* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Artificial Small-Molecule Peptide Synthesizer
Survey
Document related concepts
Ancestral sequence reconstruction wikipedia , lookup
Citric acid cycle wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein structure prediction wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Expanded genetic code wikipedia , lookup
Self-assembling peptide wikipedia , lookup
Biochemistry wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Transcript
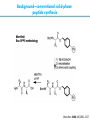
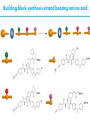
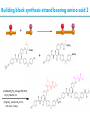
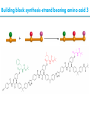
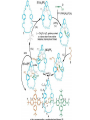
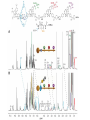
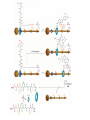
Artificial small-molecule peptide synthesizer Leigh, D.A. et al. Science. 2013,339,189-193 Lingbowei Hu Burke Group Literature Seminar 4.12.2014 Framework of the small-molecule machine Background—nonribosomal peptide synthesis PCP: peptidyl carrier protein Chem. Rev. 2005,105,715-738 Background—conventional solid-phase peptide synthesis Chem.Rev. 2000,100,2091-2157 Framework of the small-molecule machine Building block synthesis-strand bearing amino acid Building block synthesis-strand bearing amino acid 2 + + i) Cu(MeCN)4PF6,TentagelTM TBTA, CH2Cl2:tBuOH=1:1 ii) AgNO3, acetone:H2O=4:1, 43% over, 2 steps Building block synthesis-strand bearing amino acid 3 + Building block synthesis—rotaxane formation Cu(MeCN)4PF6, CH2Cl2:tBuOH=1:1 7d, 32% yield Building block synthesis—rotaxane formation 2 PhNH2(cat.), DMSO:MES-buffer=3:1 90% yield Small-molecule machine—ready, go! Reaction condition: 60oC under microwave heating, MeCN:DMF=3:1 N,N-diisopropylethylamine, tris(2-carboxyethyl)phosphine, 36h Identification of the products Peptide synthesis occurs overwhelmingly within the confines of the molecular machine • Neither peptide with different sequences nor with more or less than one Phe, Leu or Ala residue was found • In a control experiment where nonthreaded strand and macrocycle were used, several products were found but no desired product was detected Recent progress • The yield of rotaxane was increased through a change of sequence in the synthetic route • A seven peptide containing chain was synthesized Leigh,D.A. et al. JACS, 2014. ASAP Discussion • Demonstrated more or less the potential of small-molecule machines • The design could be employed in other types of reactions • Application? • Long reaction time due to the movement of the macrocycle, low efficiency, rotaxane building is labor intense -12h per amide bond, 30% yield (53%yield in subsequent work) -Ribosome:15 to 20 amide bonds per second -Conventional solid-phase peptide synthesis: 2 to 4 hours per bond Discussion 2 • The first three Cys-Gly-Gly is required for successful synthesis • The size of the oligopeptide chain is restrict by the design of the machine Discussion 3 • Strand has to be synthesized separately for different peptides, combined in desired sequence manually and the sequence information is lost after it is translated into the product Discussion 4 • The scope of amino acid substrate was not demonstrated. There might be problems with certain amino acids such as Lys, Arg, Cys Summary • The first small-molecule peptide synthesizer which resembles peptide synthesis of ribosome was synthesized and proved to work • Up to 4 amino acids can be attached in specific sequence to an certain 3-amino-acid peptide chain, with a rate of approximately 12h per amino acid • Although inspiring, the further development of this system faces a lot restrictions and challenges Welcome to wonderland