* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Genetically engineered single-chain antibody fusion proteins
Survey
Document related concepts
Gene expression wikipedia , lookup
SNARE (protein) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein moonlighting wikipedia , lookup
Gene regulatory network wikipedia , lookup
Protein adsorption wikipedia , lookup
Proteolysis wikipedia , lookup
Expression vector wikipedia , lookup
Protein–protein interaction wikipedia , lookup
DNA vaccination wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Transcript
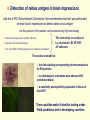
Genetically Engineered Single-Chain Antibody Fusion Proteins for Detection of Rabies Virus Antigen Dr Mohamed MOUSLI Groupe Immuno-Biotechnologie Laboratoire LIVGM Institut Pasteur de Tunis The most widely used tests for the detection of rabies antigens are Flurorescent antibody test (FAT) Immunohistochemistry Enzyme-linked immunosorbent assay (ELISA) These tests are easy, sensitive, currently recommended by the WHO Expert Committee on Rabies However, these routine laboratory tests present drawbacks: requires expensive reagents and instruments; the procedures are relatively long; well-trained technicians; is carried out with primary or secondary antibodies, that are labeled with sensitive reporter molecules, like fluorescent dyes colorimetric enzymes the chemical labelling is the conventional method for obtaining the conjugates. The chemical cross-linking methodology present some difficulties such as a random cross-linking chemical reaction; is usually not specific; produce heterogeneous conjugates e.g. enzyme-enzyme conjugates, antibody-antibody conjugates leads to side reactions that damage the combining site; and reduce activity; require several purification steps; sometimes producing important variations from batch to batch. To address these problems Recombinant DNA Technology has provided new facilities single-chain antibody gene proteic tracer gene Fusion gene Transformed bacteria Fusion protein Genetic engineering has provided a way to create a chimeric bifunctional molecules in which the variable domains of an antibody are genetically linked to unrelated proteic tracers and produced by recombinant bacteria. The gene fusion approach is simple, easy and reproducible; it gives a control molar ratio between antibody and labelling group; The recombinant immunoconjugate molecule expressed in bacteria systems is rapidly grow up them on an industrial scale; rapidly purified in one-step; and with a well-controlled quality; The genetic approach makes possible the improvement of the antibody affinity by genetic engineering in order to reach or exceed the sensitivity level. Here we describe the generation of a recombinant scFv from the 50AD1 anti-Rabies Virus Glycoprotein hybridoma, The mouse hybridoma cell line 50AD1 secreting a neutralizing MAb directed against the Rabies Virus Glycoprotein MAb 50AD1 binds to conformational antigenic site III its genetic fusion with an engineered bacterial alkaline phosphatase; and the use of this recombinant colorimetric fusion protein (scFv-AP) in different assays for a one-step detection of the native form of rabies glycoprotein. Cloning of the scFv50AD1 gene VH VL PM scFv Sequencing and screen against databases Cloning scFv50AD1-AP fusion protein into pLIP6 vector allowing the periplasmic exportation of the fusion protein The nucleotide and deduced amino acid sequences of the scFv50AD1 This facilitates: disulfide bond formation solubility extraction and purification of proteins Expression and purification analyses TG1 bacterial transformation Lane 1: crude preparation periplasmic induction periplasmic proteins extraction and purification M 97 1 2 Lane 2: purified fusion protein Lane 3: non-induced cell culture 1 3 2 1 2 scFv-AP 66 45 30 20.1 SDS PAGE 12 %- silver-staining Western blot Directly revealed with Treated with Anti-AP antibody BCIP/NBT AP substrate Anti mouse-IgG-HRP Bifunctionality of the recombinant fusion protein ELISA test for evaluating the activity of scFv50AD1-AP fusion protein to RVG Microtiter plates were coated with inactivated purified rabies virus (○) rabies viral glycoprotein (●), (Platelia Rabies kit) The bound conjugate was directly revealed by the AP activity of the recombinant immunoconjugate This first result strongly indicates that the recombinant fusion conjugate is fully bifunctional had both the AP enzymatic activity The corresponding colorimetric signal increased in a dose-dependent manner with increasing amounts of scFv50AD1-AP and the antigen-binding activity against the RVG Immuno-capture ELISA test Microtiter plates were coated with standard reference serum anti-rabies treated with various amounts concentrations of rabies virus PV strain (▲) preparartion in cell culture and then with scFv50AD1-AP fusion protein Background with the blocking solution (■) revealed directly in one-step by detecting AP enzymatic activity In the presence of increasing concentrations of RV, the enzymatic activity increased in a dose dependent manner We showed that the recombinant immunoconjugate is bifunctional and the estimation of the quantity minimal detectable of the RVG content of viral suspensions is about 160 ng Dot blot assay Sensitivity of the chimeric scFv-AP fusion protein for the detection of RV antigen was tested by Dot blot assay Two-fold serial dilutions of purified rabies virus preparation in cell culture were dotted onto nitrocellulose membrane (from 5000 to 5 ng) A B The results showed that 1 7 1 7 2 8 2 8 3 9 3 9 4 10 4 10 5 11 5 11 6 NC 6 NC scFv50AD1-AP in one-step 50AD1 Mab in two-step the lower limit of RV antigen was detectable at the concentration from 156 ng approximately; were comparable when we used parental MAb in two-step procedure; no staining was observed in the negative control; The total one-step reaction procedure take no more than 2 h for evaluating the RV antigen content of viral suspensions. Cell culture test We developed immunocytochemistry system to detect viral antigen that can be used with conventional light microscopy for localizing the RV in cells Monolayers of BHK-21 cell were infected with RV suspension; 18 h p.i., the cells were fixed and endogenous alkaline phosphatase was blocked with 5 mM levamisol, The scFv50AD1-AP fusion protein were added and incubated The interaction was analysed by colorimetric BCIP/NBT AP substrate scFv50AD1-AP This photomicrographs showed that: the dark staining in cellular membrane corresponding to immunoreactive for RV particles The same pattern of staining was observed when parental 50AD1 MAb was in two-step MAb50AD1 no chromogenic substrates were observed NC This scFv50AD1-AP fusion protein has dual activity can be used for rapid and specific detection of the rabies virus in cell culture in a one-step procedure. Detection of rabies antigen in brain impressions Like the d-FAT, Recombinant Colorimetric Immunohistochemical test was performed on brain touch impressions to detect rabies virus antigen but the product of the reaction can be observed by light microscopy Mouse brain impressions with RV infection blocked with 20 mM levamisol, The scFv50AD1-AP fusion protein were added and incubated The interaction was analysed by colorimetric BCIP/NBT AP substrate The results showed that the dark staining corresponding to immunoreactive for RV particles no chromogenic substrates were observed NC (uninfected brain) a sensitivity and specificity equivalent to those of the d-FAT These qualities make it ideal for testing under Field conditions and in developing countries In conclusion The present work demonstrates that recombinant anti-RVG scFv50AD1-AP conjugate is a promising alternative new reagent for rabies virus immunodetection in one-step procedure; can be produced in homogeneous bifunctional reagent, easily, quickly, reproducibly and at low cost; could be used for quality control in the manufacturing process of rabies vaccines (ELISA, IC-ELISA or Dot-blot); and may be used directly on a smear to confirm the presence of rabies antigen in cell culture or in brain tissue of mice that have been inoculated for diagnosis. Acknowledgements Imène Turki Pr. Koussay Dellagi Mohamed Saadi Dr. Habib Kharmachi Groupe Immuno-Biotechnologie LIVGM, Institut Pasteur de Tunis Unité Spécialisée de la Rage, Laboratoire de Microbiologie Vétérinaire ; Institut Pasteur de Tunis Collaborations Drs Christine Tuffereau and Yves Gaudin, Laboratoire de Virologie Moléculaire et Structurale UMR 2472 CNRS-INRA, Gif-sur-Yvette, France Dr Frédéric Ducancel, Département d'Ingénierie et d'Etudes des Protéines CEA-Saclay, France Dr Philippe Billiald, Muséum National d'Histoire Naturelle, Paris, France This work was supported by grant from the EMRO-COMSTECH for Research in Applied Biotechnology & Genomics in Health