addition), Lane 4- C-Ins (control msulm), Lane 5- Gln-NA (GlucosammeNo addition), Lane 6- Gin-Ins (Glucosamine- Insulin), Lane 7 - C-Se (Control- selenium), Lane 8- Gln-Se (Glucosamine -selenium) After purification of O-GlcNAcylated proteins, samples were submitted to the Mass Spectrometry and Proteomics Lab at the Van Andel Research Institute for LC-MS 75 analysis for label free quantitative protein profiling. This system combines a quadrupole time-of-flight Premier mass spectrometer (Qtof-P) with a NanoAcquity ultra-high pressure liquid chromatography (UPLC) system for nanoscale separations with on-line electrospray ionization. The system represents a paradigm shift in proteomics because it employs a novel acquisition method termed LC-MS. Complex protein samples are digested into peptides using trypsin (or other enzymes) and without chemical or stable isotope labeling (label-free) peptides are analyzed to obtain both quantitative and qualitative data in a single LC-MS run. Throughout an LC-MS analysis, the Qtof-P mass spectrometer alternates the collision energy every 1 second in a high-low manner such that peptide mass, retention time, and intensity are recorded during low collision energy acquisition and peptide fragmentation is generated with high collision energy. Peptide mass and retention time are used to compare across samples while peptide intensity recorded by the mass spectrometer is used for both relative and absolute quantitation as described by Silva (Silva, Denny et al. 2005; Silva, Gorenstein et al. 2006). High collision energy acquisition generates peptide fragmentation data used to identify the protein from which the peptide originated. However, unlike all other mass spectrometers that perform conventional MS/MS data acquisition for protein identification and quantification one peptide at a time, the Qtof-P does not filter peptide ions at a given m/z value prior to fragmentation. Instead, all ions transmitting the Qtof-P, including coeluting peptides, are fragmented simultaneously, hence the term LC-MS. Using innovative software called Identity, peptide fragments within a mixture of fragment ions from many different peptides can be assigned to the parent peptide. This approach relies on the simple premise that peptide fragments generated from a given peptide in the high76 energy mode will have the same shape and retention time as its parent peptide recorded in the low energy mode. Once fragments are associated with a given peptide, the data is used to identify the protein from which the peptide originated. While the methodology described above should yield both qualitative and quantitative data in one run, attempts to receive quantitative analysis between control and experimental group did not succeed. For unexplained reasons, before trypsin digestion the amount of protein quantified was sufficient to carry out the experiments but after trypsin digestion the protein amount was too low. The digestion procedure was attempted on several sample sets and similar results were obtained. While sufficient protein was not available for quantitative analysis, a qualitative identification of protein O-GlcNAc modified was made. Since our attempt to perform quantitative analysis did not succeed, the data that was generated were analyzed comparatively. This comparative analysis led us to identify variations in glycosylation based on treatment. The following table lists the glycosylated proteins that were uniquely found in the glucosamine treated cells compared to the control, untreated cells (Table 4). 77 Table 4: O-GlcNAc modified proteins found uniquely in the glucosamine treatment as compared to control (no addition). 1 Functional Subgroup 1 Cytoskeleton 1 Actin based Intermediate filaments Microtubule based Chaperones Oxido reductase Process Accession Protein IPI00373136 IPI00365944 IPI00207014 Ankyrin repeat domain 5 Myosin light polypeptide 6 Keratin type I cytoskeletal 19 IPI00370073 IIPI00370090 IIPI00371003 IPI00421791 IIPI00421793 IIPI00421795 IIPI00421855 IIPI00454479 IPI00551558 IPI00565487 IPI00882425 IPIOO188666 IPI00471523 Keratin type I cytoskeletal 17 Keratin type I cytoskeletal 15 Keratin type I cytoskeletal 25 Keratin type I cytoskeletal 42 Type I keratin KA16 Keratin type I cytoskeletal 13 Keratin type I cytoskeletal 14 Type I keratin KA11 Keratin type II cytoskeletal 2 epidermal Keratin type I cytoskeletal 12 Keratin K6 Fragment Keratin type I cytoskeletal 27 Tubulin alpha 3 chain IPIOO189795 IPI00339167 IPI00364046 IPIOO199636 IPI00211100 Tubulin alpha 1A chain Tubulin alpha IB chain Tubulin alpha 1C chain Calnexin 3 alpha hydroxysteroid dehydrogenase IPIOO197770 IPI00205018 Aldehyde dehydrogenase mitochondrial Methylmalonate semialdehyde dehydrogenase acylating mitochondrial MHC class I RT 1 Ac heavy chain MHC class I RT1 Au heavy chain MHC class IRT1 Au heavy chain Fragment Mature alpha chain of major histocompatibility complex class I antigen Fragment Annexin A5 Basic transcription factor 3 Myoglobin Eukaryotic translation elongation factor 1 delta Immune response IPI00551631 IPI00855239 IPI00551567 IPI00896766 Apoptosis IPI00471889 IPI00372004 IPI00214517 IPI00471525 Heme binding Elongation factor 78 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 | Table 4: Continued 1 Growth factor activity Metal binding Cell diviion Protease Other IPI00204130 Hepatoma derived growth factor related protein 3 IPIOO195036 IPI00231107 IPI00210009 IPIOO191748 IPI00763910 IPI00765011 IPI00566672 IPI00210090 IPI111 111 13 IPI00365582 Metallothionein 2 Parathymosin Nuclear migration protein nudC Proteasome subunit alpha type 1 Similar to 60 kDa heat shock protein mitochondrial precursor Similar to Actin cytoplasmic 2 Similar to heat shock protein 8 SP120 Serum albumin precursor Allergen Bos d 6 1 Carbamoyl phosphate synthetase 2 aspartate transcarbamylase and 1 dihydroorotase Isoform 2 of Basigin 1 Isoform 2 of Haptoglobin 1 IPIOO193425 IPI00382202 Surprisingly, the analysis of the proteins that were glycosylated under the glucosamine conditions did not reveal any modification to signaling proteins or metabolic enzymes but interestingly literature search on all of the above proteins shows that none of the above mentioned proteins have so far been identified to be glycosylated. So, to the best of my knowledge all the above mentioned proteins have been identified as glycosylated proteins for the first time in this study. Eventhough, the identified proteins did not reveal any modification to signaling proteins or metabolic enzymes, some functions were of interest. For example, 3 alpha hydroxysteroid dehydrogenase is an enzyme that catalyzes the following chemical reaction Androsterone + NAD (P) + ^5alpha-androstane-3, 17-dione + NAD (P) H + H+ 79 It belongs to the family of oxidoreductases, and participates in 3 metabolic pathways: bile acid biosynthesis, c21-steroid hormone metabolism and androgen and estrogen metabolism. The literature suggests that 3-a-hydroxysteroid dehydrogenase is involved in regeneration of L-Ascorbic acid (AA) in diabetic animals. L-Ascorbic acid is an antioxidant that is responsible for the scavenging of toxic free radicals in both plasma and tissues. AA is oxidized to monodehydroascorbic acid and dehydroascorbic acid (DHA). It has been reported that AA levels in plasma and tissues are lower than normal in diabetic animals (Will and Byers 1996). AA also increases the stability of blood vessels in patients with diabetes and a decrease in AA might contribute to the diabetic complications found at the late stages of the disease. The levels of AA are maintained in plasma and tissues by the intracellular reduction of an oxidized form of AA. This reductive regeneration is catalyzed by NADPH-dependant regenerating enzymes such as 3-a-hydroxysteroid dehydrogenase (AKR) and thioredoxin reductase (TR). The study carried out by Kashiba revealed that low AA levels in plasma and tissue resulting from a decrease in AA regeneration may be caused by a decrease in 3-a-hydroxysteroid dehydrogenase and this might increase oxidative stress in Goto - Kakizaki (GK) diabetic rats (Kashiba, Oka et al. 2000). Another protein modified is aldehyde dehydrogenase mitochondria (ALDH2) and is responsible for acetaldehyde oxidation in ethanol metabolism. It is involved in the oxidation and detoxification of aromatic and aliphatic aldehydes such as 4-hydroxy-2-nonenal (Bosron and Li 1986; Ohsawa, Nishimaki et al. 2003; Vasiliou and Nebert 2005). Association of aldehyde dehydrogenase mitochondrial with inheritance of non-insulin dependent diabetes mellitus (NIDDM) was shown by Matsuoka. In that study, they investigated the influence of the ALDH2 genotype on 80 diabetes. The preliminary study on Japanese patients with NIDDM suggests a relationship between alcohol intolerance and inheritance of diabetes (Suzuki, Muramatsu et al. 1996). Recent work carried out by Wang has shown that hyperglycemia-induced oxidative stress could reduce the activity and expression of ALDH2 in streptozotocininduced diabetic rat heart, and antioxidant could ameliorate these effects. They suggested that ALDH2 inhibition aggravates mitochondrial impairment in response to hyperglycemia, which could be the mechanism underlying left ventricle contractile dysfunction in diabetic rats. Further we compared the glucosamine plus insulin treatment to insulin only control. The following table lists the proteins that are uniquely glycosylated in the glucosamine plus insulin treatment compared to insulin only control (Table 5). 81 Table 5: O-GlcNAc modified proteins found uniquely in glucosamine plus insulin treatment compared to insulin 1 Functional Subgroup Accession Protein IPI00197888 Isoform 1 of Tropomyosin alpha 1 chain IPIOO187731 Isoform 2 of Tropomyosin beta chain IPI00207964 Isoform 4 of Tropomyosin alpha 1 chain IPI00365944 Myosin light polypeptide 6 IPI00370090 Keratin type I cytoskeletal 15 IPI00480679 Keratin type I cytoskeletal 18 IPI00207014 Keratin type I cytoskeletal 19 1 IPI00421802 Keratin type I cytoskeletal 40 1 IPI00421791 Keratin type I cytoskeletal 42 1 IPI00393340 Keratin type II cytoskeletal 6A 1 Microtubule based IPI00213299 Chaperones IPI00215463 Microtubule associated protein RP EB 1 family member 1 Calmodulin like protein 3 1 IPIOO196751 Heat shock 70 kDa protein 1A IB 1 IPI00870885 Cdk5 and Abl enzyme substrate 1 1 Elongation factor IPI00471525 Metal binding IPI00231107 Eukaryotic translation elongation factor 1 delta Parathymosin 1 IPI00230788.6 Carbonic anhydrase 3 1 Protease IPI00191748 Proteasome subunit alpha type 1 1 Other IPI00371266 Nascent polypeptide associated complex alpha polypeptide Predicted isoform CRA b | 1 Cyto skeleton 1 Actin based 1 Intermediate filaments 1 82 In order to assess whether or not selenium could potentially reverse or prevent the glycosylation of proteins that might be responsible for the glucosamine induced insulin resistance, the glycosylation of proteins with selenium treatment was evaluated. The following table lists the proteins that are found uniquely in glucosamine plus selenium treatment compared to selenium (Table 6). Table 6: O-GlcNAc modified proteins found uniquely in glucosamine plus selenium treatment compared to selenium Functional Subgroup Accession Protein Cyto skeleton Actin based IPI00360356 Actin beta like 2 IPI00454431 Brain specific alpha actinin 1 isoform IPI00231563 Isoform 3 of Tropomyosin alpha 1 chain IPI00421782 Keratin 75 IPI00421802 Keratin type I cytoskeletal 40 IPI00421857 Keratin type II cytoskeletal 1 IPI00421788 Keratin type II cytoskeletal 7 IPI00421784.1 Type II keratin Kb24 IPI00558057 Similar to keratin Kb40 Ligase IPIOO188989 Long chain fatty acid CoA ligase 1 Chaperones IPI00206660 DnaJ homolog subfamily C member 3 IPI00207355 Heat shock related 70 kDa protein 2 IPI00566672 Similar to heat shock protein 8 IPIOO197770 Aldehyde dehydrogenase mitochondrial IPI00369579 Isoform 1 of ERO1 like protein alpha Intermediate filaments Oxid reductase Process 83 Table 6: Continued 1 Immune response IPI00551567 MHC class I RT1 Au heavy chain Fragment 1 Initiation factor IPI00206152 Eukaryotic translation initiation factor 1A I Protease IPI00758468 Plasma glutamate carboxypeptidase IPIOO191748 Proteasome subunit alpha type 1 IPI00369093 Cytochrome b cl complex subunit 6 mitochondrial IPI00421517 Desmin IPI00231827 Nucleolin IPI00361827 Pgm2 protein IPIOO192140 Protein Niban IPI00363569 RAN binding protein 1 IPI00734740 Similar to 60S acidic ribosomal protein P2 1 IPI00559713 Similar to Ferritin light chain 2 1 IPIOO189682 Similar to Glycine cleavage system H protein 1 mitochondrial precursor IPI00362649 Similar to paladin IPI00368613 Similar to ring finger and KH domain containing 3 IPI00476033 Ubc protein IPI00207657 Ubiquitin specific peptidase 5 Other 1 1 After comparing the list of O-GlcNAc modified proteins uniquely present in the glucosamine plus insulin treatment with those uniquely found with glucosamine 84 treatment, we found the following proteins are present in both glucosamine and glucosamine plus insulin treatment (Table 7). Table 7: O-GlcNAc modified proteins found in both glucosamine and glucosamine plus insulin treatment Functional Subgroup Accession Protein Elongation factor IPI00471525 Eukaryotic translation elongation factor 1 delta Metal binding IPI00231107 Parathymosin Protease IPIOO191748 Proteasome subunit alpha type 1 Among the O-GlcNAcylated proteins under glucosamine selenium treatment, the following two proteins that are present in glucosamine plus insulin treatment are not present in glucosamine plus selenium treatment (Table 8). Table 8: O-GlcNAc modified proteins found uniquely in glucosamine plus insulin treatment but not present in glucosamine plus selenium treatment Functional Subgroup Accession Protein Elongation factor IPI00471525 Eukaryotic translation elongation factor 1 delta Metal binding IPI00231107 Parathymosin It is attractive to speculate that this glycosylation reversal facilitates in some way the regulation of key metabolic enzymes even in an insulin resistant state. 85 However, further investigation is required to show that selenium can decrease or reverse glucosamine induced O-GlcNAcylation. Functions of the identified O-GlcNAc modified proteins Eukaryotic translation elongation factor delta 1 encodes a subunit of the elongation factor-1 complex, which is responsible for the enzymatic delivery of aminoacyl tRNA to the ribose. This subunit functions as a guanine nucleotide exchange factor (Sanders, Brandsma et al. 1996). The next protein parathymosin is also called 11.5KDa zn 2+ binding protein (ZnBP) or macromolecular translocation inhibitor II (MTI-II). This protein is almost ubiquitous. It has the highest concentration in liver, brain, adrenal gland and smooth muscle and the lowest level in skeletal muscle. It inhibits the glycolytic enzyme phosphofructokinase-lin vitro. This inhibition is reversible and results from a dissociation of the tetrameric enzyme into its inactive protomers and is dependent on the presence of zinc (Brand, Heinickel et al. 1988). Four clusters of acidic amino acid residues responsible for zinc binding and inactivation of phosphofructokinase (PFK) were identified between positions 35 and 78 of parathymosin. By further proteolytic cleavage the two specific zinc-binding sites were located to amino acid residues between positions 51 and 72, whereas the region between positions 35 and 50 is necessary for binding and inactivation of phosphofructokinase (Brand, Heinickel et al. 1988). Phosphofructokinase is a key regulator of glycolysis. It is a kinase that phosphorylates fructose-6-phosphate (F6P) in glycolysis. As mentioned before, one of the sites by which glucose enters into 86 hexosamine pathway is by conversion of F6P to glucosamine-6-phosphate. So, inhibition of PFK may shift fructose-6-phosphate from the glycolytic pathway to HBP. So, if parathymosin inhibits PFK it can increase the carbon flux into the HBP. It has also been shown by parathymosin-Sephrose affinity chromatography that the interaction of parathymosin with many enzymes of carbohydrate metabolism is zinc specific. From liver cytosol the following enzymes were retained: hexokinase/glucokinase, glucose-6-phosphate dehydrogenase, phosphofructokinase-1, aldolase, glycerol-3-phosphate dehydrogenase, glyceral-3-phosphate dehydrogenase, fructose-1, 6-bisphosphatase, and the phosphorylated form of pyruvate kinase L (Brand, Heinickel et al. 1991). So it is possible that parathymosin, due to interaction with enzymes at the branching points of carbohydrate metabolism, could shift the glycolytic flux in the directions to other metabolic pathways. However, more experiments are required to support this idea. These include demonstration of parathymosin containing complexes in intact cells, study of kinetic effects of complex formation on defined metabolic fluxes. No reports exist on potential phosphorylation or any other posttranslational modifications of parathymosin except the acetylation of its N terminus (Brand, Heinickel et al. 1991). So we have shown for the first time the glycosylation of parathymosin. So our result shows that parathymosin is glycosylated under glucosamine treatment as well as glucosamine plus insulin treatment but not in control. But glycosylated parathymosin is not present in glucosamine plus selenium 87 treatment which again attracts us to speculate that this glycosylation reversal facilitates in some way the regulation of PFK. It is also shown to enhance glucocorticoid dependent transcription via a direct interaction with the glucocorticoid receptor in vivo. But so far there is no literature report on influence of parathymosin on glucocorticoid regulation of metabolic genes. Without glucocorticoid hormone, the glucocorticoid receptor (GR), a member of the nuclear receptor family, is localized in the cytosol through its association with a variety of heat shock/chaperone proteins like heat shock protein 90 (hsp90), heat shock protein 60 and protein FKBP52. These chaperone proteins were not identified in our study. Upon hormone binding, GR dissociates from the chaperone proteins and translocates from the cytosol to the nucleus. In the nucleus, the ligand-bound GR binds to specific DNA sequences termed glucocorticoid response elements (Elchebly, Payette et al. 2000), where it recruits a coactivator complex containing the pi60 steroid receptor coactivator (SRC), acetyltransferases (CBP, p300, and p/CAF), and methyltransferases (CARM1 and PRMT1). In association with this coactivator complex, GR directly activates gene transcription (Okamoto and Isohashi 2000). The next protein is proteasome subunit alpha type 1.There is not much information on the function of proteasome subunit alpha type 1. No reports exist on potential glycosylation modification of these proteins. So, we have shown here for the first time the glycosylation of the proteasome subunit alpha type 1 88 CHAPTER 4 DISCUSSION The discovery of insulin by Banting and colleagues in the early 1920s, stands as one of the greatest scientific achievements of the 20th century. Few scientific endeavors have had such scientific impact. Insulin was first isolated in 1921, by Banting, Best, Collip and Macleod following the observations of Von Mering and Minkowski that removal of the pancreas made dogs severely diabetic (Banting, Best et al. 1991). Banting and Best made a pancreatic extract that reduced glucose levels when injected into these diabetic dogs. Since then, insulin administration has become a common treatment for both Type 1 and Type 2 diabetes. Prior to this discovery the prospects for patients afflicted with diabetes were grim. It is one of the most decorated molecules in biology and the work on insulin (directly or indirectly) has yielded six Nobel prizes. Even though 90 years have passed since the discovery of insulin, the molecular actions of insulin have only begun to be understood over the past 25 years with the cloning of the insulin receptor, the start of transgenic technology and the development of phosphotyrosine-specific antibodies. 89 The primary metabolic action of insulin is to facilitate the postprandial disposition of glucose via its actions on three key target tissues, suppression of glucose output from the liver and stimulation of glucose uptake and metabolism in skeletal muscle and adipose tissue. Defects in insulin secretion and insulin action on its target tissues manifest clinically as diabetes mellitus. Diabetic Mellitus is classified primarily as either Type 1 or Type 2. Type 1 diabetes, comprises only 10% of those affected by the disease and results from autoimmune destruction of the Pcells of the pancreas. The remaining 90% of patients, classified as type 2, are affected by a complex disorder characterized by insulin resistance. Insulin resistance is a condition in which normal insulin secretion from the pancreas is insufficient to induce a biological response in the peripheral tissues (liver, muscle, adipose). In the early stage of the disease progression, the pancreas enhances insulin secretion to maintain normal blood glucose levels; however at later stages pancreatic function becomes impaired, which leads to hyperglycemia. Sustained hyperglycemia has been shown to induce insulin resistance and this has been suggested to be an adaptive mechanism in protecting cells from the oxidative stress that results from excess nutrients (Buse 2006). How hyperglycemia mediates insulin resistance at the cellular level remained unanswered for many years. One of the mechanisms that appear to be involved in the adverse effects of hyperglycemia is the O-GlcNAc modification of intracellular proteins on serine and threonine residues. OGlcNAc modification of proteins are dependent on the concentration of UDP-GlcNAc 90 produced by the HBP, which itself depends on the concentration of glucose in the cell. An increase in glucose uptake is thought to lead to increased flux through the HBP, resulting in increased UDP-GlcNAc levels which directly cause an increase in protein O-GlcNAcylation. O-GlcNAc was discovered in 1983, when purified bovine milk galactosyltransferase and its radiolabeled donor substrate (UDP-[3H] galactose) were used to probe for GlcNAc-terminating glycoconjugates in living murine thymocytes, splenic B- and T-lymphocytes, and macrophages (Torres and Hart 1984). As mentioned before, enzymes catalyzing O-GlcNAc addition are referred to as OGlcNAc transferases (OGT) and enzymes that remove O-GlcNAc are referred to as O-GlcNAcases. Both are found in the cytosol and nucleus. O-GlcNAcylated proteins have been identified from all cellular compartments representing nearly all functional class of proteins. O-GlcNAc modified proteins are found in the nuclear pore complex (Holt and Hart 1986) and on many cytoplasmic, mitochondrial and membrane associated proteins (Love, Kochan et al. 2003). These proteins belong to functional groups like transcription factors (c-myc) (Chou, Hart et al. 1995), polymerases (RNA Pol II) (Kelly, Dahmus et al. 1993), chaperones (Heat shock protein 70 (HSP70) (Walgren, Vincent et al. 2003), Heat shock protein 27 (HSP 27) (Roquemore, Dell et al. 1992), cytoskeletal proteins (Synapsin) (Cole and Hart 1999), phosphatases (Nuclear tyrosine phosphatases p65) (Meikrantz, Smith et 91 al. 1991) and kinases and adapter proteins (casein kinase II) (Lubas and Hanover 2000); (Insulin receptor substrate 1 and 2) (Vosseller, Wells et al. 2002). This dynamic addition of O-GlcNAc to proteins has been involved in modulating protein behavior via different mechanisms. For example, in vitro studies on RNA polymerase II (RNA pol II) showed that reciprocal glycosylation and phosphorylation of RNA Pol II may regulate transcription to prevent premature transcriptional initiation (Comer and Hart 2001). Similarly, the reciprocal modification by phosphorylation or O-GlcNAcylation at Ser 16 on the estrogen receptor beta modifies the receptor's behavior by altering the rate of degradation. When the estrogen receptor is phosphorylated, the protein is rapidly targeted for degradation, while the O-GlcNAcylated form is degraded much more slowly (Cheng and Hart 2001). Modification of the proteasome in vitro by O-GlcNAc reduces degradation by inhibition of the ATPase activity (Zhang, Su et al. 2003). O-GlcNAc can also modulate protein-protein interaction, for example, O-GlcNAc modification of a zinc finger DNA-binding transcription factor, YY1 causes its dissociation from retinoblastoma protein (pRb) allowing it to bind DNA(Hiromura, Choi et al. 2003). The major work so far to test the hypothesis, that increased flux through the hexosamine biosynthetic pathway can induce insulin resistance has been carried out in adipocytes, muscles and pancreatic P cells and less attention has been focused on liver. As mentioned in the introduction, the liver plays an important role in coordinating the whole body metabolism, including carbohydrate, lipid and protein 92 metabolism and detoxification. The various functions of the liver are carried out by the liver cells or hepatocytes. Some of the major metabolic functions of the liver include gluconeogenesis, glycogenolysis, glycogenesis, lipogenesis (Leclercq, Da Silva Morais et al. 2007). Hepatic insulin resistance is due to impaired suppression of hepatic glucose production, which accounts for hyperglycemia and glucose intolerance (Reaven 1995). Failure of insulin to inhibit hepatic gluconeogenesis and glycogenolysis, is responsible for the development of fasting hyperglycemia and persistent stimulation of insulin production by pancreatic P-cells. For example, mice lacking the insulin receptor (IR) in hepatocytes exhibit insulin resistance, severe glucose intolerance and failure of insulin to regulate hepatic gene expression and to suppress hepatic glucose output (Michael, Kulkarni et al. 2000). In contrast, normal glucose and insulin levels are found in mice with a deletion of IR in skeletal muscle (Bruning, Michael et al. 1998). Deletion of the IR in the adipose tissue was shown to be associated with low insulin levels suggesting improved insulin sensitivity (Kim, Michael et al. 2000; Bluher, Michael et al. 2002). When the IR is simultaneously knocked down in fat and muscle, there is no change in insulin or glucose levels. Thus, hepatic insulin resistance, but not peripheral insulin resistance, is necessary to develop hyperglycemia and glucose intolerance. McClain was the first to examine the effect of increased hexosamine flux in liver. They overexpressed GFAT in transgenic mice using the PEPCK promoter. 93 They observed that older transgenic mice gained more weight compared to the control mice, and also developed glucose intolerance with a significant decrease in glucose disposal rates (Veerababu, Tang et al. 2000). In our study, we have used glucosamine to induce insulin resistance in primary rat hepatocytes. Glucosamine enters the hexosamine biosynthetic pathway downstream of GFAT, bypassing the rate limiting step. It has been used to evaluate the role of hexosamine flux in the development of insulin resistance. For example, in vitro studies carried out in rat skeletal muscle indicates that glucosamine induces insulin resistance and may modulate insulin and glucose effects on pyruvate kinase and glycogen synthase (Traxinger and Marshall 1992; Robinson, Sens et al. 1993). Similarly, infusion of glucosamine in Wistar rats induces insulin resistance of skeletal muscle through decreased phosphorylation of insulin receptor substrate-1 (IRS-1) and its association with PI3-Kinase and inturn results in impaired translocation of GLUT4 to the plasma membrane (Baron, Zhu et al. 1995). In a similar experiment in rat adipocytes incubated in 5mM glucosamine for 4h the activity of GLUT4 is decreased and longer incubation (16h) leads to a decrease in the amount of GLUT4 in the plasma membrane (Chen, Ing et al. 1997). In a recent study, incubation of HEK293 cells with 25mM glucose for 24hr or 5mM glucosamine for 2hr resulted in increase in FoxOl O-GlcNAc modification which in turn increased the expression of G6pase gene (Kuo, Zilberfarb et al. 2008). 94 The major pitfall to earlier experiments using glucosamine was the use of very high concentrations (5mM-50mM) of glucosamine that could obscure its main action. For example studies carried out by Mueckler et al concluded that glucosamineinduced insulin resistance in 3T3-L1 cells is caused by depletion of intracellular ATP (Hresko, Heimberg et al. 1998). Subsequent investigators such as those described by Gulve et al have shown that this conclusion was derived from a toxic adverse effect of glucosamine that could be minimized by the judicious use of glucosamine (Ross, Chen et al. 2000). In our study, we utilized ImM glucosamine and evaluated whether or not there was any effect of glucosamine on cell viability or cytotoxicity using the LDH assay. Our results show that there is no significant difference in the level of LDH in glucosamine treated cells versus control, which suggests the cells are not compromised by glucosamine treatment. One of the molecular mechanisms responsible for the insulin resistant condition in type 2 diabetes is alteration at one or more levels of the insulin-signaling cascade in the insulin sensitive tissues: skeletal muscles, adipocytes, and liver. The protein kinase Akt, downstream to PI3-kinase, is an important mediator of insulin signaling in the regulation of cell survival and metabolism (Chang, Chiang et al. 2004; Zdychova and Komers 2005). Several studies have demonstrated linkage between the HBP, the development of insulin resistance, and altered Akt signaling. For example, treatment of 3T3-L1 adipocytes with PUGNAc (an inhibitor of OGlcNAcase) reduces Akt phosphorylation, resulting in reduction of insulin stimulated 95 2-deoxyglucose uptake (Vosseller, Wells et al. 2002; Park, Ryu et al. 2005). However, the involvement of Akt in insulin resistance from O-GlcNAc modification is controversial. In a study carried out by Patti et al, it was observed that glucosamine treatment in rat skeletal muscle caused reduced tyrosine phosphorylation of IRS-1 and 2 but attenuation of insulin stimulated phosphorylation of Akt was not observed (Patti, Virkamaki et al. 1999). Similarly, the elevated O-GlcNAc modification by PUGNAc induced insulin resistance in skeletal muscle was found to be independent of attenuated phosphorylation of Akt and GSK3a/p (Kim, Zhu et al. 1999; Arias, Kim et al. 2004). These contradictory results among insulin sensitive tissues led us to further investigate the effect of glucosamine on the insulin induced phosphorylation of Akt in primary rat hepatocytes. Hence, this was the first target for the investigation. In our study we found that the insulin stimulated phosphorylation of Akt (Ser 473) was drastically decreased by 4 fold in the presence of glucosamine when compared to the phosphorylation of Akt with insulin which is in agreement with the study carried out by Vosseller et al, which reported that PUGNAc decreased insulin stimulated activation of Akt in 3T3-L1 adipocytes. In our study the treatment of glucosamine caused drastic reductions in insulin stimulated phosphorylation of Akt, clearly suggesting that one of the mechanisms through which glucosamine interferes with insulin signaling is through inhibiting insulin stimulated Akt phosphorylation at 96 Ser-307 in primary rat hepatocytes. It is possible that different cells or tissues respond differently under various experimental conditions, explaining the controversial results. Difference in HBP induced changes in Akt phosphorylation or activity could be attributed to changes in the insulin signaling upstream of Akt. For example, a study in rat insulinoma P-cells has shown that HBP flux can impair insulin-induced tyrosine phosphorylation of IRS-1, PI 3-kinase activity, and subsequent Akt activation demonstrating that impaired upstream signaling may lead to impaired Akt activation (Andreozzi, DAlessandris et al. 2004). In contrast to the above study, McClain showed an increase in Akt signaling resulting from overexpression of O-GlcNAcase; however they did not observe an increase in PI 3-kinase activity (Soesanto, Luo et al. 2008). Selenium, an essential trace element, has been shown to mimic some of insulin's actions both in vivo and in vitro. For example, incubation of rat adipocytes with selenium stimulated glucose transport activity, due to the translocation of two types of glucose transporters (GLUT1 and GLUT2) to the membrane surface (Ezaki 1990). In addition to glucose uptake, selenium has been shown to regulate the activity and expression of various enzymes involved in the processes of glycolysis, gluconeogenesis, fatty acid synthesis and the pentose phosphate pathway. Oral administration of selenate to diabetic animals partly reversed abnormal liver expression of both glycogenic and gluconeogenic enzymes (Becker, Reul et al. 1996). Regulation by insulin-mimetics of the expression of lipogenic enzymes was also 97 found to be similar to insulin. Treatment of the diabetic animals or rat hepatocytes in culture with selenate restored the expression of both FAS and G6PDH, demonstrating that selenate was capable of stimulating lipogenesis in the liver (Berg 1995; Ghosh 1994). The literature indicates that like insulin, selenium treatment also triggers a variety of phosphoproteins of insulin signaling pathway. For example, treatment of NIH3T3 HIR 3.5 cells with selenate was found to stimulate phosphorylation of the insulin receptor (Pillay and Makgoba 1992). In a later study, incubation of either primary rat hepatocytes or 3T3-LI adipocytes in culture with selenate caused not only a concentration and time dependent increase in phosphorylation of the P subunit of the insulin receptor but also IRS-1 as determined through immunoprecipitation studies (Stapleton, Garlock et al. 1997). To determine whether selenium can act as an antidiabetic agent in this model system, we first investigated the effect of selenium on Akt phosphorylation. Previously we had shown selenium caused increases in phosphorylation of signal proteins early in the PI3 kinase cascade (Stapleton, Garlock et al. 1997). If selenium truly acts as a mimetic, then an increase in phosphorylation of proteins downstream of PI3 kinase such as Akt with selenium should also occur. Our results show that selenium treatment increased the phosphorylation of Akt by 13 fold as compared to the basal level in control. Unlike what we observed with the quenching effect of glucosamine on the insulin induced phosphorylation of Akt, selenium induced phosphorylation of Akt is unaffected in the presence of glucosamine. This suggests 98 that selenium can act as a potent insulin - mimetic not only under normal/ control conditions but also under insulin resistant condition as well. Selenium was first shown to stimulate PI3 kinase activity in 3T3-L1 adipocytes by Heart and Sung. They reported that selenium stimulates Akt phosphorylation and this selenium action is downstream of PI3 kinase as in insulin signaling pathways. They showed this by using the PI3 kinase inhibitor Wortmanin which completely abolished Akt phosphorylation by selenium (Heart and Sung 2003). Further studies carried out in non-obese diabetic mice (NOD), showed that expression level of Akt and phosphoAkt significantly increased in selenium treated diabetic NOD mice (Hwang, Seo et al. 2007). Increase in the flux through HBP has been shown to cause an increase in OGlcNAc modified proteins (Vosseller, Wells et al. 2002; DAlessandris, Andreozzi et al. 2004; Park, Ryu et al. 2005; Akimoto, Hart et al. 2007). Studies from various labs suggest that numerous components of the insulin signaling pathway that are involved in glucose metabolism are O-GlcNAcylated. For example, studies carried out by Gandy et al showed that Aktl was modified by O-GlcNAc. In this study it was observed that treatment of neuroblastoma cells with 0-(2-acetamido-2-deoxy-dglucopyranosylidene) amino-iV-phenylcarbamate (PUGNAc), which inhibits the enzymatic removal of O-GlcNAc from proteins, increased cytosolic O-GlcNAc Aktl levels (Gandy, Rountree et al. 2006). A similar study carried out in rat primary 99 adipocytes showed that treatment with PUGNAc increased O-GlcNAc modification of IRS-1 and Akt2, accompanied by a partial reduction of insulin stimulated phosphorylation of IRSl and Akt2. PUGNAc also decreased insulin stimulated 2deoxyglucose (2DG) uptake and GLUT4 translocation in adipocytes (Park, Ryu et al. 2005). Other signaling proteins that have been shown to be O-GlcNAcylated are: Glutl(Buse, Robinson et al. 2002), casein-kinase II, glycogen synthase kinase 3 (Lubas and Hanover 2000) and IRSl/2(Patti, Virkamaki et al. 1999). We found that glucosamine and glucosamine plus insulin treatment showed an increase in proteins that are O-GlcNAcylated compared to control by immunoblotting with anti OGlcNAc antibody. In a recent report, Yang et al. demonstrates that upon insulin stimulation OGT can be translocated to the plasma membrane through interaction with PI(3,4,5)P3, demonstrating a potential mechanism by which Akt can be OGlcNAcylated (Yang 2008). We were unable however to demonstrate anti O-GlcNAc antibody cross reactivity with immunoprecipitated Akt (data not shown). However, this does not exclude the possibility that it can be modified by O-GlcNAc. The stochiometry of the modification might have been below the limit of detection by the specific antibody chosen for identifying O-GlcNAcylation, 110.6. Since we found an increase in proteins that are O-GlcNAcylated under glucosamine and glucosamine plus insulin treatment, we speculated that this modification of key proteins might be linked to the induction of insulin resistance. In order to verify that hypothesis, we attempted to identify the O-GlcNAc modified proteins. For this, O-GlcNAcylated 100 proteins were isolated and purified using wheat germ agglutinin column. Purified proteins were verified using SDS PAGE and samples were submitted to the Mass Spectrometry and Proteomics Lab at Van Andel research institute, Grand Rapids for LC-MS analysis. Our attempt to receive quantitative analysis between control and experimental group was unsuccessful. However, this experiment generated a list of O-GlcNAc modified proteins. We generated a list of proteins that are uniquely found in the glucosamine treated cells compared to control, untreated cells. Surprisingly, analysis of the proteins that were glycosylated under the glucosamine conditions did not reveal any modification to signaling proteins or metabolic enzymes. Most of the proteins that were identified are chaperones and cytoskeletal proteins. Selenium as well as insulin alone also causes unique glycosylation events. It was attractive to speculate that proteins glycosylated under insulin plus glucosamine are reversed with selenium treatment. We observed in the glucosamine plus insulin treatment the following uniquely identified proteins, eukaryotic translation elongation factor 1 delta, parathymosin and proteasome subunit alpha type 1. Among these three OGlcNAcylated proteins, eukaryotic translation elongation factor 1 delta and parathymosin were the two proteins that are not glycosylated under glucosamine selenium treatment. Given observation it is attractive to speculate that selenium might be reversing the glycosylation event allowing regulation of metabolism to occur. However, further quantitative studies are required to validate our observation. 101 Insulin, for example regulates many cellular processes and the expression of several genes through the activation of various signaling proteins and pathways. If the activation of the signal proteins is affected, then the effect on gene expression should also be altered. So, we examined the effect of glucosamine on the insulin regulated expression of key metabolic enzymes of pentose phosphate pathway, fatty acid biosynthesis and gluconeogenesis. G6PDH is a lipogenic enzyme that catalyses the first reaction in the pentose phosphate pathway. The pentose phosphate pathway is an anabolic pathway that utilizes excess glucose present in the liver. The primary function of this pathway is to generate reducing equivalents in the form of NADPH, which is necessary for biosynthetic reactions including fatty acid metabolism, and to produce ribose-5phosphate (R5P), which is necessary for the synthesis of nucleotides, the building blocks of nucleic acids. Thus G6PDH is important in both carbohydrate and fatty acid metabolism. The G6PDH gene has been considered to be a "housekeeping" gene, but several studies have shown its expression to be highly regulated. The expression of G6PDH has been shown to be increased by insulin in primary rat hepatocytes (Kurtz and Wells 1981; Stapleton, Stevens et al. 1993) and by oxidative stress in cells in culture (Ursini, Parrella et al. 1997; Xu, Maki et al. 2003). Our result shows that under glucosamine treatment, insulin stimulated gene expression of G6PDH was decreased significantly compared to insulin stimulated 102 G6PDH gene expression in control, suggesting that insulin is no longer able to fully regulate G6PDH expression. Since the pentose phosphate pathway is responsible for most of the NADPH production, and NADPH is needed by fatty acid biosynthesis (Mourrieras, Foufelle et al. 1997), it stands to reason that the decrease in the insulin induced gene expression of G6PDH under glucosamine treatment might lead to a decrease in fatty acid synthesis. If insulin sensitivity is truly impacted then insulin induced expression of FAS should be altered. Our results show that insulin significantly increased the gene expression of FAS by 1.6 fold when compared to control, which is comparable to published reports (Paulauskis and Sul 1988; Katsurada, Iritani et al. 1990) On the other hand, insulin stimulated expression of FAS under glucosamine treatment decreased significantly compared to insulin stimulated FAS gene expression in control. This indicates that glucosamine inhibits insulin stimulated FAS gene expression. In contrast to our studies, Marshall studies in primary rat adipocytes reported that in insulin treated primary rat adipocytes, glucosamine unregulated FAS mRNA levels (Rumberger, Wu et al. 2003). As mentioned before, like insulin selenium can affect the gene expression of lipogenic enzymes, G6PDH and FAS in streptozotocin induced type 1 diabetic model (Berg, Wu et al. 1995). In the presence of glucosamine we investigated the effect of selenium on the gene expression of G6PDH and FAS. Our results show that selenium induces the gene expression of G6PDH by approximate 1.5 fold and FAS by 1.3 fold 103 at 6hr respectively. Unlike our findings with insulin in the presence of glucosamine, selenium induction of G6PDH and FAS was similar in the presence or absence of glucosamine. This result is comparable to our previous study in which it was found that administration of selenate or vanadate to streptozotocin- induced diabetic rats restored the expression of FAS and G6PDH (Berg, Wu et al. 1995). Hepatic glucose output is mainly from gluconeogenesis (Nordlie, Foster et al. 1999) and plays an important role in diabetes. As mentioned before, hepatic insulin resistance is due to impaired suppression of hepatic glucose production (Reaven 1995). One of the key rate limiting enzymes of gluconeogenesis is PEPCK. The control of hepatic gluconeogenesis is regulated, in large part, by altering the rate of transcription of the PEPCK gene (Pilkis and Gramier 1992). Glucagon and glucocorticoids induce transcription of the PEPCK gene and insulin inhibits its induction (Sutherland, O'Brien et al. 1996). To address the importance of insulin signaling in the control of gluconeogenesis in vivo, liver specific disruption of the insulin receptor was performed in mice (LIRKO mice). Disruption of insulin action in the liver of LIRKO mice lead to severe glucose intolerance, resistance to the blood glucose lowering effect of insulin, and non inhibited hepatic glucose production due to elevated liver G6Pase and PEPCK expression(Michael, Kulkarni et al. 2000). To study the role of the expression of PEPCK gene in the development of type 2 diabetes many transgenic mice expressing a PEPCK gene have been generated and studied. For example, studies carried out by Valera et al, showed that overexpression of the 104 PEPCK gene leads to alterations in liver glycogen content and muscle glucose transporter GLUT-4 gene expression and increased glucose production in hepatocytes in primary culture. When intraperitoneal glucose tolerance tests were performed, blood glucose levels were higher than those detected in normal mice. This study clearly showed that primary alterations in the rate of liver glucose production may induce insulin resistance and type 2 diabetes (Valera, Pujol et al. 1994). We investigated the effect of glucosamine on the insulin regulation of this key enzyme of gluconeogenesis. We used cAMP to induce the gene expression of PEPCK. Glucagon increases PEPCK gene expression through the second messenger cAMP (Lamers, Hanson et al. 1982). Upon glucagon binding to the glucagon receptor, cAMP stimulates a series of reactions that leads to mobilization of glucose from the liver: overall decreases in glycolysis and glycogen synthesis, and a increases in glycogenolysis and gluconeogenesis. Our results show that the addition of cAMP for 1 hr in the control group caused an increase in PEPCK gene expression by 27 fold, which was comparable to previous studies (Beale, Andreone et al. 1984; Imai, Miner et al. 1993). Following treatment of insulin after cAMP a 25% decrease was observed in the cAMP induced gene expression. In the glucosamine treated cells, the inhibition of cAMP induced PEPCK expression by insulin was not observed, which suggests that expression of PEPCK is resistant to down regulation by insulin. It was recently demonstrated that adenoviral-mediated overexpression of OGT in mouse liver attenuated the ability of insulin to suppress hepatic glucose production. 105 This study demonstrated a mechanism by which upon hormone stimulation OGT is recruited to the plasma membrane in the vicinity of the proteins involved in early steps of insulin signaling. In the basal state, OGT is localized to the nucleus and cytoplasm (Kreppel, Blomberg et al. 1997). Upon insulin stimulation phosphatidylinositol 3-phosphate (PIP3) production by PI-3 kinase induces the recruitment of OGT to the plasma membrane, causing GlcNAcylation of the insulin receptor and insulin receptor substrate. It was observed that Akt phosphorylation and Akt activity was decreased resulting in upregulation of the expression of gluconeogenic enzymes PEPCK and glucose -6-phosphatase (G6pase). In the same paper it was reported that the exposure of 3T3-L1 adipocytes to high glucose concentration or PUGNAc decreases the phosphorylation of Akt at threonine 308. In contrast, the phosphorylation level of the MAPK pathway, particularly the Erkl/2 kinases, is unaffected. The phosphorylation of IRSl is also modified under these conditions, since the treatment of adipocytes with PUGNAc or the adenovirus mediated transfection of adipocytes and Fao hepatoma cells with Ad-OGT increases the phosphorylation of IRSl at Ser307 and Ser632/635, three sites known to downregulate the insulin signaling pathway. Taken as a whole, this paper demonstrates that OGT participates in the termination of the insulin signaling pathway by interacting with PIP3 at the plasma membrane. OGT then O-GlcNAcylates numerous components of the insulin signaling pathway, lowering the response to insulin; this 106 supports the role of OGT in the physiopathology of insulin resistance (Whelan, Lane et al. 2008; Yang, Ongusaha et al. 2008). Further validating the importance of O-GlcNAc on hepatic insulin signaling, recent studies focusing on the transcription factors FoxOl and CRTC2/TORC2 have shown that O-GlcNAcylation also leads to insulin resistance and glucose toxicity through the control of gluconeogenesis (Dentin, Hedrick et al. 2008; Housley, Rodgers et al. 2008; Kuo, Zilberfarb et al. 2008). FoxOl is responsible for the expression of two enzymes of gluconeogenesis, G6pase and PEPCK. Recent data demonstrated that FoxOl is O-GlcNAcylated in liver cells, resulting in an increase in its transcriptional activity and in turn increasing the transcriptional activity of target genes including PEPCK and G6pase. In yet another report, it has been shown that the transcriptional co-activator PGC-la, which is itself O-GlcNAcylated, regulates FoxOl O-GlcNAcylation, and consequently its transcriptional activity, by recruiting and targeting OGT to FoxOl (Housley, Udeshi et al. 2009). Interestingly, both glucose-6-phosphatase and PEPCK are transcriptionally regulated by CRTC2, which is also controlled by OGlcNAcylation unlike FoxOl, regulation occur through nuclear re-location since the two major OGlcNAcylated sites that have been mapped sequester CRTC2 in the cytoplasm by a phosphorylation-dependent mechanism. Under normal conditions, CRTC2 is phosphorylated at Ser 70 and Ser 171 in the cytoplasm by 14-3-3- protein. Upon high glucose treatment, CRTC2 becomes GlcNAcylated at Ser 70 and Ser 171, 107 allowing nuclear translocation and transcription of gluconeogenic genes in hepatocytes (Dentin, Hedrick et al. 2008). In the last several decades, most studies regarding the mechanism of hormone action on PEPCK gene expression have focused on mapping out homione response units (HRU) on this gene promoter. Studies in this field began to focus on the identification of coregulators of the hormone-activated PEPCK gene promoter. Afterward, the functional importance of certain transcription factors and coregulators on mediating PEPCK gene expression were studied through the expression of dominant negative forms of these proteins (Wang, Stafford et al. 2000). In the early 1990s, studies from the Granner lab reported that insulin represses glucocorticoid and cAMP-induced PEPCK gene transcription through a DNA element located between -416bp and -402bp of this gene promoter, called the insulin response sequence (IRS)(0'Brien, Lucas et al. 1990). Their group further demonstrated that transcription factor Forkhead box a2 (Foxa2), also called hepatic nuclear factor 1 beta (HNF3P) and CCAAT/enhancer-binding protein p (C/EBPP), can associate with the IRS of the PEPCK gene promoter in vitro. However, only Foxa2 is functionally required for glucocorticoid induced PEPCK gene expression (O'Brien, Noisin et al. 1995). O'Brien et al found that the insulin-like growth factor-binding protein l(IGFBP-l) gene can be stimulated by glucocorticoids and inhibited by insulin. 108 Analyses of this promoter by transfection of IGFBP-1 chloramphenicol acetyltransferase fusion genes into rat hepatoma cells has led to the identification of insulin response sequences (IRSs) in this gene (O'Brien, Noisin et al. 1995). By comparison, the IRS, T (G/A) TTTTG, is the same both in PEPCK and IGFBP-1 genes. The IGFBP-1 IRS and PEPCK IRS both bind the alpha and beta forms of HNF-3p also called Foxal and Foxa2. Mutational analyses of the PEPCK and IGFBP-1 IRSs revealed mutations that do not affect the binding of HNF3 proteins to these elements but do eliminate the ability of the IRSs to mediate an insulin response. These data led O'Brien et al to postulate that insulin mediates its negative effect on glucocorticoid-induced PEPCK and IGFBP-1 gene transcription indirectly by inhibiting HNF-3 binding (Foxa2) (O'Brien, Noisin et al. 1995). Later Wang et al found that expression of a dominant negative Foxa2, in which the transactivation domain is deleted, reduced glucocorticoid-mediated PEPCK gene expression and hepatic gluconeogenesis (Wang, Stafford et al. 2000). Studies from the Stoffel group showed that Foxa2 (HNF3P) physically interacts with Akt. Foxa2 is phosphorylated at a single conserved site (T156). The insulin induced phosphorylation of Foxa2 through Akt mediates the translocation of this protein from nucleus to cytosol (Wolfrum, Besser et al. 2003). Our next objective was to examine whether or not insulin mediated reduced expression of PEPCK could attribute to reduced expression level of Foxa2 and if so what effect it would have. Our results show that the addition of 500uM of cAMP for 109 lhr in the control group caused a 2.3 fold increase in Foxa2 gene expression which is in accord with studies carried out by Imae et al where they analyzed the effect of insulin, dexamethasone and protein malnutrition on the hepatic mRNA levels of HNF3a, HNF3P and HNF3y. They found that daily administration of dexamethasone to Wistar rats caused the mRNA levels of HNF3a and HNF3p to increase (Imae, Inoue et al. 2000). Following treatment of insulin after cAMP an approximately 40% decrease in Foxa2 expression was observed. Glucosamine had no effect on Foxa2 expression or alter the effect of cAMP. With glucosamine treatment, there was no significant difference between cAMP induced Foxa2 gene expression with or without insulin. This result shows that under glucosamine treatment insulin no longer is able to inhibit the induction of cAMP induced gene expression of Foxa2. Our results so far indicate that under glucosamine treatment, all the key enzymes studied are unresponsive to insulin. The first animal study to investigate the antidiabetic mechanisms of selenate was shown in type 2 diabetic db/db mice. They reported that selenate administration to the db/db mice led to a marked downregulation of the gluconeogenic enzyme PEPCK in comparison to selenium deficient mice (Mueller and Pallauf 2006). We investigated the effect of selenium on the expression of PEPCK in our model system. It was found that addition of cAMP for 1 hr in control group caused an increase in PEPCK gene expression by 27 fold. Selenium treatment decreased cAMP induced gene expression of PEPCK significantly and expression was near basal level by 6hr. 110 Again, like with FAS and G6PDH, selenium had similar effects on the expression of PEPCK in the presence of glucosamine suggesting its actions are not sensitive to glucosamine. We further found that selenium treatment under the insulin resistant state could inhibit the induction of cAMP induced gene expression of Foxa2. Again, as with FAS, G6PDH, PEPCK, selenium had similar effects on the expression of Foxa2 in the presence of glucosamine suggesting its actions are not sensitive to glucosamine. In summary, we have established an insulin resistance model in primary rat hepatocytes by using glucosamine, a precursor of HBP. We speculate this could be due to increased O-GlcNAc modified proteins that were observed under glucosamine compared to control however, the proteins we observed to be O-GlcNAcylated were not as predicted. Another interesting observation of this study is that selenium is able to act as an insulin-mimetic not only under the control conditions but also under insulin resistant condition as well. We also observed glycosylation of proteins under selenium treatment. However, further studies are necessary to determine if selenium's actions are indeed mediated by altering the glycosylation state of the protein. Our work is only the beginning of an intricate and interesting story. Further studies are crucial in order decipher what other players are involved in glucosamine induced insulin resistance. Ill APPENDIX IACUC approval form for animal studies WESTERN MICHIGAN UNIVERSITY hstiiutiaiidl Animal Care antJ Use ComnuUoB Dale March 30, 2010 To Susan Stapleton, Principal Investi/a/or/, From Robert Evcrsole, ('hair Re IACUC Protocol No 10-02-01 Your piolocol tilled "Regulation of Gene Expression in Hepatoc) les"' ha.s received npprovnl irora the Institutional Ajnmal Tare and Vw Committee. The conditions and duration of this approved aie specified in the Policies of Western Michigan Umvcrail) You may now begin to implement the research as described in the application The Board wishes you success in the pursuit of youi research goals Appi ovi«l Termination Match 30,2011 Wa «od Hall Mim««» W mj% 54W 112 BIBLIOGRAPHY Accili, D. (2004). "Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic." Diabetes 53(7): 1633-1642. Aguirre, V., T. Uchida, L. Yenush, R. Davis and M. F. White (2000). "The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307)." J Biol Chem 275(12): 9047-9054. Aguirre, V., E. D. Werner, J. Giraud, Y. H. Lee, S. E. Shoelson and M. F. White (2002). "Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action." J Biol Chem 277(2): 1531-1537. Akimoto, Y., G. W. Hart, L. Wells, K. Vosseller, K. Yamamoto, E. Munetomo, M. Ohara-Imaizumi, C. Nishiwaki, S. Nagamatsu, H. Hirano and H. Kawakami (2007). "Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats." Glycobiology 17(2): 127-140. Altomare, D. A., K. Guo, J. Q. Cheng, G. Sonoda, K. Walsh and J. R. Testa (1995). "Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene." Oncogene 11(6): 1055-1060. Andrali, S. S., Q. Qian and S. Ozcan (2007). "Glucose mediates the translocation of NeuroDl by O-linked glycosylation." J Biol Chem 282(21): 15589-15596. Andreozzi, F., C. DAlessandris, M. Federici, E. Laratta, S. Del Guerra, S. Del Prato, P. Marchetti, R. Lauro, F. Perticone and G. Sesti (2004). "Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser307 and Ser612 and impairs the phosphatidylinositol 3kinase/Akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic beta-cells." Endocrinology 145(6): 2845-2857. Arias-Salgado, E. G., F. Haj, C. Dubois, B. Moran, A. Kasirer-Friede, B. C. Furie, B. Furie, B. G. Neel and S. J. Shattil (2005). "PTP-1B is an essential positive regulator of platelet integrin signaling." J Cell Biol 170(5): 837-845. 113 Arias, E. B., J. Kim and G. D. Cartee (2004). "Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle." Diabetes 53(4): 921-930. Au, S. W., S. Gover, V. M. Lam and M. J. Adams (2000). "Human glucose-6phosphate dehydrogenase: the crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency." Structure 8(3): 293303. Avruch, J. ((2001)). The molecular basis of insulin action and insulin resistance. Genetics ofDiabetes Mellitus, Kluwer Academic Publishers,MA. Ayaz, M., B. Can, S. Ozdemir and B. Turan (2002). "Protective effect of selenium treatment on diabetes-induced myocardial structural alterations." Biol Trace Elem Res 89(3): 215-226. Ball, L. E., M. N. Berkaw and M. G. Buse (2006). "Identification of the major site of O-linked beta-N-acetylglucosamine modification in the C terminus of insulin receptor substrate-1." Mol Cell Proteomics 5(2): 313-323. Banting, F. G., C. H. Best, J. B. Collip, W. R. Campbell and A. A. Fletcher (1991). "Pancreatic extracts in the treatment of diabetes mellitus: preliminary report. 1922." CMAJ 145(10): 1281-1286. Barbour, L. A., J. Shao, L. Qiao, W. Leitner, M. Anderson, J. E. Friedman and B. Draznin (2004). "Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle." Endocrinology 145(3): 1144-1150. Baron, A. D. (1994). "Hemodynamic actions of insulin." Am J Physiol 267(2 Pt 1): El 87-202. Baron, A. D., J. S. Zhu, J. H. Zhu, H. Weldon, L. Maianu and W. T. Garvey (1995). "Glucosamine induces insulin resistance in vivo by affecting GLUT 4 translocation in skeletal muscle. Implications for glucose toxicity." J Clin Invest 96(6): 2792-2801. Battell, M. L., H. L. Delgatty and J. H. McNeill (1998). "Sodium selenate corrects glucose tolerance and heart function in STZ diabetic rats." Mol Cell Biochem 179(1-2): 27-34. 114 Beale, E., T. Andreone, S. Koch, M. Granner and D. Granner (1984). "Insulin and glucagon regulate cytosolic phosphoenolpyruvate carboxykinase (GTP) mRNA in rat liver." Diabetes 33(4): 328-332. Becker, D. J., B. Reul, A. T. Ozcelikay, J. P. Buchet, J. C. Henquin and S. M. Brichard (1996). "Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats." DiabetolQgia 39(1): 3-11. Berg, E. A., J. Y. Wu, L. Campbell, M. Kagey and S. R. Stapleton (1995). "Insulinlike effects of vanadate and selenate on the expression of glucose-6-phosphate dehydrogenase and fatty acid synthase in diabetic rats." Biochimie 77(12): 919924. Biddinger, S. B., M. Miyazaki, J. Boucher, J. M. Ntambi and C. R. Kahn (2006). "Leptin suppresses stearoyl-CoA desaturase 1 by mechanisms independent of insulin and sterol regulatory element-binding protein-lc." Diabetes 55(7): 20322041. Bjornholm, M., Y. Kawano, M. Lehtihet and J. R. Zierath (1997). "Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation." Diabetes 46(3): 524-527. Bosron, W. F. and T. K. Li (1986). "Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism." Hepatology 6(3): 502-510. Bost, F., M. Aouadi, L. Caron, P. Even, N. Belmonte, M. Prot, C. Dani, P. Hofman, G. Pages, J. Pouyssegur, Y. Le Marchand-Brustel and B. Binetruy (2005). "The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis." Diabetes 54(2): 402-411. Bouzakri, K., M. Roques, P. Gual, S. Espinosa, F. Guebre-Egziabher, J. P. Riou, M. Laville, Y. Le Marchand-Brustel, J. F. Tanti and H. Vidal (2003). "Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes." Diabetes 52(6): 1319-1325. Brand, I. A., A. Heinickel, H. Kratzin and H. D. Soling (1988). "Properties of a 19kDa Zn2+-binding protein and sequence of the Zn2+-binding domains." Eur J Biochem 177(3): 561-568. 115 Brodbeck, D., P. Cron and B. A. Hemmings (1999). "A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain." J Biol Chem 274(14): 9133-9136. Buse, M. G. (2006). "Hexosamines, insulin resistance, and the complications of diabetes: current status." Am J Physiol Endocrinol Metab 290(1): E1-E8. Buse, M. G., K. A. Robinson, B. A. Marshall, R. C. Hresko and M. M. Mueckler (2002). "Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUTl-overexpressing muscles." Am J Physiol Endocrinol Metab 283(2): E241-250. Calera, M. R., C. Martinez, H. Liu, A. K. Jack, M. J. Birnbaum and P. F. Pilch (1998). "Insulin increases the association of Akt-2 with Glut4-containing vesicles." J Biol Chem 273(13): 7201-7204. Carlson, C. J., M. F. White and C. M. Rondinone (2004). "Mammalian target of rapamycin regulates IRS-1 serine 307 phosphorylation." Biochem Biophys Res Commun 316(2): 533-539. Caro, J. F., M. K. Sinha, S. M. Raju, O. Ittoop, W. J. Pories, E. G. Flickinger, D. Meelheim and G. L. Dohm (1987). "Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes." J_ Clin Invest 79(5): 1330-1337. Chang, L., S. H. Chiang and A. R. Saltiel (2004). "Insulin signaling and the regulation of glucose transport." Mol Med 10(7-12): 65-71. Cheatham, B. and C. R. Kahn (1995). "Insulin action and the insulin signaling network." Endocr Rev 16(2): 117-142. Chen, G., P. Liu, D. C. Thurmond and J. S. Elmendorf (2003). "Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Muncl8c." FEBS Lett 534(1-3): 54-60. Chen, H., B. L. Ing, K. A. Robinson, A. C. Feagin, M. G. Buse and M. J. Quon (1997). "Effects of overexpression of glutamine: fructose-6-phosphate amidotransferase (GFAT) and glucosamine treatment on translocation of GLUT4 in rat adipose cells." Mol Cell Endocrinol 135(1): 67-77. Chen, R. H., Y. H. Su, R. L. Chuang and T. Y. Chang (1998). "Suppression of transforming growth factor-beta-induced apoptosis through a 116 phosphatidylinositol 3-kinase/Akt-dependent pathway." Oncogene 17(15): 1959-1968. Cheng, X. and G. W. Hart (2001). "Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: post-translational regulation of turnover and transactivation activity." J Biol Chem 276(13): 10570-10575. Chou, T. Y., G. W. Hart and C. V. Dang (1995). "c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas." J_ Biol Chem 270(32): 18961-18965. Cichy, S. B., S. Uddin, A. Danilkovich, S. Guo, A. Klippel and T. G. Unterman (1998). "Protein kinase B/Akt mediates effects of insulin on hepatic insulin-like growth factor-binding protein-1 gene expression through a conserved insulin response sequence." J Biol Chem 273(11): 6482-6487. Coffer, P. J., J. Jin and J. R. Woodgett (1998). "Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation." Biochem J 3 3 5 ( P t l ) : 1-13. Cohen, P., D. R. Alessi and D. A. Cross (1997). "PDK1, one of the missing links in insulin signal transduction?" FEBS Lett 410(1): 3-10. Cole, R. N. and G. W. Hart (1999). "Glycosylation sites flank phosphorylation sites on synapsin I: O-linked N-acetylglucosamine residues are localized within domains mediating synapsin I interactions." J Neurochem 73(1): 418-428. Combs, G. F., Jr., L. C. Clark and B. W. Turnbull (2001). "An analysis of cancer prevention by selenium." Biofactors 14(1-4): 153-159. Comer, F. I. and G. W. Hart (2001). "Reciprocity between O-GlcNAc and Ophosphate on the carboxyl terminal domain of RNA polymerase II." Biochemistry 40(26): 7845-7852. DAlessandris, C, F. Andreozzi, M. Federici, M. Cardellini, A. Brunetti, M. Ranalli, S. Del Guerra, D. Lauro, S. Del Prato, P. Marchetti, R. Lauro and G. Sesti (2004). "Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic betacells." FASEB J 18(9): 959-961. DeFronzo, R. A. (1988). "Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM." Diabetes 37(6): 667-687. 117 DeFronzo, R. A., R. C. Bonadonna and E. Ferrannini (1992). "Pathogenesis of NIDDM. A balanced overview." Diabetes Care 15(3): 318-368. DeFronzo, R. A., E. Jacot, E. Jequier, E. Maeder, J. Wahren and J. P. Felber (1981). "The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization." Diabetes 30(12): 1000-1007. Delibegovic, M., D. Zimmer, C. Kauffman, K. Rak, E. G. Hong, Y. R. Cho, J. K. Kim, B. B. Kahn, B. G. Neel and K. K. Bence (2009). "Liver-specific deletion of protein-tyrosine phosphatase IB (PTPIB) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress." Diabetes 58(3): 590-599. Dentin, R., S. Hedrick, J. Xie, J. Yates, 3rd and M. Montminy (2008). "Hepatic glucose sensing via the CREB coactivator CRTC2." Science 319(5868): 14021405. Dey, D., M. Mukherjee, D. Basu, M. Datta, S. S. Roy, A. Bandyopadhyay and S. Bhattacharya (2005). "Inhibition of insulin receptor gene expression and insulin signaling by fatty acid: interplay of PKC isoforms therein." Cell Physiol Biochem 16(4-6): 217-228. Dimitriadis GD, R. s. a. N. E. p.-. (2000). Integration od Some Biochemical and Physiologic effects of Insulin that may play a role in the Control of Blood Glucose Concentration. Chapter 15. in Diabetes Mellitus:A fundamental and Clinical Text. % LipponcottWilliams & Wilkins, PA. Dong, D. L. and G. W. Hart (1994). "Purification and characterization of an OGlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol." J_ Biol Chem 269(30): 19321-19330. Dudek, H., S. R. Datta, T. F. Franke, M. J. Birnbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan and M. E. Greenberg (1997). "Regulation of neuronal survival by the serine-threonine protein kinase Akt." Science 275(5300): 661665. Eguez, L., A. Lee, J. A. Chavez, C. P. Miinea, S. Kane, G. E. Lienhard and T. E. McGraw (2005). "Full intracellular retention of GLUT4 requires AS 160 Rab GTPase activating protein." Cell Metab 2(4): 263-272. 118 Elchebly, M., P. Payette, E. Michaliszyn, W. Cromlish, S. Collins, A. L. Loy, D. Normandin, A. Cheng, J. Himms-Hagen, C. C. Chan, C. Ramachandran, M. J. Gresser, M. L. Tremblay and B. P. Kennedy (1999). "Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-IB gene." Science 283(5407): 1544-1548. Elstrom, R. L., D. E. Bauer, M. Buzzai, R. Karnauskas, M. H. Harris, D. R. Plas, H. Zhuang, R. M. Cinalli, A. Alavi, C. M. Rudin and C. B. Thompson (2004). "Akt stimulates aerobic glycolysis in cancer cells." Cancer Res 64(11): 3892-3899. Emanuelli, B., P. Peraldi, C. Filloux, C. Chavey, K. Freidinger, D. J. Hilton, G. S. Hotamisligil and E. Van Obberghen (2001). "SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice." J Biol Chem 276(51): 47944-47949. Ezaki, O. (1990). "The insulin-like effects of selenate in rat adipocytes." J Biol Chem 265(2): 1124-1128. Felig, P., E. Marliss, O. E. Owen and G. F. Cahill, Jr. (1969). "Blood glucose and cluconeogenesis in fasting man." Arch Intern Med 123(3): 293-298. Flakoll PJ, C. M. a. C. A. (2000). Physiological Actions of Insulin.Chapter 14. Diabetes MellitusiA fundamental and Clinical Text., Lipponcott Williams & Wilkins, PA Forest, C. D., R. M. O'Brien, P. C. Lucas, M. A. Magnuson and D. K. Granner (1990). "Regulation of phosphoenolpyruvate carboxykinase gene expression by insulin. Use of the stable transfection approach to locate an insulin responsive sequence." Mol Endocrinol 4(9): 1302-1310. Frame, S., P. Cohen and R. M. Biondi (2001). "A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation." Mol Cell 7(6): 1321-1327. Friedman, J. E., T. Ishizuka, S. Liu, C. J. Farrell, D. Bedol, R. J. Koletsky, H. L. Kaung and P. Ernsberger (1997). "Reduced insulin receptor signaling in the obese spontaneously hypertensive Koletsky rat." Am J Physiol 273(5 Pt 1): E1014-1023. 119 Fukuda, H., A. Katsurada and N. Iritani (1992). "Nutritional and hormonal regulation of mRNA levels of lipogenic enzymes in primary cultures of rat hepatocytes." J_ Biochem 111(1): 25-30. Furnsinn, C, R. Englisch, K. Ebner, P. Nowotny, C. Vogl and W. Waldhausl (1996). "Insulin-like vs. non-insulin-like stimulation of glucose metabolism by vanadium, tungsten, and selenium compounds in rat muscle." Life Sci 59(23): 1989-2000. Gandy, J. C, A. E. Rountree and G. N. Bijur (2006). "Aktl is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1." FEBS Lett 580(13): 3051-3058. Gao, Y., J. Miyazaki and G. W. Hart (2003). "The transcription factor PDX-1 is posttranslationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 betacells." ArchBiochem^^ 155-163. Gao, Y., L. Wells, F. I. Comer, G. J. Parker and G. W. Hart (2001). "Dynamic Oglycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain." J Biol Chem 276(13): 9838-9845. Gao, Z., D. Hwang, F. Bataille, M. Lefevre, D. York, M. J. Quon and J. Ye (2002). "Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex." J Biol Chem 277(50): 48115-48121. Gerich, J. E. (1993). "Control of glycaemia." Baillieres Clin Endocrinol Metab 7(3): 551-586. Ghosh, R., B. Mukherjee and M. Chatterjee (1994). "A novel effect of selenium on streptozotocin-induced diabetic mice." Diabetes Res 25(4): 165-171. Goodyear, L. J., F. Giorgino, L. A. Sherman, J. Carey, R. J. Smith and G. L. Dohm (1995). "Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects." J Clin Invest 95(5): 21952204. Gottstein, U. (1979). "[Chewing and brain circulation]." Dtsch Med Wochenschr 104(50): 1768. 120 Gould, G. W. and G. D. Holman (1993). "The glucose transporter family: structure, function and tissue-specific expression." Biochem J 295 ( Pt 2): 329-341. Hajduch, E., D. R. Alessi, B. A. Hemmings and H. S. Hundal (1998). "Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells." Diabetes 47(7): 1006-1013. Hall, R. K., D. K. Scott, E. L. Noisin, P. C. Lucas and D. K. Granner (1992). "Activation of the phosphoenolpyruvate carboxykinase gene retinoic acid response element is dependent on a retinoic acid receptor/coregulator complex." Mol Cell Biol 12(12): 5527-5535. Haltiwanger, R. S., M. A. Blomberg and G. W. Hart (1992). "Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptidebeta-Nacetylglucosaminyltransferase." J Biol Chem 267(13): 9005-9013. Hanson, R. W. and L. Reshef (1997). "Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression." Annu Rev Biochem 66: 581-611. Haxhiu, M. A., I. A. Dreshaj, C. B. McFadden, B. O. Erokwu and P. Ernsberger (1999). "Moxonidine acting centrally inhibits airway reflex responses." AnnN Y Acad Sci 881: 372-382. Heart, E. and C. K. Sung (2003). "Insulin-like and non-insulin-like selenium actions in 3T3-L1 adipocytes." J Cell Biochem 88(4): 719-731. Hei, Y. J., S. Farahbakhshian, X. Chen, M. L. Battell and J. H. McNeill (1998). "Stimulation of MAP kinase and S6 kinase by vanadium and selenium in rat adipocytes." Mol Cell Biochem 178(1-2): 367-375. Hiromura, M., C. H. Choi, N. A. Sabourin, H. Jones, D. Bachvarov and A. Usheva (2003). "YY1 is regulated by O-linked N-acetylglucosaminylation (OglcNAcylation)." J Biol Chem 278(16): 14046-14052. Hirosumi, J., G. Tuncman, L. Chang, C. Z. Gorgun, K. T. Uysal, K. Maeda, M. Karin and G. S. Hotamisligil (2002). "A central role for JNK in obesity and insulin resistance." Nature 420(6913): 333-336. Holt, G. D. and G. W. Hart (1986). "The subcellular distribution of terminal Nacetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc." J Biol Chem 261(17): 8049-8057. 121 Holt, G. D., C. M. Snow, A. Senior, R. S. Haltiwanger, L. Gerace and G. W. Hart (1987). "Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine." J Cell Biol 104(5): 1157-1164. Housley, M. P., J. T. Rodgers, N. D. Udeshi, T. J. Kelly, J. Shabanowitz, D. F. Hunt, P. Puigserver and G. W. Hart (2008). "O-GlcNAc regulates FoxO activation in response to glucose." J Biol Chem 283(24): 16283-16292. Housley, M. P., N. D. Udeshi, J. T. Rodgers, J. Shabanowitz, P. Puigserver, D. F. Hunt and G. W. Hart (2009). "A PGC-lalpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose." J Biol Chem 284(8): 5148-5157. Hresko, R. C, H. Heimberg, M. M. Chi and M. Mueckler (1998). "Glucosamineinduced insulin resistance in 3T3-L1 adipocytes is caused by depletion of intracellular ATP." J Biol Chem 273(32): 20658-20668. Hwang, D., S. Seo, Y. Kim, C. Kim, S. Shim, S. Jee, S. Lee, M. Jang, M. Kim, S. Yim, S. K. Lee, B. Kang, I. Jang and J. Cho (2007). "Selenium acts as an insulin-like molecule for the down-regulation of diabetic symptoms via endoplasmic reticulum stress and insulin signalling proteins in diabetes-induced non-obese diabetic mice." J Biosci 32(4): 723-735. Imae, M., Y. Inoue, Z. Fu, H. Kato and T. Noguchi (2000). "Gene expression of the three members of hepatocyte nuclear factor-3 is differentially regulated by nutritional and hormonal factors." J Endocrinol 167(1): Rl-5. Imai, E., J. N. Miner, J. A. Mitchell, K. R. Yamamoto and D. K. Granner (1993). "Glucocorticoid receptor-cAMP response element-binding protein interaction and the response of the phosphoenolpyruvate carboxykinase gene to glucocorticoids." J Biol Chem 268(8): 5353-5356. Ishihara, H., P. Maechler, A. Gjinovci, P. L. Herrera and C. B. Wollheim (2003). "Islet beta-cell secretion determines glucagon release from neighbouring alphacells." Nat Cell Biol 5(4): 330-335. Iynedjian, P. B., A. Gjinovci and A. E. Renold (1988). "Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats." J Biol Chem 263(2): 740-744. Jones, P. F., T. Jakubowicz and B. A. Hemmings (1991). "Molecular cloning of a second form of rae protein kinase." Cell Regul 2(12): 1001-1009. 122 Kamemura, K., B. K. Hayes, F. I. Comer and G. W. Hart (2002). "Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens." J Biol Chem 277(21): 19229-19235. Katsurada, A., N. Iritani, H. Fukuda, Y. Matsumura, N. Nishimoto, T. Noguchi and T. Tanaka (1990). "Effects of nutrients and hormones on transcriptional and posttranscriptional regulation of fatty acid synthase in rat liver." Eur J Biochem 190(2): 427-433. Kelly, W. G., M. E. Dahmus and G. W. Hart (1993). "RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc." J_ Biol Chem 268(14): 10416-10424. Kerouz, N. J., D. Horsch, S. Pons and C. R. Kahn (1997). "Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse." J_ Clin Invest 100(12): 3164-3172. Kile, B. T., B. A. Schulman, W. S. Alexander, N. A. Nicola, H. M. Martin and D. J. Hilton (2002). "The SOCS box: a tale of destruction and degradation." Trends Biochem Sci 27(5): 235-241. Kim, Y. B., S. E. Nikoulina, T. P. Ciaraldi, R. R. Henry and B. B. Kahn (1999). "Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes." J Clin Invest 104(6): 733-741. Kim, Y. B., O. D. Peroni, T. F. Franke and B. B. Kahn (2000). "Divergent regulation of Aktl and Akt2 isoforms in insulin target tissues of obese Zucker rats." Diabetes 49(5): 847-856. Kim, Y. B., J. S. Zhu, J. R. Zierath, H. Q. Shen, A. D. Baron and B. B. Kahn (1999). "Glucosamine infusion in rats rapidly impairs insulin stimulation of phosphoinositide 3-kinase but does not alter activation of Akt/protein kinase B in skeletal muscle." Diabetes 48(2): 310-320. Klaman, L. D., O. Boss, O. D. Peroni, J. K. Kim, J. L. Martino, J. M. Zabolotny, N. Moghal, M. Lubkin, Y. B. Kim, A. H. Sharpe, A. Stricker-Krongrad, G. I. Shulman, B. G. Neel and B. B. Kahn (2000). "Increased energy expenditure, 123 decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase IB-deficient mice." Mol Cell Biol 20(15): 5479-5489. Klippel, A., W. M. Kavanaugh, D. Pot and L. T. Williams (1997). "A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain." Mol Cell Biol 17(1): 338-344. Kohn, A. D., S. A. Summers, M. J. Birnbaum and R. A. Roth (1996). "Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation." J Biol Chem 271(49): 31372-31378. Kole, H. K., S. Kole, B. P. Mallory, P. M. Li, B. J. Goldstein and M. Bernier (1998). "Inhibition of the transmembrane protein tyrosine phosphatase lar by 3Speptide-I enhances insulin receptor phosphorylation in intact cells." J Basic Clin Physiol Pharmacol 9(2-4): 111-126. Kolterman, O. G., R. S. Gray, J. Griffin, P. Burstein, J. Insel, J. A. Scarlett and J. M. Olefsky (1981). "Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus." J Clin Invest 68(4): 957969. Kulik, G., A. Klippel and M. J. Weber (1997). "Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt." Mol Cell Biol 17(3): 1595-1606. Kuo, M., V. Zilberfarb, N. Gangneux, N. Christeff and T. Issad (2008). "Oglycosylation of FoxOl increases its transcriptional activity towards the glucose 6-phosphatase gene." FEBS Lett 582(5): 829-834. Kurtz, J. W. and W. W. Wells (1981). "Induction of glucose-6-phosphate dehydrogenase in primary cultures of adult rat hepatocytes. Requirement for insulin and dexamethasone." J Biol Chem 256(21): 10870-10875. Kuwajima, M., S. Golden, J. Katz, R. H. Unger, D. W. Foster and J. D. McGarry (1986). "Active hepatic glycogen synthesis from gluconeogenic precursors despite high tissue levels of fructose 2,6-bisphosphate." J Biol Chem 261(6): 2632-2637. Lamers, W. H., R. W. Hanson and H. M. Meisner (1982). "cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei." Proc Natl Acad Sci U S A 79(17): 5137-5141. 124 Le Roith, D. and Y. Zick (2001). "Recent advances in our understanding of insulin action and insulin resistance." Diabetes Care 24(3): 588-597. Liao, J., A. Barthel, K. Nakatani and R. A. Roth (1998). "Activation of protein kinase B/Akt is sufficient to repress the glucocorticoid and cAMP induction of phosphoenolpyruvate carboxykinase gene." J Biol Chem 273(42): 27320-27324. Lizcano, J. M. and D. R. Alessi (2002). "The insulin signalling pathway." Curr Biol 12(7): R236-238. Love, D. C, J. Kochan, R. L. Cathey, S. H. Shin and J. A. Hanover (2003). "Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase." J Cell Sci 116(Pt 4): 647-654. Lubas, W. A. and J. A. Hanover (2000). "Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity." J Biol Chem 275(15): 10983-10988. Lucas, P. C, R. M. O'Brien, J. A. Mitchell, C. M. Davis, E. Imai, B. M. Forman, H. H. Samuels and D. K. Granner (1991). "A retinoic acid response element is part of a pleiotropic domain in the phosphoenolpyruvate carboxykinase gene." Proc Natl Acad Sci U S A 88(6): 2184-2188. Lum, J. J., T. Bui, M. Gruber, J. D. Gordan, R. J. DeBerardinis, K. L. Covello, M. C. Simon and C. B. Thompson (2007). "The transcription factor HIF-lalpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis." Genes Dev 21(9): 1037-1049. Maegawa, H., Y. Shigeta, K. Egawa and M. Kobayashi (1991). "Impaired autophosphorylation of insulin receptors from abdominal skeletal muscles in nonobese subjects with NIDDM." Diabetes 40(7): 815-819. Magun, R., B. M. Burgering, P. J. Coffer, D. Pardasani, Y. Lin, J. Chabot and A. Sorisky (1996). "Expression of a constitutively activated form of protein kinase B (c-Akt) in 3T3-L1 preadipose cells causes spontaneous differentiation." Endocrinology 137(8): 3590-3593. Majumder, P. K., P. G. Febbo, R. Bikoff, R. Berger, Q. Xue, L. M. McMahon, J. Manola, J. Brugarolas, T. J. McDonnell, T. R. Golub, M. Loda, H. A. Lane and W. R. Sellers (2004). "mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways." Nat Med 10(6): 594-601. 125 Mandarino, L. J., A. Consoli, A. Jain and D. E. Kelley (1993). "Differential regulation of intracellular glucose metabolism by glucose and insulin in human muscle." Am J Physiol 265(6 Pt 1): E898-905. Marshall, S., V. Bacote and R. R. Traxinger (1991). "Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance." J Biol Chem 266(8): 4706-4712. McClain, D. A. (2002). "Hexosamines as mediators of nutrient sensing and regulation in diabetes." J Diabetes Complications 16(1): 72-80. McGarry, J. D., M. Kuwajima, C. B. Newgard, D. W. Foster and J. Katz (1987). "From dietary glucose to liver glycogen: the full circle round." Annu Rev Nutr 7:51-73. McNeill, J. H., H. L. Delgatty and M. L. Battell (1991). "Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats." Diabetes 40(12): 1675-1678. Meier, R. and B. A. Hemmings (1999). "Regulation of protein kinase B." J Recept Signal Transduct Res 19(1-4): 121-128. Meikrantz, W., D. M. Smith, M. M. Sladicka and R. A. Schlegel (1991). "Nuclear localization of an O-glycosylated protein phosphotyrosine phosphatase from human cells." J Cell Sci 98 ( Pt 3): 303-307. Meshkani, R., M. Taghikhani, B. Larijani, S. Khatami, E. Khoshbin and K. Adeli (2006). "The relationship between homeostasis model assessment and cardiovascular risk factors in Iranian subjects with normal fasting glucose and normal glucose tolerance." Clin Chim Acta 371(1-2): 169-175. Michael, M. D., R. N. Kulkarni, C. Postic, S. F. Previs, G. I. Shulman, M. A. Magnuson and C. R. Kahn (2000). "Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction." Mol Cell 6(1): 87-97. Mooney, R. A., J. Senn, S. Cameron, N. Inamdar, L. M. Boivin, Y. Shang and R. W. Furlanetto (2001). "Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance." J Biol Chem 276(28): 25889-25893. 126 Moore, M. C, P. S. Hsieh, P. J. Flakoll, D. W. Neal and A. D. Cherrington (1999). "Net hepatic gluconeogenic amino acid uptake in response to peripheral versus portal amino acid infusion in conscious dogs." J Nutr 129(12): 2218-2224. Mourrieras, F., F. Foufelle, M. Foretz, J. Morin, S. Bouche and P. Ferre (1997). "Induction of fatty acid synthase and S14 gene expression by glucose, xylitol and dihydroxyacetone in cultured rat hepatocytes is closely correlated with glucose 6-phosphate concentrations." Biochem J 326 ( Pt 2): 345-349. Moustaid, N., R. S. Beyer and H. S. Sul (1994). "Identification of an insulin response element in the fatty acid synthase promoter." J Biol Chem 269(8): 5629-5634. Mueller, A. S. and J. Pallauf (2006). "Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice." J Nutr Biochem 17(8): 548-560. Nakatani, K., H. Sakaue, D. A. Thompson, R. J. Weigel and R. A. Roth (1999). "Identification of a human Akt3 (protein kinase B gamma) which contains the regulatory serine phosphorylation site." Biochem Biophys Res Commun 257(3): 906-910. Nordlie, R. C, J. D. Foster and A. J. Lange (1999). "Regulation of glucose production by the liver." Annu Rev Nutr 19: 379-406. O'Brien, R. M., P. C. Lucas, C. D. Forest, M. A. Magnuson and D. K. Granner (1990). "Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription." Science 249(4968): 533-537. O'Brien, R. M., E. L. Noisin, A. Suwanichkul, T. Yamasaki, P. C. Lucas, J. C. Wang, D. R. Powell and D. K. Granner (1995). "Hepatic nuclear factor 3- and hormone-regulated expression of the phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein 1 genes." Mol Cell Biol 15(3): 17471758. Obici, S., Z. Feng, G. Karkanias, D. G. Baskin and L. Rossetti (2002). "Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats." Nat Neurosci 5(6): 566-572. Ohsawa, I., K. Nishimaki, C. Yasuda, K. Kamino and S. Ohta (2003). "Deficiency in a mitochondrial aldehyde dehydrogenase increases vulnerability to oxidative stress in PC12 cells." J Neurochem 84(5): 1110-1117. 127 Okamoto, K. and F. Isohashi (2000). "Purification and primary structure of a macromolecular-translocation inhibitor II of glucocorticoid-receptor binding to nuclei from rat liver. Inhibitor II is the 11.5-kDa Zn2+-binding protein (parathymosin)." Eur J Biochem 267(1): 155-162. Ottensmeyer, F. P., D. R. Beniac, R. Z. Luo and C. C. Yip (2000). "Mechanism of transmembrane signaling: insulin binding and the insulin receptor." Biochemistry 39(40): 12103-12112. Park, E. A., D. C. Jerden and S. W. Bahouth (1995). "Regulation of phosphoenolpyruvate carboxykinase gene transcription by thyroid hormone involves two distinct binding sites in the promoter." Biochem J 309 ( Pt 3): 913919. Park, S. Y., J. Ryu and W. Lee (2005). "O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes." Exp Mol Med 37(3): 220-229. Parker, G. J., K. C. Lund, R. P. Taylor and D. A. McClain (2003). "Insulin resistance of glycogen synthase mediated by o-linked N-acetylglucosamine." J Biol Chem 278(12): 10022-10027. Patti, M. E., A. Virkamaki, E. J. Landaker, C. R. Kahn and H. Yki-Jarvinen (1999). "Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle." Diabetes 48(8): 1562-1571. Paulauskis, J. D. and H. S. Sul (1988). "Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3-L1 cells." J Biol Chem 263(15): 7049-7054. Pederson, T. M., D. L. Kramer and C. M. Rondinone (2001). "Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation." Diabetes 50(1): 24-31. Pilkis, S. J. and D. K. Granner (1992). "Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis." Annu Rev Physiol 54: 885-909. Pillay, T. S. and M. W. Makgoba (1992). "Enhancement of epidermal growth factor (EGF) and insulin-stimulated tyrosine phosphorylation of endogenous substrates by sodium selenate." FEBS Lett 308(1): 38-42. 128 Racek, J., V. Holecek and L. Trefil (2000). "[Antioxidative properties of ascorbic acid]." Cas Lek Cesk 139(19): 583-587. Rae, T. (1977). "Tolerance of mouse macrophages in vitro to barium sulfate used in orthopedic bone cement." J Biomed Mater Res 11(6): 839-846. Rank, K. B., P. K. Harris, L. C. Ginsberg and S. R. Stapleton (1994). "Isolation and sequence of a rat glucose-6-phosphate dehydrogenase promoter." Biochim Biophvs Acta 1217(1): 90-92. Rayman, M. P. (2002). "The argument for increasing selenium intake." Proc Nutr Soc 61(2): 203-215. Reaven, G. M. (1995). "Pathophysiology of insulin resistance in human disease." Physiol Rev 75(3): 473-486. Rencurel, F. and J. Girard (1998). "Regulation of liver gene expression by glucose." Proc Nutr Soc 57(2): 265-275. Rieusset, J., K. Bouzakri, E. Chevillotte, N. Ricard, D. Jacquet, J. P. Bastard, M. Laville and H. Vidal (2004). "Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients." Diabetes 53(9): 2232-2241. Robinson, K. A., D. A. Sens and M. G. Buse (1993). "Pre-exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rat skeletal muscles. Study of mechanisms in muscle and in rat-1 fibroblasts overexpressing the human insulin receptor." Diabetes 42(9): 13331346. Rognstad, R. (1979). "Rate-limiting steps in metabolic pathways." J Biol Chem 254(6): 1875-1878. Roquemore, E. P., A. Dell, H. R. Morris, M. Panico, A. J. Reason, L. A. Savoy, G. J. Wistow, J. S. Zigler, Jr., B. J. Earles and G. W. Hart (1992). "Vertebrate lens alpha-crystallins are modified by O-linked N-acetylglucosamine." J Biol Chem 267(1): 555-563. Ross, S. A., X. Chen, H. R. Hope, S. Sun, E. G. McMahon, K. Broschat and E. A. Gulve (2000). "Development and comparison of two 3T3-L1 adipocyte models of insulin resistance: increased glucose flux vs glucosamine treatment." Biochem Biophvs Res Commun 273(3): 1033-1041. 129 Rui, L., M. Yuan, D. Frantz, S. Shoelson and M. F. White (2002). "SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRSl and IRS2." J Biol Chem 277(44): 42394-42398. Rumberger, J. M., T. Wu, M. A. Hering and S. Marshall (2003). "Role of hexosamine biosynthesis in glucose-mediated up-regulation of lipogenic enzyme mRNA levels: effects of glucose, glutamine, and glucosamine on glycerophosphate dehydrogenase, fatty acid synthase, and acetyl-CoA carboxylase mRNA levels." J Biol Chem 278(31): 28547-28552. Sabio, G., M. Das, A. Mora, Z. Zhang, J. Y. Jun, H. J. Ko, T. Barrett, J. K. Kim and R. J. Davis (2008). "A stress signaling pathway in adipose tissue regulates hepatic insulin resistance." Science 322(5907): 1539-1543. Saltiel, A. R. and C. R. Kahn (2001). "Insulin signalling and the regulation of glucose and lipid metabolism." Nature 414(6865): 799-806. Sanders, J., M. Brandsma, G. M. Janssen, J. Dijk and W. Moller (1996). "Immunofluorescence studies of human fibroblasts demonstrate the presence of the complex of elongation factor-1 beta gamma delta in the endoplasmic reticulum." J Cell Sci 109 ( Pt 5): 1113-1117. Sano, H., S. Kane, E. Sano, C. P. Miinea, J. M. Asara, W. S. Lane, C. W. Garner and G. E. Lienhard (2003). "Insulin-stimulated phosphorylation of a Rab GTPaseactivating protein regulates GLUT4 translocation." J Biol Chem 278(17): 14599-14602. Sasaki, K., T. P. Cripe, S. R. Koch, T. L. Andreone, D. D. Petersen, E. G. Beale and D. K. Granner (1984). "Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin." J Biol Chem 259(24): 15242-15251. Scott, D. K., P. E. Stromstedt, J. C. Wang and D. K. Granner (1998). "Further characterization of the glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. The role of the glucocorticoid receptor-binding sites." Mol Endocrinol 12(4): 482-491. Semenza, G. L., P. H. Roth, H. M. Fang and G. L. Wang (1994). "Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1." J Biol Chem 269(38): 23757-23763. 130 Shaw, P., J. Freeman, R. Bovey and R. Iggo (1996). "Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus." Oncogene 12(4): 921-930. Shi, H., I. Tzameli, C. Bjorbaek and J. S. Flier (2004). "Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling." J Biol Chem 279(33): 34733-34740. Silva, J. C, R. Denny, C. A. Dorschel, M. Gorenstein, I. J. Kass, G. Z. Li, T. McKenna, M. J. Nold, K. Richardson, P. Young and S. Geromanos (2005). "Quantitative proteomic analysis by accurate mass retention time pairs." Anal Chem 77(7): 2187-2200. Silva, J. C, M. V. Gorenstein, G. Z. Li, J. P. Vissers and S. J. Geromanos (2006). "Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition." Mol Cell Proteomics 5(1): 144-156. Sindelar, D. K., J. H. Balcom, C. A. Chu, D. W. Neal and A. D. Cherrington (1996). "A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog." Diabetes 45(11): 15941604. Sindelar, D. K., C. A. Chu, M. Rohlie, D. W. Neal, L. L. Swift and A. D. Cherrington (1997). "The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog." Diabetes 46(2): 187-196. Singh, R., Y. Wang, Y. Xiang, K. E. Tanaka, W. A. Gaarde and M. J. Czaja (2009). "Differential effects of JNKl and JNK2 inhibition on murine steatohepatitis and insulin resistance." Hepatology 49(1): 87-96. Sinha, M. K., W. J. Pories, E. G. Flickinger, D. Meelheim and J. F. Caro (1987). "Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM." Diabetes 36(5): 620-625. Soesanto, Y. A., B. Luo, D. Jones, R. Taylor, J. S. Gabrielsen, G. Parker and D. A. McClain (2008). "Regulation of Akt signaling by O-GlcNAc in euglycemia." Am J Physiol Endocrinol Metab 295(4): E974-980. Stapleton, S. R., G. L. Garlock, L. Foellmi-Adams and R. F. Kletzien (1997). "Selenium: potent stimulator of tyrosyl phosphorylation and activator of MAP kinase." Biochim Biophvs Acta 1355(3): 259-269. 131 Stapleton, S. R., G. J. Stevens, J. F. Teel, K. B. Rank, E. A. Berg, J. Y. Wu, L. C. Ginsberg and R. F. Kletzien (1993). "Effects of acetaldehyde on glucosesphosphate dehydrogenase activity and mRNA levels in primary rat hepatocytes in culture." Biochimie 75(11): 971-976. Sundqvist, A., M. T. Bengoechea-Alonso, X. Ye, V. Lukiyanchuk, J. Jin, J. W. Harper and J. Ericsson (2005). "Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7)." Cell Metab 1(6): 379-391. Sutherland, C, I. A. Leighton and P. Cohen (1993). "Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling." Biochem J 296 ( Pt 1): 15-19. Sutherland, C, R. M. O'Brien and D. K. Granner (1996). "New connections in the regulation of PEPCK gene expression by insulin." Philos Trans R Soc Lond B Biol Sci 351(1336): 191-199. Suzuki, Y., T. Muramatsu, M. Taniyama, Y. Atsumi, R. Kawaguchi, S. Higuchi, K. Hosokawa, T. Asahina, C. Murata and K. Matsuoka (1996). "Association of aldehyde dehydrogenase with inheritance of NIDDM." Diabetologia 39(9): 1115-1118. Terauchi, Y., Y. Tsuji, S. Satoh, H. Minoura, K. Murakami, A. Okuno, K. hiukai, T. Asano, Y. Kaburagi, K. Ueki, H. Nakajima, T. Hanafusa, Y. Matsuzawa, H. Sekihara, Y. Yin, J. C. Barrett, H. Oda, T. Ishikawa, Y. Akanuma, I. Komuro, M. Suzuki, K. Yamamura, T. Kodama, H. Suzuki, S. Koyasu, S. Aizawa, K. Tobe, Y. Fukui, Y. Yazaki and T. Kadowaki (1999). "Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase." Nat Genet 21(2): 230-235. Torisu, T., N. Sato, D. Yoshiga, T. Kobayashi, T. Yoshioka, H. Mori, M. Iida and A. Yoshimura (2007). "The dual function of hepatic SOCS3 in insulin resistance in vivo." Genes Cells 12(2): 143-154. Torres, C. R. and G. W. Hart (1984). "Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc." J Biol Chem 259(5): 3308-3317. Traxinger, R. R. and S. Marshall (1992). "Insulin regulation of pyruvate kinase activity in isolated adipocytes. Crucial role of glucose and the hexosamine 132 biosynthesis pathway in the expression of insulin action." J Biol Chem 267(14): 9718-9723. Ueki, K., T. Kondo and C. R. Kahn (2004). "Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms." Mol Cell Biol 24(12): 5434-5446. Ueki, K., R. Yamamoto-Honda, Y. Kaburagi, T. Yamauchi, K. Tobe, B. M. Burgering, P. J. Coffer, I. Komuro, Y. Akanuma, Y. Yazaki and T. Kadowaki (1998). "Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis." J Biol Chem 273(9): 5315-5322. Ursini, M. V., A. Parrella, G. Rosa, S. Salzano and G. Martini (1997). "Enhanced expression of glucose-6-phosphate dehydrogenase in human cells sustaining oxidative stress." Biochem J 323 ( Pt 3): 801-806. Valera, A., A. Pujol, M. Pelegrin and F. Bosch (1994). "Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulindependent diabetes mellitus." Proc Natl Acad Sci U S A 91(19): 9151-9154. Vanderford, N. L., S. S. Andrali and S. Ozcan (2007). "Glucose induces MafA expression in pancreatic beta cell lines via the hexosamine biosynthetic pathway." J Biol Chem 282(3): 1577-1584. Vanhaesebroeck, B. and D. R. Alessi (2000). "The PI3K-PDK1 connection: more than just a road to PKB." Biochem J 346 Pt 3: 561-576. Vasiliou, V. and D. W. Nebert (2005). "Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family." Hum Genomics 2(2): 138-143. Veerababu, G., J. Tang, R. T. Hoffman, M. C. Daniels, L. F. Hebert, Jr., E. D. Crook, R. C. Cooksey and D. A. McClain (2000). "Overexpression of glutamine: fructose-6-phosphate amidotransferase in the liver of transgenic mice results in enhanced glycogen storage, hyperlipidemia, obesity, and impaired glucose tolerance." Diabetes 49(12): 2070-2078. Vosseller, K, L. Wells, M. D. Lane and G. W. Hart (2002). "Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes." Proc Natl Acad Sci US A 99(8): 5313-5318. 133 Vuguin, P., E. Raab, B. Liu, N. Barzilai and R. Simmons (2004). "Hepatic insulin resistance precedes the development of diabetes in a model of intrauterine growth retardation." Diabetes 53(10): 2617-2622. Wagle, A., S. Jivraj, G. L. Garlock and S. R. Stapleton (1998). "Insulin regulation of glucose-6-phosphate dehydrogenase gene expression is rapamycin-sensitive and requires phosphatidylinositol 3-kinase." J Biol Chem 273(24): 14968-14974. Walgren, J. L., T. S. Vincent, K. L. Schey and M. G. Buse (2003). "High glucose and insulin promote O-GlcNAc modification of proteins, including alpha-tubulin." Am J Physiol Endocrinol Metab 284(2): E424-434. Wang, D. and H. S. Sul (1998). "Insulin stimulation of the fatty acid synthase promoter is mediated by the phosphatidylinositol 3-kinase pathway. Involvement of protein kinase B/Akt." J Biol Chem 273(39): 25420-25426. Wang, J. C , J. M. Stafford, D. K. Scott, C. Sutherland and D. K. Granner (2000). "The molecular physiology of hepatic nuclear factor 3 in the regulation of gluconeogenesis." J Biol Chem 275(19): 14717-14721. Weinhouse, S. (1976). "Regulation of glucokinase in liver." Curr Top Cell Regul 11: 1-50. Weyer, C , C. Bogardus, D. M. Mott and R. E. Pratley (1999). "The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus." J Clin Invest 104(6): 787-794. Whelan, S. A., M. D. Lane and G. W. Hart (2008). "Regulation of the O-linked betaN-acetylglucosamine transferase by insulin signaling." J Biol Chem 283(31): 21411-21417. White, M. F. (1997). "The insulin signalling system and the IRS proteins." Diabetologia 40 Suppl 2: S2-17. Will, J. C. and T. Byers (1996). "Does diabetes mellitus increase the requirement for vitamin C?" NutrRev 54(7): 193-202. Wolfrum, C , D. Besser, E. Luca and M. Stoffel (2003). "Insulin regulates the activity of forkhead transcription factor Hnf-3beta/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization." Proc Natl Acad Sci U S A 100(20): 11624-11629. 134 Wroblewski, F. and J. S. Ladue (1955). "Lactic dehydrogenase activity in blood." Proc Soc Exp Biol Med 90(1): 210-213. Xu, J., D. Maki and S. R. Stapleton (2003). "Mediation of cadmium-induced oxidative damage and glucose-6-phosphate dehydrogenase expression through glutathione depletion." J Biochem Mol Toxicol 17(2): 67-75. Yang, X., P. P. Ongusaha, P. D. Miles, J. C. Havstad, F. Zhang, W. V. So, J. E. Kudlow, R. H. Michell, J. M. Olefsky, S. J. Field and R. M. Evans (2008). "Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance." Nature 451(7181): 964-969. Zabolotny, J. M., F. G. Haj, Y. B. Kim, H. J. Kim, G. I. Shulman, J. K. Kim, B. G. Neel and B. B. Kahn (2004). "Transgenic overexpression of protein-tyrosine phosphatase IB in muscle causes insulin resistance, but overexpression with leukocyte antigen-related phosphatase does not additively impair insulin action." J Biol Chem 279(23): 24844-24851. Zdychova, J. and R. Komers (2005). "Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications." Physiol Res 54(1): 1-16. Zhang, F., K. Su, X. Yang, D. B. Bowe, A. J. Paterson and J. E. Kudlow (2003). "OGlcNAc modification is an endogenous inhibitor of the proteasome." Cell 115(6): 715-725. Zierath, J. R. and H. Wallberg-Henriksson (2002). "From receptor to effector: insulin signal transduction in skeletal muscle from type II diabetic patients." Ann N Y Acad Sci 967: 120-134. 135