* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Neurotoxic Effect of Paracetamol Overdose on Rat Brain Amina E
Neural oscillation wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neuroinformatics wikipedia , lookup
Dendritic spine wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Causes of transsexuality wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Human brain wikipedia , lookup
Neurolinguistics wikipedia , lookup
Donald O. Hebb wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neural engineering wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroeconomics wikipedia , lookup
Brain morphometry wikipedia , lookup
Environmental enrichment wikipedia , lookup
Synaptic gating wikipedia , lookup
Brain Rules wikipedia , lookup
Selfish brain theory wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Spike-and-wave wikipedia , lookup
History of neuroimaging wikipedia , lookup
Development of the nervous system wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuropsychology wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Aging brain wikipedia , lookup
Optogenetics wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Neuroplasticity wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Channelrhodopsin wikipedia , lookup
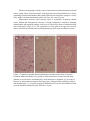
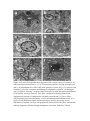
Neurotoxic Effect of Paracetamol Overdose on Rat Brain Amina E Essawy1, Ahmed A Soffar1, Afrah F Alkhuriji2 1 Department of Zoology, Faculty of Science, Alexandria University, Alexandria, Egypt. 2 Department of Zoology, College of Science, King Saud University, Saudi Arabia. Corresponding author: [email protected] Abstract Objective:Paracetamol (Acetaminophen) is one of the most popular over the counter medications that is commonly used an anti-inflammatory, pain killer and to relieve fever and headaches. Despite the several therapeutic benefits, it is well known that an overdose of Paracetamolcan lead to hepatic and renal damage. Also, considering that brain cells is one of the main targets for Paracetamol in the body, the effect of an overdosage of Paracetamol on the brainhas been poorly investigated. Methods: Adult male Wistar rats were grouped into 2 groups of 8 each – control and Paracetamol-treated groups. The control group was administered orally with distilled water. The experimental group was administered orally with Paracetamol(650mg/kg b.wt.) for 15 days. Blood was collected to determine AchE level and brain was dissected out for neuroanatomical, histological, and ultrastructural studies. Results:In the present study, treatment of an overdose of Paracetamol caused significant elevation in the level of AchE in the plasma of treated male rats. Moreover, rapid Golgi-cox staining method showed that, Paracetamolinduced morphological aberrations and a dramatic reduction in the density of dendritic spines in cortical brain cells. Histological and ultrastructure alterations were also recorded in the neurons ofParacetamol-treated rats. These alterations included both nucleus and cytoplasm. Conclusion:Taken together,results of this work point to a clear neurotoxic effect of Paracetamol over-dosage. Keywords: Paracetamol, rats, neurotoxicity, AchE, histopathology Introduction Paracetamol (Acetaminophen, N-Acetyl-p-Aminophenol)is widely used for its analgesic and antipyretic properties in many over-the-counter formulations in both adults and children1. As an OTC, the main problem is the misuse of Paracetamol through intentional or unintentional uptake of supratherapeuticdoses, which usually leads to hepatic and renal adverse effects in both humans and experimental animals2. Paracetamol is mainly metabolized in the liver via conjugation with glucuronic acid and sulphate and finally excreted in urine. Although, Paracetamol metabolism yields a cytochrome P450-dependent highly reactive metabolite, N-acetyl-pbenzoquinonimine (NAPQI) which reacts with glutathione (GSH) to form a non-toxic conjugate that will be excretedvia the kidney3. However, overdosages ofParacetamol saturates these essential metabolic routes thus the rate of production of NAPQI exceeds the capacity to detoxification. The excess NAPQIbinds to cellular proteins, including mitochondrial proteins leading to liver damage associated with oxidative stress4.Cytochrome P450 isoform CYP2E1 is also expressed in the brain, suggesting that Paracetamol might be metabolized by neurons producing the toxic metabolite NAPQI Despite intensive investigations of the toxic effect on liver and kidney tissues, information about pathological effects of Paracetamol on the brain and neuronal cells is relatively rare.Hence it is important to investigate the possible adverse effects of Paracetamol overdosageon morphological, histological and subcellular structures of the brain tissue. Also, we investigated the effect of Paracetamol on the physiological activity of Acetylcholinesterase (AchE). Materials and methods A total of 16 adult male Wistaralbino rats of 150-160 g body weight were used in this study. They were housed in standard metallic cages (4 rats per cage) and kept in a temperature-controlled environment (22 ± 2°C) with an alternating 12 h light-dark cycle. Rats were acclimatizedto the lab environment for 1week prior to the experiment.The animals had free access to commercial food pellets and clean drinking water. The experiments were done in compliance with the Guide for the Care and Use of Laboratory Animals. Paracetamol (Panadol665, GlaxoSmithKlineAustralia Pty Limited, Australia) was obtained from a local medical store (Alexandria, Egypt). Kits for the measurement of serum AchE were purchased from Span Diagnostics Ltd. (Surat India). Animals were randomly divided into 2 groups of 8 rats each.Group 1: Animals were orally given distilled water 2ml/kg b.wt. for 15 days and served as controls.Group 2: Animals were orally given Paracetamol (650mg/kg b.wt. dissolved in 2ml distilled water) daily for 15 days5. After 15 days of treatment, blood samples were collected from both control and experimental animals for determination of AchE activity,then animals were sacrificed andthe brain wasquickly removed and prepared for neuroanatomical, histological and ultrastructural studies. Serum was separated out from blood samples by centrifugation at 3000 rpm for 10 min.AchE activity was determined in plasma using acetylcholine iodide as a substrate6. According to this method,AchE in samples hydrolyzes acetylthiocholine iodide into thiocholine and butyric acid. The thiocholine reacts with 5,5′-dithiobis-2-nitrobenzoic acid to form 5-thio-2nitrobenzoic acid. The developed yellow color is measured spectrophotometrically at 412 nm (Elico-SL177, Elico LTD. Hyderabad Andra Pradesh, India). For detection of the morphological changes in dendrites and axons, rapid Golgi-cox staining technique was used. Animals from control and Paracetamol-treated groups were deeply anesthetized with diethyl either. Brains were carefully removed from skull and immersed en-block in rapid Golgi solution in colored bottles for 1 day and replaced with fresh fixative. The composition of Golgi solution was: 5% potassium dichromate, 5% chloral hydrate, 4% Glutaraldehyde, 2.5% formaldehyde, 0.5% DMSO.Twenty-four hours later, the fixative in which the tissue was immersed on day 1 was slowly poured out. The tissue was rinsed for 2 days by adding a small amount of fresh fixative and kept in the dark chamber every 24 hours. The tissue blocks remained undisturbed in the fixative for another 24 hours and then were rinsed for 4 days in 0.75% aqueous solution of silver nitrate (AgNO3) till the reddish brown color of the potassium dichromate-silver complex disappeared. Tissue pieces were placed in a petri dish and silver deposits were gently brushed off. After dehydration, the tissue blocks were carefully mounted onto block holders with required orientation and embedded with paraffin wax. The brain tissue was cut into 100μm thick sections in horizontal plane. The sections were collected in a petri-dish containing 100% alcohol and transferred to xylene for clearing and finally were mounted serially on slides with DPX.Slides images were captured using an Olympus Fluorescent Microscope imaging system. For histopathological study, autopsy samples were taken from the brain of rats of control and Paracetamol-treated animals and fixed in 10% formalin for 24 h. Washing was doneby rinsing the sample in tap water for 24 h. Sample dehydration was performed using serial dilutions of ethanol. Specimens were cleared in xylene and embedded in paraffin. Paraffin sections (5m) were stained by hematoxylin and eosin stains for histopathological examination. For ultrastructural examination, small pieces of brain were immediately immersed in 4F1G in phosphate buffer (pH 7.2) fixation mixture for 3 hr at 4°C then post-fixed in 2% OsO4 (Osmium tetroxide) at 4°C for 2 hr. The specimens were dehydrated in graded series of ethanol, and then embedded in Epon-araldite mixture in labeled beam capsules. LKB ultramicrotome was used to obtain ultrathin sections (50 nm thick) which were picked upon 200 mesh naked copper grids. Grids were double stained with uranyl acetate for ½ h and lead citrate for 20-30 min. Scoping the grids was achieved by using Jeol 100 CX Transmission electron microscope (TEM). The results were expressed as mean ± SD were analyzed by student’st-test using Microsoft excel® 2016. Values of p< 0.05 were considered to be statistically significant. Results Activity of AchE The activity of AchE in plasma of control and Paracetamol- treated rats are shown in Figure (1). We found a significant (p<0.05) 3-fold increase in the level of AchEactivity in Paracetamol-treated rats as compared to control rats (174.7 ± 58.8 & 53 ± 12.5 unit/L respectively). Figure 1. Serum levels of acetylcholinesterase (AChE) activity in control- and Paracetamol-treated rats. Values are mean ± SD for six rats in each group. *p < 0.05. Neuroanatomical observations In the brain sections of control rats (Figs 2a, b), the neuronal cell bodies together with their projections are stained dark brown in the preparation impregnated by the rapid Golgi method.Theneural spines are well recognizable and appeared as membranous protrusions arise from the neuronal surface of the dendrites. Abnormalities in the morphology and dramatic reduction in the density of these dendritic spines were observed in brain sections from animals treated with Paracetamol (Figs 2c&d). Figure 2. light micrographs of cortical neurons from control and Paracetamol-treated rats (Golgi-cox stain). (a) A control neuron from control rat with normal architecture of perikaryon, axon and dendrites. (b) A typical dendron of a neuron of control rat carrying many dendritic spines (enlarged part). (c) A neuron from Paracetamol-treated rat showing degenerated parts a long its axon (arrow). (d) A dendron from Paracetamol-treated rat showing dramatic reduction in spine number as compared with control neurons. (a - d), Scale bar, 10 μm; enlarged parts, 5 μm. Histological and ultrastructural observations Normal histological architecture of the brain tissue was shown in brain sections from controlrats (Fig. 3a). However, brain of Paracetamol-treated rats showed severalhistopathologicalalterationsin both neurons and neurogliacells.The neurons appeared withsigns of degeneration, including cytolysis and chromatolysis (Fig. 3b). Also, spongiform necrosis and nuclear pyknosiswere observed (Fig. 3b). Degenerated neuroglia appeared containing pyknotic nuclei while congested cerebral blood vessels showed detached endothelial wall (Fig. 3c). Electron micrographs of brain cortex in untreated rats showed normal neuronal somas, along with a normal neuropile with myelinated and unmyelinated nerve fibers containing normal mitochondria and normal light and electron dense synaptic vesicles (Figs 4a&b). Normal protoplasmic glial cells were also seen (Fig. 4a). Degenerated neurons with complete lyses of cytoplasm, irregularly shaped nuclei, dilated and disorganized cisternae of rough endoplasmic reticulum, damaged mitochondria and ruptured synaptic vesicles were observed in brain of animals treated with Paracetamol (Figs 4c-e). The cytoplasm of some glial cells appeared electron dense, with altered mitochondria and disorganization of the inner membrane system. Figure 3. Light micrographs showing histological changes in the brain of rat after treatment with Paracetamol (a) a group of cortical neurons of control rat with large globular or ovoid nuclei surrounded by a small amount of cytoplasm. (b) A group of neurons of Paracetamol-treated rat showing signs of degeneration including cytolysis and chromatolysis. (c) Cerebral congested, dilated blood vessel in Paracetamol-treated rat with detached endothelial wall. Scale bar, 20 μm. Figure 4. Electron micrographs showing parts of the cerebral cortex of control (a, b) and Paracetamol-treated rats (c - f). (a) Two neurons and their serving microglia cell (MC). (b) Myelinated nerve fiber (NF) with synaptic vesicles (SV). (c) A neuron with complete lysis of the cytoplasm and damaged cytoplasmic organelle. (d) Enlarged part of a neuron, showing , irregular-shaped nucleus (N) with indentation and dilation of the nuclear envelope (arrows). Note also: cytoplasm containing dilated and disorganized cisternae of endoplasmic reticulum (arrowheads). (e) Nerve fibers (NF)containing degenerated synaptic vesicles and damaged mitochondria (M). (f) A glial cell with abnormal outline and electron dense nucleus possessing severe indentation of nuclear envelope and peripherally located nucleolus (Nu). Arrowheads indicate fragments of dilated rough endoplasmic reticulum. Scale bar, 500 nm. Discussion In the present study, overdose treatment with Paracetamol (650 mg/kg b.wt.) caused a significant elevation in the level of AchE in the plasma of treated rats. Moreover, it produced morphological, histological and ultrastructure alterations which indicates a possible brain injury. The elevated activity of AchE could be attributed to neuronal membrane damage due to increased lipid peroxidation which is one of the main manifestations of oxidative damage7.An overdose treatment of Paracetamolcaused a significant increase in malondialdehyde (MDA) which is associated with a remarkable decrease of total antioxidant capacity in the brain of male albino rats 8. The elevated level of MDA in the brain following ahighParacetamol dose correlates with an increased rate of lipid peroxidation.The increasedAchE activity following administration of high doses of Paracetamol positively reduce the cholinergic neurotransmission efficiency by decreasing the level of acetylcholine in the synaptic cleft, but, on the other hand, may leadto anoxidative stress-mediated structural alteration in neurons.On the contrary, a low dose of Paracetamol may protect cerebral cortical neurons from oxidative stress and limits neuronal inflammation by reducing menadione- mediated oxidative neurotoxicity9. In the present work, neuroanatomical study using rapid Golgi staining technique revealed a dramatic reduction in the number of neural spines upon overdose Paracetamol treatment. Dendritic spines are small membrane protrusions from dendritic shafts.They contain several essential compartments for synaptic function and plasticity such as glutamate receptors, and signaling systems and they are the primary locations of excitatory synapses10.Defect in morphology or alterations in density of neural spines is associated with a growing number of neurological disorders, includingautism spectrum disorders (ASD), schizophrenia and Alzheimer's disease1113 . Our histological and ultrastructure investigation revealed different degenerative changes in brain cells in response to Paracetamol treatment. These findings are in agreement with Posadas et al.14, who reported a deleterious effect ofParacetamolon cortical neurons, both in vivo and in vitro. In addition, Paracetamol induces neuronal damage in cerebral granular cells15. Paracetamol is able to activate the neuronal CYP2E1 thus generating toxic metabolites such as NAPQI. The formation of NAPQI metabolite decreases GSH levels leading to oxidative stress and neurotoxicity.Overdose of Paracetamol decreases the levels of anti-oxidative stress candidates such as glutathione, ascorbic acid and is associated with decreased superoxide dismutase activity16. Excessive production of reactive oxygen species in brain and the disturbance in balance between oxidative species antioxidant defenses are associated to pathological changes in neurodegenerative diseases . In conclusion, the results of this study point to the fact that administration of overdose ofParacetamol is neurotoxic. The remarkable degenerative changes upon Pacarcetamol administration in brain cells of albino rats may be attributed for oxidative stress. Future studies are necessary to clarify the exact neurotoxic mechanism of Paracetamol in case of being used at high doses. References 1. Sood S, Howell J, Sundararajan V, et al. Paracetamol overdose in Victoria remains a significant health-care burden. J Gastroenterol Hepatol . 2013;28:1356–60. 2. Gulnaz H, Tahir M, Munir B, Sami W. Protective effects of garlic oil on Acetamophine induced nephrotoxicity in male albino rats. Biomedica. 2010;26:9-15. 3. Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499-516. 4. Jóźwiak-Bebenista M, Nowak JZ. Paracetamol: Mechanism of action, application and safety concern. Acta Poloniae Pharmaceutica and Drug Research. 2014; 71(1): 11-23. 5. Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat.Food Chem Toxicol. 2010; 48(11): 3246-3261. 6. Jamshhidzade A, Nicknahad H, Mohammadi- Bardbori A, Talati M . Comparative measurement of serum Acetyl Cholinesterase Enzyme using three different methods. Iranian Journal of Toxicology. 2009; 2(4): 268-272. 7. Samanta P , Sandipan Pal S, MukherjeeAK, Ghosh AR. Biochemical Effects of Glyphosate Based Herbicide, Excel Mera 71 on Enzyme Activities of Acetylcholinesterase (AChE), Lipid Peroxidation (LPO), Catalase (CAT), Glutathione-S-transferase (GST) and Protein Content on Teleostean Fishes. Ecotox Environ Safe. 2014; 107:120-125 . 8. Mohammed, ET, Safwat, GM. Assessment of the ameliorative role of selenium nanoparticles on the oxidative stress of acetaminophen in some tissues of male albino rats.Beni-Suef University Journal of Basic and Applied Sciences. 2013;2(2):80-85. 9. Tripathy D, and Grammas P. Acetaminophen inhibits neuronal inflammation and protects neurons from oxidative stress. J Neuroinflam. 2009; 6:10-17. 10. Yu w Lu B . Synapses and Dendritic Spines as Pathogenic Targets in Alzheimer’s Disease.Neural Plasticity. 2012 ; doi:10.1155/2012/24715.Article ID 2471500. 11. Tackenberg C, Ghori A, Brandt R. Thin, stubby or mushroom: spine pathology in Alzheimer’s disease. Curr. Alzheimer Res. 2009; 6:261–268. 12. Lin, Y. C. & Koleske, A. J. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu. Rev. Neurosci. 2010; 33: 349–378. 13. Lai KO, Jordan BA, Ma XM, Srivastava DP, Tolias KF. Molecular mechanisms of dendritic spine development and plasticity.Neural Plast. 2016; 2078121. doi: 10.1155/2016/2078121. 14. Posadas I. Santos P. Blanco A. Munzo-Fernadez M. Cena V. Acetaminophen induces apoptosis in rat cortical neurons. PLoS One. 2010; 5:15360. 15. Mohamed H M, Hazem A M and Ashraf HA. Effects of paracetamol and Monosodium Glutamate on cerebellar granule cells of the adult male albino rats: A histological and morphometric study. Med. J. Cairo Univ. 2014;82(2):289-302. 16. Hamza R , Al-Harbi M.Amelioration of paracetamol hepatotoxicity and oxidative stress on mice liver withsilymarin and Nigella sativa extract supplements. Asian Pac J Trop Biomed .2015; 5(7): 521-531