* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Disease Modeling Using Embryonic Stem Cells
Neuroregeneration wikipedia , lookup
Electrophysiology wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Environmental enrichment wikipedia , lookup
Axon guidance wikipedia , lookup
Neural oscillation wikipedia , lookup
Brain-derived neurotrophic factor wikipedia , lookup
Mirror neuron wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neural coding wikipedia , lookup
Synaptogenesis wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Central pattern generator wikipedia , lookup
Subventricular zone wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Multielectrode array wikipedia , lookup
Nervous system network models wikipedia , lookup
Synaptic gating wikipedia , lookup
Development of the nervous system wikipedia , lookup
Circumventricular organs wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Neuroanatomy wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Optogenetics wikipedia , lookup
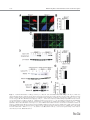
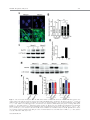
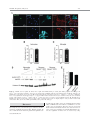
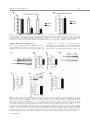
EMBRYONIC STEM CELLS/INDUCED PLURIPOTENT STEM CELLS Disease Modeling Using Embryonic Stem Cells: MeCP2 Regulates Nuclear Size and RNA Synthesis in Neurons MORTEZA YAZDANI,a RUBÉN DEOGRACIAS,a JACKY GUY,b RAYMOND A. POOT,c ADRIAN BIRD,b YVES-ALAIN BARDEa a Biozentrum, University of Basel, Basel, Switzerland; bWellcome Trust Centre for Cell Biology, University of Edinburgh, Kings Buildings, Mayfield Road, Edinburgh, United Kingdom; cDepartment of Cell Biology, Erasmus Medical Center (MC), Rotterdam, The Netherlands Key Words. Rett syndrome • Nuclear size • Transcription • Astrocytes • Brain-derived neurotrophic factor • Synaptophysin ABSTRACT Mutations in the gene encoding the methyl-CpG-binding protein MECP2 are the major cause of Rett syndrome, an autism spectrum disorder mainly affecting young females. MeCP2 is an abundant chromatin-associated protein, but how and when its absence begins to alter brain function is still far from clear. Using a stem cell-based system allowing the synchronous differentiation of neuronal progenitors, we found that in the absence of MeCP2, the size of neuronal nuclei fails to increase at normal rates during differentiation. This is accompanied by a marked decrease in the rate of ribonucleotide incorporation, indicating an early role of MeCP2 in regulating total gene transcription, not restricted to selected mRNAs. We also found that the levels of brain-derived neurotrophic factor (BDNF) were decreased in mutant neurons, while those of the presynaptic protein synaptophysin increased at similar rates in wild-type and mutant neurons. By contrast, nuclear size, transcription rates, and BDNF levels remained unchanged in astrocytes lacking MeCP2. Re-expressing MeCP2 in mutant neurons rescued the nuclear size phenotype as well as BDNF levels. These results reveal a new role of MeCP2 in regulating overall RNA synthesis in neurons during the course of their maturation, in line with recent findings indicating a reduced nucleolar size in neurons of the developing brain of mice lacking Mecp2. STEM CELLS 2012;30:2128–2139 Disclosure of potential conflicts of interest is found at the end of this article. INTRODUCTION Rett syndrome (RTT) is a progressive neurodevelopmental disorder observed in about one in 10,000 females at birth [1, 2]. Affected girls show a variety of neurological symptoms including microcephaly early in life, autistic features, breathing arrhythmia, and mental retardation [2]. Mutations in the X-linked gene MECP2 encoding the methyl-CpG-binding protein 2 are the major cause of RTT [3]. As in humans, Mecp2 is X-linked in the mouse and the selective deletion of Mecp2 in postmitotic forebrain neurons has been shown to recapitulate important aspects of the disease [4, 5]. Most interestingly, global or neuron-selective re-expression of MeCP2 in animals deprived of MeCP2 for a few weeks after birth markedly improves the symptoms of affected animals and significantly increases their lifespan, suggesting that the lack of MeCP2 even early during brain development does not cause irreparable brain damage [6, 7]. Yet in spite of important progress in modeling RTT in mice, the functional role of MeCP2 in the developing brain is still unclear. MeCP2 is expressed at high levels in adult neurons, comparable to those of histone H1 [8], suggesting a general function in the arrangement of the chromatin. While mRNA-based microarray studies revealed that almost 10% of annotated genes in the mouse genome is, for the most part, underexpressed in the adult brain of Mecp2/y mice [9, 10], no studies so far analyzed and compared total transcriptional activity of wild-type versus mutant neuronal nuclei. Such measurements necessitate access to uniform populations of neurons. Indeed, nuclear size has long been known to correlate with transcriptional activity and neurons with different identities typically have nuclei of different sizes and therefore rates of transcription that may vary by several folds [11]. In addition, nuclear size rapidly increases during neuronal differentiation such that developmental synchrony is a prerequisite for meaningful comparisons to be made between wild-type and mutant neurons. To circumvent the problems resulting from temporal and cellular heterogeneity, we used here an in vitro system based on the differentiation of mouse embryonic stem cells (ESCs). We previously reported that homogenous populations of neuronal progenitors can be generated from mouse ESCs [12, 13], thus allowing a precise monitoring of the earliest steps of neuronal differentiation. This synchronous system can then be used to measure and quantify the impact of the lack of MeCP2 by comparing mutant with wild-type ESCs. Causality can also be firmly established in this system by restoring MeCP2 expression in mutant neurons. Authors contributions: M.Y., R.D., and Y.-A.B.: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; J.G. and A.B.: provision of material, data analysis, and manuscript writing; R.A.P.: data analysis and interpretation and manuscript writing. M.Y. and R.D. contributed equally to this article. Correspondence: Yves-Alain Barde, M.D., Biozentrum, University of Basel, 50/70 Klingelbergstrasse, CH 4056 Basel, Switzerland. Telephone: þ41612672230; Fax: þ41612672208; e-mail: [email protected] Received December 20, 2011; accepted for publication C AlphaMed Press 1066-5099/2012/$30.00/0 doi: 10.1002/ June 30, 2012; first published online in STEM CELLS EXPRESS August 3, 2012. V stem.1180 STEM CELLS 2012;30:2128–2139 www.StemCells.com Yazdani, Deogracias, Guy et al. RESULTS Generation of Neurons from Wild-Type and Mecp22/y ESCs To study the role of MeCP2 during the earliest stages of neuronal maturation, we used E14 TG2a wild-type (Mecp2þ/y) and Mecp2/y ESCs [4]. Since the success of our neuronal differentiation procedure relies on the uniformity and pluripotency of the ESC populations used [12, 13], we first examined and quantified the expression of Oct-4 and Nanog in wild-type and Mecp2/y ESCs. As illustrated in Figure 1A and 1B, the vast majority of wild-type and Mecp2/y ESCs were colabeled with both Oct-4 and Nanog, suggesting that the lack of MeCP2 does not affect pluripotency. Both ESC lines were then used to generate cellular aggregates that were treated with retinoic acid as previously reported [12, 13]. This procedure has been shown to generate essentially pure populations of Pax6-positive progenitor cells [12]. As observed with other ESC lines [13], both wild-type and ESCs lacking MeCP2 generated neuronal cultures of high purity, with more than 90% of the cells expressing neuronal markers after a few days in culture. These neurons were previously characterized as glutamatergic using antibodies to a glutamate vesicular transporter and to form functional contacts [12]. Three days after dissociation of the progenitor aggregates, MeCP2 antibodies revealed a distinct nuclear staining that was absent in Mecp2/y neurons (Fig. 1C). To test whether wild-type and Mecp2/y ESC-derived neurons begin to express synaptic markers at similar rates, we monitored between day 1 and day 21 the levels of synaptophysin, a protein associated with presynaptic vesicles. As previously reported, these levels increase dramatically during the second week of neuronal maturation (Fig. 1D, 1E), reflecting synaptogenesis and functional connectivity during this period [12]. No significant differences were found between wild-type and Mecp2/y neurons (Fig. 1E). In this system, synaptic transmission can be readily detected by electrophysiological recording at 12 days following progenitor plating [12] as was found to also be the case with neurons lacking MeCP2 (data not shown). Western blots were also performed with nuclei isolated both from cultured cortical and ESC-derived neurons (Fig. 1F) and when normalized for histone H3, the levels of MeCP2 were found to be very similar between these two cell types. While no differences were observed in the levels of histone H3 between wild-type and mutant neurons, those of histone H1 were markedly elevated in neurons lacking MeCP2 10 days after progenitor plating (Fig. 1H, 1I: 100% 6 7.18%, n ¼ 3 vs. 217.8% 6 18.7%, n ¼ 3, t test p ¼ .021), in line with previous in vivo findings [8]. Nuclear Size of ESC-Derived Neurons As chromosomes typically attach to the nuclear membrane, Hoechst staining (Fig. 2A) of cultured cells can be used to monitor nuclear diameter as verified using antibodies to Lamin B (Supporting Information Fig. S1). We found that 3 days after plating, the size of both wild-type and mutant neuronal nuclei is indistinguishable (Fig. 2B). Note that the levels of MeCP2 are still low at this stage (Fig. 2C). Subsequently, there is a marked increase in MeCP2 levels and nuclear size in wild-type neurons (Fig. 2B–2D), but in the absence of MeCP2, the size of the nuclei failed to increase at the same rate as wild types. Even at 26 days, the latest time point analyzed, the nuclei of Mecp2/y neurons were still significantly smaller than those of wild-type neurons (Fig. 2B). To rule out the possibility that the smaller nuclear size observed in neurons lacking MeCP2 may result from additional differences www.StemCells.com 2129 between the mutant and wild-type ESCs used to generate neurons, we examined two additional mutant lines: Mecp2lox/y ESCs carrying a Cre-excisable (‘‘floxed’’) version of the gene [4] and Mecp2stop/y with a floxed stop cassette inserted before exon 3 preventing the expression of the gene [6]. Western blot analyses showed a roughly 40% reduction of MeCP2 levels in Mecp2loxP/y neurons and negligible levels in Mecp2stop/y neurons (Fig. 2E, 2F). While 3 days after progenitor plating, the nuclei of neurons generated from wild-type and the three mutant ESC lines were essentially of the same size (Fig. 2G), after 8 days both Mecp2/y and Mecp2stop/y neurons had smaller nuclei compared with age-matched wild-type neurons. The addition of brain-derived neurotrophic factor (BDNF) (40 ng/ml added every other day starting 2 days after progenitor plating) did not increase nuclear size when measured 10 days following aggregate dissociation, neither with wild-type nor with Mecp2/y neurons (Supporting Information Fig. S1). These measurements also revealed that the size of the nuclei of Mecp2loxP/y neurons expressing intermediate levels of MeCP2 was smaller compared with the size of wild-type neuronal nuclei but larger than the size of Mecp2/y neurons (Fig. 2G). MeCP2 levels are reduced by 40%–50% in the brains of mice carrying the same floxed allele as used here and the animals show neurological symptoms and breathing abnormalities [14]. Re-Expression of MeCP2 Rescues the Small Nuclei Phenotype in a Cell-Autonomous Fashion We then tested whether the small nuclear size phenotype detected in the Mecp2stop/y cells could be rescued by the reexpression of MeCP2 following the removal of the stop cassette. To this end, Mecp2stop/y cells were infected with lentiviruses containing a dual Synapsin promoter construct driving the neuronal expression of both Cre and green fluorescent protein (GFP) [15] (see Materials and Methods). This approach ensures a very high rate of infection as evidenced by the observation that the majority of neurons expressed GFP (data not shown). Western blot analyses revealed that Cre-mediated excision led to a re-expression of MeCP2 from its endogenous promoter back to normal levels (Fig. 3A) and that these neurons had significantly bigger nuclei compared with cells infected with GFP-encoding lentiviruses used as control (Fig. 3B) (22.01 lm2 6 0.4949 lm2, n ¼ 49, vs. Cre viruses: 31.87 lm2 6 0.8158 lm2, n ¼ 51). In a complementary approach, we expressed MeCP2 in neurons generated from Mecp2/y ESCs using the same viral delivery system. To also test whether MeCP2 regulates nuclear size in a cell-autonomous fashion, we used low viral titers so that only about half of the neurons would be infected. Neurons were infected with either a control virus (GFP only) or a virus encoding both MeCP2 and GFP (see Materials and Methods) and nuclear size was measured 18 days following infection. While the nuclear size of the Mecp2/y neurons expressing MeCP2 had enlarged (31.16 lm2 6 0.6361 lm2 n ¼ 132), the nuclei of neighboring noninfected neurons remained small (22.02 lm2 6 0.4081 lm2; n ¼ 89), as small as the nuclei of control neurons from noninfected sister cultures (22.67 lm2 6 0.4320 lm2; n ¼ 108) (Fig. 3C). These results indicate that the regulation of the nuclear size by MeCP2 is cell-autonomous. No Changes in the Size of Glial Cells Nuclei Lacking MeCP2 Given newly proposed role(s) for MeCP2 in glial cells (see below and Discussion), the nuclear size of these cells was also measured in primary cultures of astrocytes and of 2130 MeCP2 Regulates Neuronal Nuclear Size and Transcription Figure 1. Neuronal differentiation of wild-type and Mecp2/y embryonic stem cells (ESCs). Cultures of wild-type and Mecp2/y ESCs were stained with Oct4 and Nanog antibodies and the nuclear dye Hoechst. Arrowheads point to Oct4- and Nanog-negative nuclei of fibroblasts (A). The number of double-positive nuclei for Oct4 and Nanog was quantified and the significance of differences was assessed by Student’s t test (n ¼ 400 for each cell type) (B). Three DIV ESC-derived wild-type or Mecp2/þ neurons were analyzed by immunostaining using MeCP2 and Tuj-1 antibodies (C). Neuronal lysates of wild-type and Mecp2/y ESC-derived neurons were tested at 1, 3, 8, 14, and 21 DIV for Synaptophysin and b-III-Tubulin (Tuj-1) expression by Western blot (D). The signal intensity of synaptophysin was quantified and normalized to Tuj-1 levels (n ¼ 3) (E). Quantitative Western blot with dilution series using 0.1, 0.2, and 0.4 million nuclei isolated from either cortical neurons or ESC-derived neurons labeled here as ESCD neurons (F, G). Note that the levels of MeCP2 normalized to histone H3 levels are similar when 16 DIV cortical neurons and 8 DIV ESC-derived neurons are compared. However, while the levels of histone H3 remain constant in the absence of MeCP2, those of H1 remain more than double (mean 6 SEM, n ¼ 3, **, p < .01, Student’s t test) (H, I). Nuclei of Mecp2/y were used as negative loading control. Abbreviations: DIV, days in vitro; ESCD, ESC-derived. Yazdani, Deogracias, Guy et al. 2131 Figure 2. The size of nuclei in embryonic stem cell (ESC)-derived neurons correlates with their levels of MeCP2. ESC-derived neurons were stained at DIV 8 with antibodies against the neuron-specific marker b-III Tubulin (Tuj-1) and Hoechst. The nuclei of the Mecp2/y neurons are smaller than those of wild-types neurons (A). Nuclear area of neurons was measured during the course of neuronal maturation (mean 6 SEM, ***, p < .0001, Student’s t test) (B). Mecp2, Tuj-1, and actin levels were analyzed by immunoblotting using lysates of wild-type ESC-derived neurons (1, 3, and 8 DIV) and Mecp2/y ESC-derived neurons (8 DIV) (C). Mecp2 levels were quantified and normalized to actin levels (D). MeCP2 protein levels in neurons generated from four ESC lines with various mutations in the Mecp2 gene (wild-type: Mecp2þ/y, Mecp2/y, Mecp2loxP/y, and Mecp2stop/y) were assessed by Western blot (E). Levels of MeCP2 were normalized to Tuj-1 levels (F). Quantification of nuclear size of wild-type and Mecp2 mutant neurons during the course of neuronal maturation at 3 and 8 DIV (mean 6 SEM, ***, p < .0001, Student’s t test) (G). Abbreviation: DIV, days in vitro. www.StemCells.com 2132 MeCP2 Regulates Neuronal Nuclear Size and Transcription and mutant neurons with either wild-type or mutant astrocytes. We found that the size of neuronal nuclei was solely determined by the genotype of the neurons, with the genotype of the astrocytes not playing any measurable role on the size of nuclear neurons (Supporting Information Fig. S3). In Vivo Measurements of Nuclei Size Figure 3. Restoration of MeCP2 in MeCP2-deficient neurons rescues the small nucleus phenotype in a cell-autonomous fashion. Infection of Mecp2stop/y neuronal culture at days in vitro (DIV) 2 with Cre-encoding lentiviruses led to the re-expression of MeCP2 from the endogenous promoter as assessed by Western blot at DIV 8, while control virus (GFP) did not affect the expression of MeCP2 (A). Quantification of nuclear area at DIV 8 in Mecp2stop/y neurons infected with either GFP or Cre-encoding lentiviral particles (mean 6 SEM, ***, p < .0001, Student’s t test) (B). The nuclear size of Mecp2/y neurons at DIV 20 in noninfected cultures (control) was compared to that of the Mecp2/y neurons DIV 20 infected with MeCP2 encoding lentiviruses at DIV 2 and the nuclear size of the neighbor noninfected Mecp2/y neurons in the same culture (mean 6 SEM, ***, p < .0001, Student’s t test) (C). Abbreviation: GFP, green fluorescent protein. microglial cells isolated from the brains of wild-type and of Mecp2/y mice. Immunostaining using MeCP2 antibodies revealed a clear staining of the nuclei of glial fibrillary acidic protein (GFAP)-positive astrocytes (Fig. 4A). Unlike with neurons, the size of the astrocytic nuclei was not affected by the lack of MeCP2 (Fig. 4B): wild type: 79.50 lm2 6 1.126 lm2, n ¼ 85, Mecp2/y: 77.32 lm2 6 1.140 lm2, n ¼ 82. CD11b-positive microglial cells expressed MeCP2 at very low levels (Fig. 4A) and the size of their nuclei was unaffected by the lack of MeCP2 (Fig. 4C): wild type: 29.76 lm2 6 0.518 lm2, n ¼ 62, Mecp2/y 29.81 lm2 6 0.614 lm2, n ¼ 69. To test the possibility that MeCP2 may fail to regulate the size of astrocytic nuclei because of lower levels of MeCP2 expression compared with neurons, we analyzed by Western blotting lysates from dilution series of nuclei isolated from cultured astrocytes as well as from wild-type and of Mecp2/y neurons (Fig. 4D). Surprisingly in view of the lack of differences in nuclear size in wild-type versus mutant astrocytes, the levels of MeCP2 in astrocytes were found to be comparable with those observed in ESC-derived neurons 8 days after progenitor plating (Fig. 4E). We also tested the possibility that astrocytes may express a different splice variant of MeCP2, compared with neurons. To this end, we used primers allowing the amplification of the two MeCP2 main transcripts [16] using RNA extracted 14 days after plating ESC-derived progenitors and astrocytes. Both splice variants were expressed in both cell types and no measurable differences were detected in their respective expression levels (Supporting Information Fig. S2). As recently shown in experiments with transgenic animals, either form can rescue the phenotype of mice lacking MeCP2 [17]. A possible role of astrocytes in regulating neuronal nuclear size was also tested by combining cultures of wild-type The size of neuronal nuclei was then measured in the CA3 area of the hippocampus in 1-week and of 7-month-old mice. As in vitro, there is a significant increase in the size of the neuronal nuclei as they mature in vivo (1 week: 85.08 lm2 6 0.9491 lm2, n ¼ 304, 7 months: 150.2 lm2 6 2.519 lm2, n ¼ 258) (Fig. 5A, 5B). By contrast, in the same brain area of 1-month-old mice lacking MeCP2, the neuronal nuclei were significantly smaller (Fig. 5C, 5D) than wild-type animals (Mecp2þ/y: 138.2 lm2 6 2.146 lm2, n ¼ 56, Mecp2/y: 98.73 lm2 6 1.662 lm2, n ¼ 92). We also measured the nuclear size of astrocytes in hippocampus of 1-month-old mice and found that the size of the nuclei in wild-type and Mecp2/y astrocytes was similar (Fig. 5E, 5F; Mecp2þ/y: 57.71 lm2 6 1.674 lm2, n ¼ 93, Mecp2/ y : 61.99 lm2 6 1.601 lm2, n ¼ 69, p ¼ .329, Student’s t test). Reduced Rate of Transcription in MeCP2-Deficient Neurons To test whether the reduced size of nuclei in neurons lacking MeCP2 may affect global transcription, we compared radioactive ribonucleotide incorporation in nuclei isolated at different time points from neurons generated from wild-type and mutant ESCs. This procedure reflects the biosynthesis of mostly ribosomal and transfer RNA as especially ribosomal RNA is far more abundant than mRNA. After incubation of purified nuclei with unlabeled ATP, CTP, GTP, and radiolabeled [32P]-UTP, total RNA was extracted and the amount of radioactivity incorporated was measured [18]. Three days after progenitor plating, Mecp2/y neuronal nuclei were found to be transcriptionally as active as wild-type nuclei (Fig. 6A). However, neurons lacking MeCP2 had significantly reduced rate of RNA synthesis after 8 days when compared with wild-type neurons (Fig. 6A). Mecp2stop/y neurons were also significantly less transcriptionally active compared with aged-match wildtype nuclei (8 days after plating wild-type and mutant progenitors, data not shown). Given the similar levels of MeCP2 observed in cultured neurons and astrocytes (Fig. 4B), we also isolated nuclei from wild-type and Mecp2/y astrocytes. The levels of transcriptional activity were found to be indistinguishable (Fig. 6B). MeCP2 and BDNF Levels in Neurons and Astrocytes As mice lacking MeCP2 or BDNF in the CNS show striking phenotypic similarities including hind limb clasping, excessive weight in female mutants, reduced mobility, and a behavior suggestive of anxiety [4, 19], we examined the levels of BDNF in neurons derived from Mecp2/y and Mecp2stop/y ESCs and found them to be reduced in mutant neurons (Fig. 7A–7C). By contrast, the (very low) levels of BDNF in astrocytes were indistinguishable between wild-type and Mecp2/y cells (Fig. 7F, 7G). As MeCP2 re-expression in Mecp2stop/y neurons rescued the small nucleus phenotype, we investigated if this manipulation would also be accompanied by a restoration of BDNF levels. We found that Mecp2stop/y neurons infected with Cre-encoding lentiviruses had significantly higher levels of BDNF compared to neurons infected with GFP-encoding lentiviruses (Fig. 7D, 7E). Yazdani, Deogracias, Guy et al. 2133 Figure 4. MeCP2 does not regulate the nuclear size of glial cells. Immunostaining of cortical glial cultures prepared from wild-type and Mecp2/y mice using Mecp2 antibodies. Astrocytes were labeled using anti-GFAP antibodies and microglial cells with CD11b antibodies (A). Quantification of the nuclear size of astrocytes (B) and microglial cells (C) from wild-type and Mecp2/y cultures (n ¼ 350; mean 6 SEM; Student’s t test). Quantitative Western blot using 0.1, 0.2, and 0.4 million nuclei isolated from astrocytes and DIV 8 embryonic stem cell-derived neurons, either wild-type or Mecp2/y. Nuclei of Mecp2/y neurons were used as a negative control for MeCP2 detection (D). Quantification of MeCP2 levels relative to histone H3 (used as loading control) revealed that these levels are slightly higher in astrocytes than in neurons (E). Abbreviations: DIV, days in vitro; GFAP, glial fibrillary acidic protein; WT, wild type. DISCUSSION Our study uncovers several novel aspects related to the functional role of MeCP2 in neurons. First, MeCP2 regulates the size of neuronal nuclei and their global transcriptional activwww.StemCells.com ity. All previous studies on the role of MeCP2 in transcription relate to mRNA levels and neither to total RNA nor to comparative rates of biosynthesis. The large decrease observed in nuclei isolated from mutant neurons was observed in the absence of any gross retardation of neuronal differentiation. Second, the regulation of nuclear size is observed very early, 2134 MeCP2 Regulates Neuronal Nuclear Size and Transcription Figure 5. In vivo measurements of nuclear size of neurons and astrocytes in the CA3 area of the hippocampus. Hoechst staining of nuclei in the CA3 area of the hippocampus of 1 week and 7 months old wild-type mice (A). Quantification of nuclear size in CA3 area showed a significant increase in nuclear size with age (mean 6 SEM, ***, p < .0001, Student’s t test) (B). Hoechst staining of the nuclei in CA3 area of the hippocampus in 1-month-old wild-type (Mecp2þ/y) and Mecp2/y mice (C). The nuclear size in CA3 is significantly reduced in neurons lacking Mecp2/y compared with wild-type neurons (mean 6 SEM, ***, p < .0001, Student’s t test) (D). Hippocampi of a 1-month-old mouse were stained with antibodies against the astrocyte-specific protein GFAP and nuclei with Hoechst (E). Quantification of the nuclear size of astrocytes in hippocampi of 1-month-old mice revealed that wild-type and Mecp2/y astrocytes have similar nuclear sizes (mean 6 SEM; Student’s t test) (F). Abbreviation: GFAP, glial fibrillary acidic protein. even before detection of spontaneous electrical activity, during a phase of rapid growth of postmitotic neurons. MeCP2 regulates nuclear size in a cell-autonomous fashion while its absence does not affect the rate of neuronal differentiation as measured by synaptophysin expression. Third, the requirement for MeCP2 with regard to nuclear growth and transcriptional activity is specific for neurons and is not observed in astrocytes, in spite of similar levels of MeCP2 in both cultured cell types. Fourth, the restoration of BDNF levels in neurons previously lacking MeCP2 indicates that this regulation takes place directly in neurons and does not require other cell types or hormones. Yazdani, Deogracias, Guy et al. 2135 Figure 6. Transcriptional rates are reduced in nuclei isolated from Mecp2/y embryonic stem cell-derived neurons but not from Mecp2/y astrocytes. Equal numbers of nuclei at all ages were incubated with unlabeled ATP, CTP, GTP, and 1 lCi of [32P]-UTP for 0 and 30 minutes. The reaction was stopped by the addition of TRIzol and total RNA extracted. The amount of radioactivity incorporated in total RNA was measured and standardized to the values obtained from wild-type nuclei (mean 6 SEM, n ¼ 3, *, p < .05, **, p < .01 Student’s t test) (A). Similar experiments as in (A) were also performed with nuclei isolated from wild-type and Mecp2/y astrocytes (B). Abbreviation: DIV, days in vitro. An Early Role for MeCP2 in Neurons Several previous studies indicate that MeCP2-deficient neurons are smaller in some brain areas (for review, see [2]) and smaller nuclei have been noted in the adult CA1/CA2 and cortical layer V [5, 7]. A number of previous reports have also addressed the role of MeCP2 in neuronal differentiation using cultured cells, including recent reports based on differentiated mouse and human induced pluripotent (iPS) cells Figure 7. Reduced levels of BDNF in MeCP2-deficient neurons are rescued by MeCP2 re-expression. A Western blot analysis of 16 days in vitro (DIV) wild-type (Mecp2þ/y), Mecp2/y, and Mecp2stop/y neuronal lysates (A) revealed a significant reduction of BDNF levels (B). BDNF mRNA levels in neurons lacking MeCP2 at DIV 16 were analyzed by qPCR in Mecp2/y neurons and compared with BDNF mRNA levels in wild-type neurons at DIV 16 (mean 6 SEM, n ¼ 3, **, p < .01, Student’s t test) (C). Infection of Mecp2stop/y neurons with Cre-encoding lentiviruses leading to re-expression of MeCP2 from its endogenous promoter caused an increase in BDNF protein levels compared with neurons infected with GFP-encoding lentivirus (D). Quantification revealed a significant increase in BDNF protein levels (mean 6 SEM, n ¼ 3, *, p < .05, Student’s t test) (E). BDNF levels assessed by Western blots of lysates of cultured wild-type and Mecp2/y astrocytes (F). Lysates prepared from brains of mice carrying a Mapt::Cre-mediated deletion of Bdnf (cBDNF [18]) were used as negative control (F). Very low levels of BDNF protein were detected in astrocytes and no significant differences between wild-type and mutant cells. BDNF mRNA levels (G) were determined in cultured wild-type and Mecp2/y astrocytes and no significant differences were observed (mean ¼ SEM, n ¼ 3. Student’s t test). Abbreviations: BDNF, brain-derived neurotrophic factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GFP, green fluorescent protein; WT, wild type. www.StemCells.com 2136 MeCP2 Regulates Neuronal Nuclear Size and Transcription [20–24]. However, these previous studies typically reported on maturation defects in cells lacking MeCP2 based on b-IIITubulin staining [23] or else subtle changes in neurons excitability [24]. Our results show that while Mecp2/y is required in postmitotic nuclei to sustain their growth during maturation, it is not needed for the dramatic increase of the presynaptic marker synaptophysin during the course of neuronal maturation. We also note that causality can be more readily established with mouse XY ESCs, compared with human iPS cells, especially with XX cells [23, 25, 26]: re-expression of MeCP2 in postmitotic mutant neurons restored nuclear growth in a cell-autonomous fashion (Fig. 3C) as well as BDNF levels (see below). These findings fit well with the reversibility of neurological symptoms observed in mouse model of RTT [6], even when MeCP2 levels are restored only in neurons [7]. Our results also show that cell types other than neurons are not involved: neither do mutant astrocytes alter the size of wild-type neuronal nuclei nor do wild-type astrocytes rescue the small nuclear size of mutant neurons (Supporting Information Fig. S3). This is interesting in view of recent results indicating that cultured astrocytes lacking MeCP2 contribute to decreased neuronal growth [27–29], suggesting secretion of hitherto unknown toxic factors. A role for MeCP2-deficient astrocytes was also demonstrated in vivo following the selective expression of MeCP2 in astrocytes of mutant animals, a manipulation significantly improving the locomotor activity and respiratory abnormalities as well as greatly extending the lifespan of Mecp2stop/y mice [30]. In view of increasing evidence for cell types others than neurons playing a role in models of RTT, it is interesting to note that our findings indicate that the nuclear phenotype and the decreased rate of transcription caused by the absence of MeCP2 is limited to neurons and is not observed in astrocytes, in spite of comparable levels of MeCP2. Likewise, BDNF levels (see below) are decreased in mutant neurons but not in astrocytes. the absence of MeCP2. These run-off assays [18] allow changes in the rates of global transcription to be measured, an approach that differs from steady-state transcription analyses typically performed in microarrays. Our observations fit well with recent findings indicating that the size of neuronal nucleoli lacking MeCP2 is decreased early during cortical development in vivo [37]. Indeed, structural RNAs such as ribosomal RNAs contribute to the bulk of ribonucleotide incorporation in our run-off assays. Previous analyses with RNA extracted from brain tissues of Mecp2/y mice revealed that expression of approximately 2,400 genes is downregulated [9, 10]. However, these results should not be confused with our findings as all previous publications dealt with specific mRNAs and excluded structural RNAs and global transcription. MeCP2 and Nuclear Size In general, little is known about the regulation of the size and the shape of nuclei and of postmitotic neurons in particular [31–34]. Our results with astrocytes indicate that MeCP2 is not the sole regulator of nuclear size, since similarly high levels in astrocytes and neurons do not seem to be equally needed to regulate nuclear size and transcriptional activity. Another chromatin binding protein associated with nuclear size regulation is the linker histone H1 [35] and its loss in Tetrahymena results in an increase in nuclear size. Interestingly, MeCP2 is known to compete with H1 in binding to the linker DNA [36]. Recently, quantification of MeCP2 levels revealed that it is nearly as abundant as H1 in adult neuronal nuclei. Also, MeCP2 deficiency was shown to double the levels of H1 in neuronal but not in glial nuclei [8]. In line with this previous report, we found that the H1 levels are more than double in neuronal nuclei lacking MeCP2. These elevated H1 levels may enhance chromatin compaction and contribute to nuclear shrinkage [8]. Neurons have long been known to be transcriptionally highly active compared with other brain cells or even liver cells [11]. Global rates of transcription are well known to correlate with nuclear size, such that, for example, the comparatively small nuclei of cerebellar granule cells transcribe at much lower rates than those isolated from the cortex [11, 18]. In line with this, we observed that nuclei isolated from wildtype neurons significantly increase their transcriptional activity during the course of neuronal differentiation, which is accompanied by a rapid growth of the nuclei. However, during the same period of neuronal maturation, neither nuclear growth nor increased transcriptional activity is observed in MeCP2 and BDNF Early experiments with cultured neurons suggested that MeCP2 may repress the expression of BDNF, the first to establish a link between MeCP2 and BDNF [33, 34]. However, in animals lacking MeCP2, BDNF levels are decreased in several brain areas [35], including nuclei of the brain stem involved in respiratory control [36]. In addition, it has been shown by crossing mouse lines engineered to either increase or decrease BDNF levels on a Mecp2 null background that the progeny of the former showed improved symptoms resulting from the lack of MeCP2, while the progeny of the latter was worse off and died earlier [38]. In ESC-derived neurons lacking MeCP2, BDNF expression levels, both mRNA and proteins, were clearly decreased, in line with in vivo observation. Essentially, normal BDNF levels could be restored by re-expressing MeCP2 in mutant neurons. BDNF levels are much lower in cultured astrocytes compared with neurons and we did not detect any difference in BDNF levels among wildtype and Mecp2/y astrocytes, neither at the mRNA levels nor at protein levels. We believe that the extraordinary low levels of BDNF expression in astrocytes may explain the apparent discrepancy with a previous report [28]. Note that even in neurons where BDNF levels are far higher, accurate measurements by Western blot remain challenging [39]. CONCLUSIONS The use of a synchronous in vitro differentiation system allowed the demonstration that MeCP2 is required for the growth of neuronal nuclei soon after progenitors become postmitotic. Restoration of MeCP2 levels rescues these phenotypes as well as BDNF levels, in line with the previously reported behavioral rescue of mutant mice engineered to reexpress MeCP2 in adulthood. MATERIALS AND METHODS Cells Culture ESCs were cultured and differentiated into neurons as described previously [12, 13]. Briefly, this procedure selects by frequent splitting for the most rapidly dividing ESCs, ensuring their homogeneity and pluripotency (Fig. 1A, 1B). Following aggregation and 4 days of exposure to retinoic acid to trigger neural commitment, aggregates of Pax6-positive progenitor cells are dissociated and plated on a polyornithine/laminin substrate. The time points indicated in the text and days in vitro in Figures refer to the days following the plating of these dissociated progenitors counted as Day 0. Note that the serum-free, chemically defined medium used Yazdani, Deogracias, Guy et al. for progenitor and neuron differentiation contains 4 lg/ml insulin [13]. Glial cultures were prepared from P2 cerebral cortex. Briefly, cortex was dissected in phosphate buffer saline (PBS) and dissociated with 0.05% trypsin for 15 minutes at 37 C followed by mechanical dissociation with a 10-ml pipette. After centrifugation (5 minutes at 1,500 rpm), cells were plated on 10-cm cell culture dish in Dulbecco’s modified Eagle’s medium (DMEM) complemented with 20% fetal calf serum (FCS), 1% nonessential amino acids (Sigma, St. Louis, MO, www.sigma-aldrich.com), 2 mM glutamine, and Pen/Strep (Invitrogen, Grand Island, NY, www. invitrogen.com). The genotype of the pups was determined by standard genotyping methods using specific primers for Mecp2. Wild-type or mutant astrocytes were seeded on 12-well plates and maintained until confluence in DMEM supplemented with 20% FCS, 2 mM glutamine, nonessential amino acids, and Pen/ Strep. The medium was changed to neuronal growth medium (‘‘complete’’ medium [13]) 2 days before adding the neurons. Wild-type and mutant neuronal progenitors were plated on N2 medium onto polyornithine/laminin-coated coverslips that were then added after 2 days to the astrocyte-containing wells. Cells were kept in complete medium until the end of the experiment. Western Blots After washing twice with ice-cold PBS, cells were lysed for 30 minutes on ice by adding RIPA buffer (Tris-HCl 25 mM pH 7.5, NaCl 150 mM, Triton X-100 1%, sodium deoxycholate 1%, and SDS 0.1%) containing protease inhibitors (Complete; Roche, Basel, Switzerland, http://www.roche-applied-science.com/ index.jsp) and phosphatase inhibitor cocktail (PhosSTOP; Roche). Supernatants were collected after 30 minutes centrifugation at 12,000g, and protein concentration was determined by bicinchoninic acid (BCA) protein assay (BCA protein assay; Pierce, Waltham, Massachusetts, www.thermofisher.com). Equal amounts of total protein (20–25 lg) were loaded and separated by electrophoresis using NuPAGE Novex Bis-Tris 4%–12% gels (Invitrogen) and transferred to nitrocellulose membranes (GE Biosciences, Little Chalfort, United Kingdom, www.gehealthcare. com). Antibodies and concentrations were as follow: rabbit polyclonal anti-BDNF (N-20; Santa Cruz, Santa Cruz, CA, www.scbt.com) 1:500, rabbit polyclonal anti-MeCP2 (Millipore, Billerica, MA, www.millipore.com) 1:2,000, mouse monoclonal anti-b-III-Tubulin (Tuj-1 clone; Covance, Princeton, NJ, www. covance.com) 1:10,000, rabbit polyclonal anti-Histone H3 (ab7766; Abcam, Cambridge, United Kingdom, www.abcam.com) 1:3,000, rabbit polyclonal anti-Histone H1 (SantaCruz; sc-10806) 1:100, and mouse monoclonal anti-Actin (Sigma; A2228) 1:10,000. Densitometric quantification of bands was done using ImageJ software (NIH). Quantitative Real-Time PCR Total RNA (0.5 lg) was extracted using RNeasy-Plus Mini Kit (Qiagen, Venlo, Netherlands, www.qiagen.com) and reverse-transcribed using SuperScript-III Reverse Transcriptase and random primers according to manufacturer instructions (Invitrogen). The primer and probe sequences used are as follows: for Bdnf forward 50 -GGG AGC TGA GCG TGT GTG A-30 , reverse 50 -CGT CCC GCC AGA CAT GTC-30 , TaqMan probe 50 -CGA GTG GGT CAC AGC GGC AGA-30 ; and for Gapdh: forward 50 -TGT GTC CGT CGT GGA TCT GA-30 , reverse 50 -CCT GCT TCA CCA CCT TCT TGA-30 , TaqMan probe 50 -CCG CCT GGA GAA ACC TGC CAA GTA TG-30 . The levels of the Mecp2-E1 and Mecp2-E2 transcripts were assessed by PCR reaction-contained SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, www.appliedbiosystems.com), cDNA (5 ng), and the following primers (900 nM each): for Mecp2-E1 forward 50 -GGA GAG ACT GGA GGA AAA GT-30 , reverse 50 -TGC AGA ATG GTG GGG TGA AG-30 ; for Mecp2-E2 forward 50 -TGG TAG CTG GGA TGT TAG GG-30 , reverse 50 -GAA GGT TGT AGT GGC TCA TG-30 ; and for Gapdh 50 -TTG CCA TCA ACG ACC CCT www.StemCells.com 2137 TC-30 , reverse 50 -GCC TTG ACT GTG CCG TTG AA-30 . The levels of Bdnf, Mecp2-E1, and Mecp2-E2 were normalized to the levels of Gapdh transcripts according to the DCt method, and statistical significance was determined by the Student’s t test method. For the MeCP2 splice variants, specificity of the amplification reactions was assessed by melting curves to ensure single product amplifications. Immunostaining and Nuclear Size Measurement For immunocytochemistry, cells were grown on glass coverslips, washed twice with warm PBS, and fixed for 15 minutes with 4% paraformaldehyde (PFA) at 37 C. Unspecific antibody binding was prevented with blocking solution (10% horse serum, 0.1% Tween 20 in PBS) for 1 hour at room temperature. The primary antibodies were added in the blocking solution and kept overnight at 4 C. Primary antibodies and dilutions used were as follows: rabbit polyclonal anti-MeCP2 (Millipore; 07-013) 1:200, mouse monoclonal anti-GFAP (Sigma; G3893) 1:500, rat monoclonal anti-CD11b (Abcam; ab6332) 1:300, goat polyclonal anti-lamin B (SantaCruz; sc-6217) 1:100, and mouse monoclonal anti-b-III-Tubulin (Covance; MMS-435P) 1:1,000. Secondary antibodies coupled to the fluorophore were all used at a dilution of 1:500 (Invitrogen). For immunohistochemistry, animals were sedated by intraperitoneal injection of Ketalar (100 mg/kg; Parke Davies, New York, NY, www.pfizer.com)/Rompun (0.2%; Bayer Health Care, Berlin-Wedding, Germany, www.bayerscheringpharma.de) and perfused transcardially with ice-cold PBS (50–60 ml) followed by 4% PFA in 1 PBS (50–60 ml). The brains were removed and kept in fixative overnight at 4 C. Serial coronal sections (15–20lm thickness) were obtained with a cryostat (Leica, Solms, Germany, www.leica-microsystems.com). Immunostaining of brain sections was as with cultured cells. The freely available software ImageJ (NIH) was used for nuclear size measurement. All the pictures were taken with a 40 objective and in the software setting, one pixel was set equal to 0.115 lm. With a line drawn around the Hoechst nuclear staining, the software then calculates the surface of the nuclei automatically. Student’s t test was used for statistical analysis. Nuclei Purification and In Vitro Transcription Assay Cells were washed twice with PBS, collected in 1 ml of PBS containing protease inhibitors using a cell scraper, and pelleted at 800g for 5 minutes. To lyse the cell membrane, the cellular pellets were resuspended in 10 volume of 250-STM buffer (250 mM sucrose, 50 mM Tris-HCl pH 8.0, 5 mM MgCl2, and protease inhibitors) with 0.1% NP40 and kept on ice for 10 minutes, then centrifuged at 800g for 10 minutes. Nuclear pellets were resuspended in 1 ml of 2 M-STM buffer (2 M sucrose, 50 mM Tris-HCl pH 8.0, 5 mM MgCl2, and protease inhibitors) and centrifuged at 45,000g for 30 minutes to separate nuclei from contaminating cytoplasmic organelles. The pellets, verified to contain highly purified nuclei by phase contrast microscopy, were then resuspended in 600 ll 2 elongation buffer (100 mM Tris-HCl pH 8.0, 5 mM MgCl2, 5 mM MnCl2, b-mercaptoethanol 10 mM, and protease inhibitors). Nuclei were counted using a cell counter (NucleoCounter, Chemometec, Allerod, Denmark, www. chemometec.com). The number of nuclei was adjusted, with 2 elongation buffer, to have desired numbers in 100 ll, typically 250,000 nuclei. In vitro transcription was initiated by adding 100 ll of nuclei suspension to 100 ll of the reaction mixture (ATP, CTP, and GTP ribonucleotides each at a final concentration of 0.6 mM, 1 lCi of [32P]-UTP, and 40 units of RNase Inhibitor from Roche) in water. The reaction was performed at 37 C for 0 and 30 minutes and terminated by adding 800 ll TRIzol (Invitrogen) to each tube. The RNA purification was performed according to the manufacturer’s instructions (TRIzol, Invitrogen). The radioactivity incorporated into total RNA was measured using tricarb liquid scintillation counter (Packard, Ramsey, MN, www.gmi-inc.com). Statistical differences were determined by the Student’s t test. 2138 MeCP2 Regulates Neuronal Nuclear Size and Transcription Mecp2 Mutant Mice 30 (bold letters: NotI restriction site). Similar to CRE, the PCR product was digested with BglII and NotI and ligated into the BglII/NotI double-digested pLL-Syn-DsRed-Syn-EGFP vector. Lentiviral particles were produced by cotransfecting the pLLSyn-Cre-Syn-EGFP or pLL-Syn-Mecp2-Syn-EGFP vectors with the pMD2.G and psPax2 vectors (kindly provided by D. Trono, Ecole Polytechnique Federale de Lausanne, Switzerland) into 60% confluent HEK-293T cells. Three days after transfection, the lentiviral particles were concentrated from the medium using the Lenti-X concentrator system according to the manufacturer instructions (Clontech, Mountain View, CA, www.clontech.com), resuspended in complete medium, and kept at 80 C until used. Viruses were added 2 days after plating the progenitor cells on the polyornithine/laminin substrate. Mecp2 mutant mice [4] were obtained from the Jackson Laboratory (stock number 003890; B6.129P2(C)-Mecp2Ptm1.1BirdP/J; Bar Harbor, ME, www.jax.org). Male mutants were used in our studies with their wild-type littermates as control. All experiments involving wild-type and mutant animals were performed following the rules of the University of Basel. Plasmids and Lentivirus Generation In order to express Cre and Mecp2 specifically in neurons, we used the dual synapsin promoter lentiviral vector pLL-SynDsRed-Syn-EGFP [15]. Due to the dual synapsin promoter system, only neurons express the proteins of interest plus the reporter EGFP. The pLL-Syn-CRE-Syn-EGFP and pLL-Syn-Mecp2myc-SynEGFP vectors were generated by cloning the respective PCR products into the pLL-Syn-DsRed-Syn-EGFP vector using the BamHI and NotI sites. The Cre sequence was PCR amplified from the pMC-CRE vector using the following primers: Cre forward 50 -GG AGATCT ATG CCC AAG AAG AAG AGG AAG-30 (bold letters: BglII restriction site), Cre reverse 50 -CG GCGGCCGC CAG ATC CTC TTC TGA GAT GAG TTT TTG TTC ATC GCC ATC TTC CAG CAG GC-30 (bold letters NotI restriction site and italic letters encode for the c-myc tag). To amplify the wild-type Mecp2, total RNA from wild-type ESC-derived neurons was extracted and reverse-transcribed into cDNA, which was subsequently used as the template in the PCR reaction. The following primer sets were used to amplify Mecp2: MeCP2 forward Primer 50 -CG AGATCT ATG GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG GTA GCT GGG ATG TTA GGG CTC AGG-30 (bold letters: BglII restriction site and italic letters c-myc tag), MeCP2 reverse primer 50 -AA GCGGCCGC TCA GCT AAC TCT CTC GGT CAC GG- REFERENCES 1 2 3 4 5 6 7 8 9 10 11 12 13 Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr 1966;116:723–726. Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron 2007;56:422–437. Amir RE, Van den Veyver IB, Wan M et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999;23:185–188. Guy J, Hendrich B, Holmes M et al. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 2001;27:322–326. Chen RZ, Akbarian S, Tudor M et al. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 2001;27:327–331. Guy J, Gan J, Selfridge J et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science 2007;315:1143–1147. Giacometti E, Luikenhuis S, Beard C et al. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci USA 2007;104:1931–1936. Skene PJ, Illingworth RS, Webb S et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell 2010;37:457–468. Ben-Shachar S, Chahrour M, Thaller C et al. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet 2009;18:2431–2442. Chahrour M, Jung SY, Shaw C et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008;320:1224–1229. Thomas JO, Thompson RJ. Variation in chromatin structure in two cell types from the same tissue: A short DNA repeat length in cerebral cortex neurons. Cell 1977;10:633–640. Bibel M, Richter J, Schrenk K et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci 2004;7: 1003–1009. Bibel M, Richter J, Lacroix E et al. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc 2007;2:1034–1043. ACKNOWLEDGMENTS We would like to thank the Rett Syndrome Research Foundation for supporting the initial phase of this work, subsequently supported by a ‘‘Sinergia’’ program of the Swiss National Foundation, CRSI33_130441, and by the International Rett Syndrome Foundation. DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST The authors indicate no potential conflicts of interest. 14 Samaco RC, Fryer JD, Ren J et al. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet 2008;17:1718–1727. 15 Gascon S, Paez-Gomez JA, Diaz-Guerra M et al. Dual-promoter lentiviral vectors for constitutive and regulated gene expression in neurons. J Neurosci Methods 2008;168:104–112. 16 Kriaucionis S, Bird A. The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucleic Acids Res 2004;32: 1818–1823. 17 Kerr B, Soto CJ, Saez M et al. Transgenic complementation of MeCP2 deficiency: Phenotypic rescue of Mecp2-null mice by isoformspecific transgenes. Eur J Hum Genet 2012;20:69–76. 18 Thompson RJ. Studies on RNA synthesis in two populations of nuclei from the mammalian cerebral cortex. J Neurochem 1973;21:19–40. 19 Rauskolb S, Zagrebelsky M, Dreznjak A et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci 2010;30:1739–1749. 20 Marchetto MC, Carromeu C, Acab A et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010;143:527–539. 21 Ananiev G, Williams EC, Li H et al. Isogenic pairs of wild type and mutant induced pluripotent stem cell (iPSC) lines from Rett syndrome patients as in vitro disease model. PLoS One 2011;6:e25255. 22 Cheung AY, Horvath LM, Grafodatskaya D et al. Isolation of MECP2-null Rett syndrome patient hiPS cells and isogenic controls through X-chromosome inactivation. Hum Mol Genet 2011;20: 2103–2115. 23 Kim KY, Hysolli E, Park IH. Neuronal maturation defect in induced pluripotent stem cells from patients with Rett syndrome. Proc Natl Acad Sci USA 2011;108:14169–14174. 24 Farra N, Zhang WB, Pasceri P et al. Rett syndrome induced pluripotent stem cell-derived neurons reveal novel neurophysiological alterations. Mol Psychiatry 2012 [Epub ahead of print]. 25 Mekhoubad S, Bock C, de Boer AS et al. Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 2012; 10:595–609. 26 Cheung AY, Horvath LM, Carrel L et al. X-Chromosome inactivation in Rett syndrome human induced pluripotent stem cells. Front Psychiatry 2012;3:24. 27 Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci 2010;30: 5346–5356. Yazdani, Deogracias, Guy et al. 28 Maezawa I, Swanberg S, Harvey D et al. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci 2009;29:5051–5061. 29 Ballas N, Lioy DT, Grunseich C et al. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci 2009;12:311–317. 30 Lioy DT, Garg SK, Monaghan CE et al. A role for glia in the progression of Rett’s syndrome. Nature 2011;475:497–500. 31 Sato S, Burgess SB, McIlwain DL. Transcription and motoneuron size. J Neurochem 1994;63:1609–1615. 32 Schmidt EE, Schibler U. Cell size regulation, a mechanism that controls cellular RNA accumulation: Consequences on regulation of the ubiquitous transcription factors Oct1 and NF-Y and the liver-enriched transcription factor DBP. J Cell Biol 1995;128:467–483. 33 Trinkle-Mulcahy L, Lamond AI. Nuclear functions in space and time: Gene expression in a dynamic, constrained environment. FEBS Lett 2008;582:1960–1970. 2139 34 Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer 2004;4:677–687. 35 Shen X, Yu L, Weir JW et al. Linker histones are not essential and affect chromatin condensation in vivo. Cell 1995;82:47–56. 36 Nikitina T, Ghosh RP, Horowitz-Scherer RA et al. MeCP2-chromatin interactions include the formation of chromatosome-like structures and are altered in mutations causing Rett syndrome. J Biol Chem 2007; 282:28237–28245. 37 Singleton MK, Gonzales ML, Leung KN et al. MeCP2 is required for global heterochromatic and nucleolar changes during activity-dependent neuronal maturation. Neurobiol Dis 2011;43: 190–200. 38 Chang Q, Khare G, Dani V et al. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 2006;49:341–348. 39 Matsumoto T, Rauskolb S, Polack M et al. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci 2008;11:131–133. See www.StemCells.com for supporting information available online.