* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Different neurotrophins are expressed and act in a developmental
Artificial general intelligence wikipedia , lookup
Convolutional neural network wikipedia , lookup
Biological neuron model wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Single-unit recording wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neuroregeneration wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neural oscillation wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Metastability in the brain wikipedia , lookup
Synaptogenesis wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Mirror neuron wikipedia , lookup
Multielectrode array wikipedia , lookup
Neural coding wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
Axon guidance wikipedia , lookup
Nervous system network models wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuroanatomy wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Synaptic gating wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Nerve growth factor wikipedia , lookup
Optogenetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
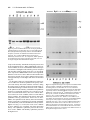
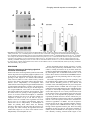
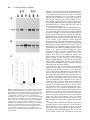
Development 118, 989-1001 (1993) Printed in Great Britain © The Company of Biologists Limited 1993 989 Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons Vladimir L. Buchman and Alun M. Davies Department of Anatomy, St. George’s Hospital Medical School, Tooting, London SW17 0RE, UK Present address: School of Biological and Medical Sciences, Bute Medical Buildings, St. Andrews University, St. Andrews, Fife KY16 9AJ, Scotland SUMMARY To investigate if different neurotrophins regulate the survival of neurons at successive developmental stages, we studied the effect of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) on the survival of mouse trigeminal neurons at closely staged intervals in development. We show that during the earliest stages of target field innervation trigeminal neurons display a transitory survival response to BDNF and NT-3. This response is lost as the neurons become NGF-dependent shortly before neuronal death begins in the trigeminal ganglion. BDNF and NT-3 mRNAs are expressed in the peripheral trigeminal target field before the arrival of the earliest axons and the onset of NGF mRNA expression. The levels of BDNF and NT-3 mRNAs peak during the early stages of target field innervation and decline shortly after the loss of neuronal responsiveness to BDNF and NT-3. Our study provides the first clear evidence that different target-derived neurotrophins can act sequentially to promote the survival of developing neurons. INTRODUCTION synthesis of NGF begins in the peripheral target field with the arrival of the earliest axons and the level of NGF mRNA increases throughout the period that trigeminal axons reach the peripheral target field (Davies et al., 1987). In the developing autonomic nervous system, NGF synthesis also begins when sympathetic axons reach their targets (Korsching and Thoenen, 1988). The demonstration that NGF is a member of an homologous family of neurotrophins (Leibrock et al., 1989; Hohn et al., 1990; Ernfors et al., 1990; Maisonpierre et al., 1990; Rosenthal et al., 1990; Jones and Reichardt, 1990; Hallbook et al., 1991; Berkemeier et al., 1991; Ip et al., 1992) has raised the issue of whether similar correlations between target field innervation, neurotrophin synthesis and neuronal responsiveness exist for these more recently identified neurotrophins. This is especially pertinent as these neurotrophins, in contrast to NGF, have additional roles in neuronal development before the onset of target field innervation. For example, there is some evidence that brainderived neurotrophin (BDNF) promotes neuronal differentiation in neural crest cell cultures (Sieber, 1991) and neurotrophin-3 (NT-3) promotes the differentiation of sensory neurons from their progenitors in early dorsal root ganglion (DRG) cell cultures (Wright et al., 1992). BDNF and NT-3 also enhance an early maturational change in DRG neurons before they become dependent on neurotrophins for survival (Wright, et al., 1992). For the above reasons, we undertook a detailed developmental study of embryonic mouse trigeminal system to Work on nerve growth factor (NGF) supports the proposal that neuronal target fields produce limiting quantities of neurotrophins that promote the survival of the appropriate number of innervating neurons during development (Davies, 1988; Barde, 1989). NGF is synthesized in the target fields of developing NGF-dependent neurons, which include sympathetic neurons and certain kinds of sensory neurons. Experimental manipulation of the availability of NGF to these neurons when they are innervating their targets influences the number of neurons that survive. Exogenous NGF prevents naturally occurring cell death in populations of NGF-dependent neurons, whereas NGF antibodies eliminate these neurons. Descriptive studies of the development of the embryonic mouse trigeminal ganglion, a population of NGF-dependent sensory neurons, and its innervation of the periphery (Davies and Lumsden, 1984, 1986; Davies, 1987a; Davies et al., 1987) have permitted the relationship between NGF synthesis and NGF receptor expression to be ascertained. When trigeminal axons are growing to their targets, the neurons express low levels of the mRNAs encoding the lowaffinity NGF receptor (p75, Wyatt et al., 1990) and the tyrosine kinase NGF receptor (trk, Wyatt and Davies, unpublished data) and are unlabelled by iodinated NGF (Davies et al., 1987). Near the time that the axons reach their targets, the levels of p75 and trk mRNAs increase markedly and the neurons become labelled by iodinated NGF. The Key words: neurotrophins, sensory neurons, trigeminal system, nerve growth factor, NGF, mouse nervous system 990 V. L. Buchman and A. M. Davies relate the spatial and temporal patterns of BDNF and NT-3 expression in this system to the phases of neuronal development and neurotrophin responsiveness. We show that trigeminal neurons exhibit a transient survival response to BDNF and NT-3 during the earliest stages of target field innervation that correlates with the early expression of BDNF mRNA and NT-3 mRNA in the peripheral field of these neurons. MATERIALS AND METHODS Dissection of embryonic tissues Embryos were obtained from overnight matings of CD1 mice. Pregnant females were killed by cervical dislocation and the stage of development of the embryos was determined by the criteria of Theiler (1972). Electrolytically sharpened tungsten needles were used to dissect trigeminal ganglia from E9.5 to E15 embryos. Maxillary processes and mystacial whisker pads (the cutaneous derivative of the maxillary process) were dissected from E10 to E16 embryos. The region of cranial ectoderm and mesenchyme from which the maxillary process develops was dissected from E9.5 embryos. Mandibular and hyoid processes were dissected from E11 embryos. Developing skin of the mandibular and ophthalmic territories was obtained at stages from E11 to E15 and the hindbrain from stages E9.5 to E15. Dissected tissue was frozen in siliconised Eppendorf tubes in liquid nitrogen and stored at −70°C. Separation of cutaneous epithelium and mesenchyme Developing skin from the maxillary, mandibular and ophthalmic territories was separated into its component epithelium and mesenchyme by treatment with dispase (Boeringer-Manheim) for 30 minutes at 4°C followed by 5 minutes at 37°C. This preferentially digests the basal lamina, allowing the sheet of epithelium to be lifted off the mesenchyme after washing the tissue in Hanks balanced salt solution (HBSS) containing 10% heat-inactivated horse serum (HIHS). Tissues were frozen and stored as above. Neuron cultures Trigeminal ganglia were incubated for 5 minutes at 37°C with 0.05% trypsin (Worthington) in calcium- and magnesium-free HBSS. After removal of the trypsin solution, the ganglia were washed twice with 10 ml of Hams F12 medium containing 10% HIHS and were gently triturated with a fire-polished, siliconised Pasteur pipette to give a single cell suspension. The cells were plated at a density of 250-500 neurons per dish in 35 mm plastic tissue-culture dishes (Nunc) that had been precoated with polyornithine (0.5 mg/ml, overnight) and laminin (20 µg/ml for 4 hours). The neurons were incubated at 37.5°C in a humidified 3.5% CO2 incubator in a defined medium consisting of Hams F14 supplemented with 2 mM glutamine, 0.35% bovine serum albumin (Pathocyte-4, ICN), 60 ng/ml progesterone, 16 µg/ml putrescine, 400 ng/ml L-thyroxine, 38 ng/ml sodium selenite, 340 ng/ml triiodo-thyronine, 60 mg/ml penicillin and 100 mg/ml streptomycin. Because morphological criteria are unreliable for identifying neurons at the earliest stages of their development, the low molecular weight neurofilament protein was localised by immunocytochemistry to identify positively and count the number of plated neurons in each experiment. To do this, the cells in several dishes were fixed with 4% paraformaldehyde 6 to 8 hours after plating. After permeablizing with 0.05% Triton-X100 in phosphatebuffered saline (PBS) for 30 minutes, the cells were incubated for 3 hours at room temperature with 1:1 dilution of 2H3 monoclonal antibody supernatant (gift of Tom Jessell). After washing, the cultures were incubated for 30 minutes with a 1:100 dilution of FITC-conjugated goat anti-mouse IgG secondary antibody and were mounted in DABCO beneath coverslips. The number of fluorescent cells within a 12×12 mm square in the centre of each dish was counted; the mean of these counts was taken as the initial number of neurons the experiment. After 24 or 48 hours incubation, neurons were clearly recognized in early trigeminal cultures by their bipolar morphology under phase-contrast optics. The percentage neuronal survival in the absence or presence of different neurotrophins was estimated at these times by counting the number of neurons in the same 12×12 mm area in each dish and expressing the results as a percentage of the initial number of neurofilament-positive cells. In each experiment, triplicate cultures were set up for all conditions. The data illustrated are compiled from the results of at least three separate experiments. Neurotrophins were added to the culture medium prior to plating the neurons. Purified mouse submandibular salivary gland NGF was a gift of Bill Mobley, UCSF. Purified recombinant full-length BDNF and NT-3 were gifts of John Winslow and Gene Burton, Genentech Inc. To determine if the increased number of early trigeminal neurons in the presence of BDNF or NT-3 was due to enhanced neuronal survival, the fate of individual neurons was monitored in culture (Vogel and Davies, 1991). This was done by recording 6 hours after plating the location of unambiguously identifiable bipolar neurons within a 9×9 mm grid scored on the undersurface of culture dishes and monitoring at 6 hourly intervals until 48 hours whether each of these neurons was surviving or had degenerated. All results are expressed as a percentage of the number of neurons in the initial neuronal cohorts at 6 hours. To avoid any effects of neurotrophins on the size of the initial cohorts, these factors were added after the identification of cohorts at 6 hours. Measurement of BDNF mRNA and NT-3 mRNA Northern blotting was used to measure the level of BDNF mRNA and NT-3 mRNA in dissected tissue. Total RNA was extracted using guanidinium isothiocyanate (Chizgwin et al., 1979). After electrophoresis in 1.2% agarose/formaldehyde gels, the RNA was blotted to Hybond-N filters (Amersham) and was crosslinked to these membranes by UV irradiation and baking at 80°C for 2 hours under vacuum. The filters were hybridized with 32P-labelled cRNA probes made by in vitro run-off transcription from either the rat NT-3 cDNA (gift of Marc Tessier-Lavigne, UCSF) or the mouse BDNF cDNA (gift of Patrick Enfors, Whitehead Institute) subcloned into the pGEM riboprobe vector downstream of the SP6 promoter. Filters were pre-hybridized at 65°C for 5 hours in the following solution: 50% formamide, 5× SSC, 50 mM sodium phosphate pH 7.0, 5 mM EDTA, 0.5% SDS, 5× Denhardt’s solution, 250 µg/ml salmon sperm DNA and 250 µg/ml E. coli tRNA. Filters were hybridized at 65°C for 15 hours in the above solution containing 107 cts/minute/ml of the 32P-labelled cRNA probe. Filters were washed twice for 15 minutes in 1× SSC with 0.1% SDS at 65°C and twice for 30 minutes in 0.1× SSC with 0.1% SDS at 65°C before exposure to Fuji X-ray film. On autoradiograms, two NT-3 mRNA transcripts of very similar size (approximately 1.4 kb) and two BDNF mRNA transcripts of 1.6 and 4 kb were detected. Band intensities on autoradiograms were measured using a Molecular Dynamics densitometer. The levels of BDNF mRNA and NT-3 mRNA in tissues were standardized to the level of β-actin mRNA. To do this, the filters were boiled for 2× 5 minutes in 0.1× SSC with 0.1% SDS to remove the BDNF cRNA or NT-3 cRNA probe and were subsequently hybridized with a 1 kb fragment of the mouse β-actin cDNA labelled by nick-translation. The filters were prehybridized at 42°C for 5 hours in a solution of the same composition as used for BDNF and NT-3 cRNA hybridizations and were hybridized at 42°C for 48 hours in the same solution containing 107 cts/minute/ml of the 32P-labelled nick-translated β-actin probe. The Changing neuronal response to neurotrophins filters were washed as described above and were exposed to X-ray film. Densitometry of the actin mRNA band intensities on the resulting autoradiograms enabled the ratio between the actin mRNA level and the previously determined BDNF or NT-3 mRNA level to be calculated for each RNA sample on a filter. To compile data on the relative BDNF or NT-3 mRNA levels in RNA samples on different filters (as in the case of RNA samples from each age series of maxillary or hindbrain tissue), several RNA samples from each age series were run on the same gel and were northern blotted onto a single filter. Subsequent hybridization of this filter with the BDNF or NT-3 probe and the actin probe followed by autoradiography and densitometry permitted standardisation of the relative BDNF or NT-3 mRNA levels in all age series. The data on the developmental changes in the relative BDNF mRNA level are compiled from three separate maxillary and hindbrain tissue age series and the NT-3 mRNA data are compiled from five separate maxillary tissue age series. Because any developmental changes in the level of actin mRNA expression in target field cells would cause corresponding shifts in the relative BDNF and NT-3 mRNA levels determined by the above method, several filters were reprobed with cDNA probes that hybridize with two other ubiquitous constitutively expressed RNAs. The L27 ribosomal protein mRNA was detected with a 0.3 kb fragment of the rat L27 cDNA that was labelled by nick-translation. This fragment of the rat L27 cDNA was isolated from rat brain cDNA by PCR amplification using primers based on the published L27 cDNA sequence (Tanaka et al., 1988). Filters were hybridized with the 32P-labelled nick-translated L27 probe as described for the β-actin probe. The 18S rRNA was detected with a 32P-labelled cRNA probe that was made by run-off transcription from the 18S rRNA cDNA (gift of Rolf Heuman) subcloned into the pGEM vector. Filters were hybridized with this probe as described previously (Harper and Davies, 1990). The developmental changes in BDNF or NT-3 mRNA levels were virtually identical when expressed relative to actin mRNA, L27 mRNA or 18S rRNA. To measure the absolute levels of BDNF mRNA and NT-3 mRNA in the maxillary process at the corresponding peaks of expression during development, the BDNF or NT-3 mRNA band intensities in northern blots of RNA samples from known numbers of maxillary processes were quantified by reference to a series of calibration standards. The calibration standards were known quantities of unlabelled, sense BDNF or NT-3 RNA transcripts that were run on gels alongside the tissue RNA samples. These figures were corrected for RNA losses during extraction, electrophoresis and blotting by reference to the band intensities of known quantities of shorter unlabelled, sense BDNF or NT-3 recovery standards that were added to tissue samples prior to RNA extraction, as described for NGF and p75 mRNAs (Heumann et al., 1984; Wyatt, 1990). The mean levels of BDNF and NT-3 mRNA per maxillary process were then related to the mean protein content of the maxillary process determined by the method of Lowry (1951) at the corresponding ages. RESULTS Early trigeminal neurons survive independently of neurotrophins When trigeminal neurons were cultured at low-density in defined medium during the earliest stages of axonal outgrowth in vivo (E10 and E11), more than 80% of the neurons identified by neurofilament staining 6 hours after plating were still alive after 24 hours incubation. In cultures set up at later stages, the proportion of neurons surviving for 991 24 hours without neurotrophins decreased to less than 5% in E14 cultures (Fig. 1). After 48 hours incubation, almost all neurons were dead in cultures of E10 and older neurons grown without neurotrophins (Fig. 1). Trigeminal neurons exhibit a transitory survival response to BDNF and NT-3 prior to naturally occurring cell death The survival effects of neurotrophins on cultured embryonic trigeminal neurons are clearly observed after 48 hours incubation when virtually all neurons have died in control cultures. Furthermore, since the serum-free medium used in these experiments is not conducive for the growth of fibroblasts, the cultures remain virtually free of non-neuronal cells. Thus, neuronal survival in this culture system is unlikely to be influenced by neurotrophins produced by non-neuronal cells. In E10 cultures supplemented with either BDNF or NT3, the number of neurons after 48 hours incubation had increased to between 120 and 150% of the number identified 6 hours after plating (Fig. 1). This effect of BDNF and NT-3 on increasing or sustaining the number of neurons in trigeminal cultures was only observed in cultures set up during the earlier stages of trigeminal ganglion development. The response to BDNF was lost most rapidly, with only 5% of the neurons surviving for 48 hours in E13 cultures containing 2 ng/ml of BDNF. The number of neurons surviving for 48 hours in the presence of 2 ng/ml of NT-3 had fallen to a similar level by E14 (Fig. 1). Fig. 2 illustrates typical dose responses of E10 and E12 trigeminal neurons to BDNF. These resembled typical NGF dose responses (see below) in that they were sigmoidal when plotted on a semi-log graph. The concentration of BDNF that promoted half-maximal survival was near the point of inflexion and had a value in E11 cultures of 1.95±0.25 pg/ml (mean±s.e.m., n=3 separate dose response experiments, each set up in triplicate). Typical dose responses of E11, E12 and E14 trigeminal neurons to NT-3 are also shown in Fig. 2. In contrast to NGF and BDNF dose responses, these were not markedly sigmoidal. Fig. 3A,B illustrates the bipolar morphology of early trigeminal neurons grown with BDNF and NT-3. Because early sensory neuron cultures contain neuron progenitor cells that can differentiate in vitro (Rohrer et al., 1985; Wright et al., 1992), it was necessary to exclude the possibility that the increased number of early trigeminal neurons surviving in the presence of BDNF or NT-3 was due to enhanced proliferation or differentiation of these progenitor cells. To do this, cohorts of neurons were identified by their bipolar morphology 6 hours after plating and the fate of the individual neurons in each cohort was monitored at intervals for the next 42 hours in vitro. BDNF or NT-3 were added to these cultures after the identification of neuronal cohorts at 6 hours incubation. Addition was made at this time because any effect of these factors on either cell attachment or neuronal differentiation would influence cohort size and confound the results. In these cohort experiments, 88%±2.8 (mean±s.e.m., n=6) of neurons grown with BDNF and 51.8%±6.4 (mean±s.e.m., n=6) of neurons grown with NT-3 survived from 6 to 48 hours in culture. In control cultures, none of the neurons 992 V. L. Buchman and A. M. Davies 24hr 100 NGF 160 48hr BDNF 140 NT-3 80 Percent survival Percent survival 120 60 40 100 80 60 40 20 20 0 0 9 10 11 12 13 14 15 Embryonic age (days) 9 10 11 12 13 14 15 Embryonic Age (days) Fig. 1. The survival of E10 to E15 trigeminal neurons in control cultures and neurotrophin supplemented cultures. The left-hand graph shows the number of neurons surviving after 24 hours (open circles) and 48 hours (closed squares) in control cultures expressed as a percentage of the number of neurons identified 6 hours after plating. The right-hand graph shows the number of neurons surviving after 48 hours incubation with 2 ng/ml NGF (open circles), 2 ng/ml BDNF (open squares) or 2 ng/ml NT-3 (closed triangles) expressed as a percentage of the number of neurons identified 6 hours after plating. The mean±s.e.m. of triplicate cultures from three separate experiments at each age are shown in both graphs. Fig. 2. Dose-responses of trigeminal neurons to BDNF and NT-3. The left-hand graph shows the number of E11 neurons (filled circles) and E12 neurons (open circles) surviving after 48 hours incubation with different concentrations of BDNF expressed as a percentage of the number of neurons identified 6 hours after plating. The right-hand graph shows the number of E11 neurons (filled circles), E12 neurons (open circles) and E14 neurons (triangles) surviving after 48 hours incubation with different concentrations of NT-3 expressed as a percentage of the number of neurons identified 6 hours after plating. The mean±s.e.m. of triplicate cultures from representative experiments are shown in both graphs. identified at 6 hours survived to 48 hours. This indicates that BDNF and NT-3 have a direct survival-promoting effect on early trigeminal neurons. NGF promotes the survival of trigeminal neurons during the period of naturally occurring cell death In contrast to the marked survival response of E10 and E11 trigeminal neurons to BDNF and NT-3, NGF promoted the survival of less than 10% of trigeminal neurons in E10 cultures and only 25% in E11 cultures. By E12, shortly before the onset of cell death in the trigeminal ganglion, NGF supported all the neurons. In cultures established throughout the phase of naturally occurring cell death (E13 to E18), NGF continued to promote the survival of the great Changing neuronal response to neurotrophins A B C D E F majority of neurons (Fig. 1 and unpublished data). Fig. 3C shows the typical bipolar morphology of E12 trigeminal neurons grown in the presence of NGF. The small percentage of E14 or E15 neurons that survived in the presence of either BDNF or NT-3 had distinctly larger cell bodies than neurons of the same age grown in the presence of NGF (Fig. 3D-F). Although NGF promoted the survival of the majority of E12 and older trigeminal neurons, there was a marked shift in the dose response of these neurons to NGF with increasing embryonic age (Fig. 4). The concentration of NGF that elicits half maximal survival (estimated by interpolation) increases by an order of magnitude from 4.7 pg/ml at E12 to 53.2 pg/ml at E15. To investigate whether NGF, BDNF and NT-3 promote the survival of the same or different subsets of neurons in the early trigeminal ganglion, E11 and E12 neurons were grown with each factor alone and with combinations of these factors. After 48 hours incubation, there was no significant additional neuronal survival in the presence of any two factors (Fig. 5). This is clearly seen in E12 cultures in which NGF promotes the survival of virtually all of the neurons (98%) and there was no significant increase in the number of neurons in the presence of either NGF + BDNF (105%) or NGF + NT-3 (95%) even though BDNF alone and NT-3 alone promoted the survival of 27% and 65% of the neurons, respectively. This indicates that the early trigeminal 993 Fig. 3. Phase-contrast photomicrographs of trigeminal neurons. The typical bipolar morphology of E11 trigeminal ganglion neurons grown for 48 hours with 2 ng/ml BDNF (A) or 2 ng/ml NT-3 (B). E12 neurons grown for 48 hours with 2 ng/ml NGF had a similar morphology (C) as did E15 neurons grown with NGF (D). The small proportion of E15 neurons surviving with 2 ng/ml of either BDNF (E) or NT-3 (F) had larger cell bodies than the majority of E15 neurons that survive in the presence of NGF (D). Many E15 neurons surviving with NGF, BDNF or NT-3 had a unipolar morphology (illustrated by the two neurons shown in micrograph E). Scale bar, 50 µm. Fig. 4. Dose responses of trigeminal neurons to NGF. The number of neurons at E12 (filled circles), E13 (open circles), E14 (filled squares) and E15 (open squares) surviving after 48 hours incubation with different concentrations of NGF is expressed as a percentage of the number of neurons identified 6 hours after plating. The mean±s.e.m. of triplicate cultures from typical experiments are shown. 994 V. L. Buchman and A. M. Davies Fig. 5. The survival of trigeminal neurons grown neurotrophins singularly and in combination. The percentage survival of E11 (left-hand bar chart) and E12 (right-hand bar chart) neurons after 48 hours incubation with neurotrophins (each at 2 ng/ml) is expressed as a percentage of the number of neurons identified 6 hours after plating. The mean±s.e.m. of triplicate cultures from typical experiments are shown. ganglion does not contain distinct subsets of neurons that respond to different factors, but that the same neurons respond to multiple factors. Developmental changes in BDNF mRNA and NT-3 mRNA expression in the peripheral target field Northern blotting revealed the presence of 1.6 kb and 4 kb A B C BDNF mRNA transcripts in the maxillary process (Fig. 6). At E9.5 and E10, low levels of these transcripts were present. From E10 to E12, the levels increased over ten-fold (relative to actin mRNA). From the peak of expression at E12, the levels fell to lower levels at E13 that remained largely unchanged through later ages (Fig. 7). Densitometry revealed that there was an approximate 1:1 ratio between the 1.6 and 4 kb transcripts throughout development (data not shown). Quantitative northern blotting showed that the mean level of BDNF mRNA per process (both transcripts together) at the peak of expression at E12 was approximately 0.9 pg. Relative to the mean protein content of the maxillary process at E12, the concentration of BDNF mRNA per process was approximately 60 pg/mg protein. Northern blotting revealed the presence of two NT-3 mRNA transcripts of very similar size that ran as a doublet of approximately 1.4 kb (Fig. 6). Low levels of these transcripts were present at E9.5, E10 and E11. The levels then increased four-fold (relative to actin mRNA) from E11 to reach a peak of expression at E13. From E13 the level subsequently fell to reach the earlier low levels by E16 (Fig. 7). Fig. 6. Developmental changes in the expression of BDNF mRNA and NT-3 mRNA in the maxillary target field. Autoradiograms of a northern blot of E9.5 to E16 maxillary total RNA hybridized sequentially with the 32P-labelled BDNF riboprobe (A), the 32Plabelled NT-3 riboprobe (B) and the 32P-labelled nick-translated actin DNA probe (C). The 1.6 and 4 kb BDNF mRNA transcripts with maximal expression at E11 and E12 are indicated by small arrows in A. A 0.95 kb BDNF RNA recovery standard is indicated by the large arrow on the left-hand side of A. 25 pg of this standard were added to each tissue same prior to RNA extraction. Different amounts (2, 5, 10 and 20 pg) of this BDNF RNA standard were run in separate lanes on the gel to calibrate the levels of the BDNF recovery standard and BDNF mRNA in each RNA sample lane. These calibration standards are indicated by a large arrow on the right-hand side of A. These calibration standards ran lower in the gel than the recovery standards because they were not retarded by the presence of additional RNA. The 1.4 kb NT-3 mRNA doublet band with maximal expression between E12 and E14 (relative to actin mRNA) is indicated by the double arrows in B. The actin mRNA band is shown in C. Changing neuronal response to neurotrophins 995 gave results that were virtually identical to those obtained by standardising the changes to actin mRNA (data not shown). Furthermore, quantitative northern blotting was used to estimate the absolute amounts of BDNF mRNA and NT-3 mRNA in the maxillary target field in two age series (E10 to E15 in each case). When the results were standardised to the mean protein content of the maxillary target field at each age, the respective developmental changes in the levels of BDNF mRNA and NT-3 mRNA were very similar to those obtained by standardising the changes to actin mRNA (data not shown). Thus, data compiled from multiple estimations of BDNF and NT-3 mRNAs levels (relative to actin mRNA) in the maxillary target field from E9.5 and E16 (Fig. 7) accurately describe the changes in the overall concentrations of these neurotrophin mRNAs in the peripheral target field prior to and throughout the phase of target field innervation. Fig. 7. Developmental changes in the levels of BDNF mRNA and NT-3 mRNA in the peripheral and central target fields of the trigeminal ganglion. The mean±s.e.m. of the BDNF mRNA level (1.6 kb + 4 kb transcripts) relative to actin mRNA in the maxillary target field (open circles, n=3) and hindbrain (open squares, n=3) are shown from E9.5 to E16 and E9.5 to E15, respectively. The mean±s.e.m. of the NT-3 mRNA level relative to actin mRNA in the maxillary target field (closed circles, n=5) are shown from E9.5 to E16. Quantitative northern blotting showed that the mean level of NT-3 mRNA per maxillary process was approximately 3.2 pg at the peak of expression at E13. Relative to the mean protein content of the maxillary process at E13, the concentration of NT-3 mRNA per process was approximately 140 pg/mg protein which is just over twice the level of BDNF mRNA at its peak of expression at E12. Because BDNF and NT-3 mRNA levels in the developing peripheral target field were standardised to the level of actin mRNA, any developmental changes in the level of actin mRNA expression in target field cells would cause corresponding shifts in the relative BDNF and NT-3 mRNA data. To exclude this possibility, several northern blots of complete age series of maxillary tissue RNA were rehybridized with 32P-labelled probes that hybridize with two other ubiquitous constitutively expressed RNAs: the L27 ribosomal protein mRNA and the 18S rRNA. Standardisation of the respective developmental changes in BDNF mRNA and NT-3 mRNA to either L27 mRNA or 18S rRNA Developmental changes in BDNF mRNA expression in the hindbrain Both the 1.6 and 4 kb BDNF mRNA transcripts were detected in northern blots of total RNA extracted from the developing hindbrain from E9.5 to E15 (the latest age studied). In contrast to BDNF mRNA expression in the peripheral target field, the levels were much lower in the central target field and there was no peak in expression between E10 and E12. Rather there was a gradual increase in both transcripts (relative to actin mRNA) from E9.5 to E15 (Figs 7 and 8). An approximate 1:1 ratio between the levels of the 1.6 and 4 kb transcripts was maintained throughout development (data not shown). In contrast to the relatively high level of NT-3 mRNA expression in the peripheral target field, NT-3 mRNA was barely detectable in northern blots of E9.5 to E15 hindbrain total RNA, yet when the same blots were re-screened with the BDNF cRNA probe, two BDNF mRNA transcripts were detected (data not shown). Regional differences in BDNF and NT-3 mRNA expression in the periphery To ascertain the relative levels of BDNF mRNA and NT-3 mRNA in the epithelium and mesenchyme of the maxillary target field, these tissue components were separated by dispase treatment at ages ranging from E11 to E15 and were assayed separately for BDNF mRNA and NT-3 mRNA by northern blotting (Fig. 9). The levels of both BDNF mRNA and NT-3 mRNA (relative to the levels of either actin mRNA or L27 mRNA) were clearly higher in the mesenchyme compared with the epithelium during the earliest stages of target field innervation at E11. By E13, however, this pattern was reversed for BDNF mRNA with higher levels in the epithelium compared with the mesenchyme and, at E14 and E15, the overall levels of BDNF mRNA were similar in both tissues. In the case of NT-3 mRNA, the overall levels of this transcript were similar in epithelium and mesenchyme from E13 to E15. To determine if there are regional differences in the levels of BDNF mRNA and NT-3 mRNA in cutaneous targets during the earliest stages of their innervation, northern blotting was used to estimate the levels of these mRNA tran- 996 V. L. Buchman and A. M. Davies A A B Fig. 8. Expression of BDNF mRNA in the developing hindbrain. Autoradiograms of a northern blot of E9.5 to E15 hindbrain total RNA (Hb) hybridized sequentially with the 32P-labelled BDNF riboprobe (A) and the 32P-labelled nick-translated actin DNA probe (B). The 1.6 and 4 kb BDNF mRNA transcripts are indicated by small arrows in A. For comparison, similar quantities of total RNA from E15 and E16 maxillary tissue (Mx) were run on the gel alongside the hindbrain RNA samples. scripts in the maxillary, mandibular and hyoid processes and in the forelimb bud at E11. When standardised to the level of actin mRNA, the levels of both BDNF mRNA transcripts were almost 3-fold higher in the maxillary process compared with the other cutaneous target tissues (Fig. 10). In contrast to the expression of BDNF mRNA, there was no significant difference in the level of NT-3 mRNA in the branchial arch target tissues and the level of NT-3 mRNA in these tissues was approximately two-fold higher than in the forelimb bud (Fig. 10). To determine if there are regional differences in the level of NT-3 mRNA in cutaneous epithelia from sparsely innervated and densely innervated cutaneous territories during the earliest stages of naturally occurring neuronal death, we used northern blotting to estimate the levels of NT-3 mRNA in the epithelia of the maxillary, mandibular and ophthalmic territories of trigeminal ganglion at E13 and E14. At the onset of neuronal death at E13, there were marked differences in the level of NT-3 mRNA (relative to actin mRNA) in these epithelia. The epithelium of the sparsely innervated ophthalmic territory possessed the lowest level of NT-3 mRNA, the epithelium of the densely innervated maxillary territory possessed a 6-fold higher level and the epithelium of the moderately innervated mandibular territory had the highest level (Fig. 11). At E14, the levels of NT-3 mRNA in these epithelia were correlated with final innervation density. The ophthalmic epithelium possessed the lowest level of NT-3 mRNA and maxillary epithelium possessed the highest level. B C D Fig. 9. Developmental changes in the expression of BDNF mRNA and NT-3 mRNA in maxillary epithelium and mesenchyme. Autoradiograms of a northern blot of E11 to E15 total RNA extracted from maxillary epithelium and mesenchyme hybridized sequentially with the 32P-labelled BDNF riboprobe (A), the 32Plabelled NT-3 riboprobe (B), the 32P-labelled nick-translated actin DNA probe (C) and the 32P-labelled nick-translated L27 DNA probe (D). The 1.6 and 4 kb BDNF mRNA transcripts are indicated by small arrows in A. Relative to actin mRNA (C) or L27 mRNA (D) band intensities, the levels of both BDNF mRNA transcripts are clearly higher in mesenchymal tissue than in epidermal tissue at E11. The 1.4 kb NT-3 mRNA doublet band is indicated by the double arrows in B. The relative level of NT-3 mRNA is also higher in mesenchymal tissue at E11. Comparison of the band intensities in C and D indicate that the levels of actin mRNA and L27 mRNA are correlated in these different tissues at developmental stages from E11 to E15. Changing neuronal response to neurotrophins 997 A D B C Fig. 10. Regional differences in the expression of BDNF mRNA and NT-3 mRNA in cutaneous tissues during the earliest stages of sensory innervation. (A-C) Autoradiograms of a northern blot of E11 total RNA extracted from the forelimb bud (FLB) and the hyoid (Hy), mandibular (Md) and maxillary (Mx) processes hybridized sequentially with the 32P-labelled BDNF riboprobe (A), the 32P-labelled NT-3 riboprobe (B) and the 32P-labelled nick-translated actin DNA probe (C). The 1.6 and 4 kb BDNF mRNA transcripts with maximal expression in the maxillary process are indicated by small arrows in A. The similar levels of the 1.4 kb NT-3 mRNA transcript in the maxillary, mandibular and hyoid processes are seen in B. The actin mRNA band is shown in C. (D) Levels of BDNF mRNA (left-hand bar chart) and NT-3 mRNA (right-hand bar chart) relative to actin mRNA in the E11 forelimb bud (FLB) and the hyoid (Hy), mandibular (Md) and maxillary (Mx) processes. DISCUSSION Changing responses of developing trigeminal neurons to neurotrophins Earlier work on the mouse trigeminal system has shown that neurite outgrowth from trigeminal ganglion explants occurs in the absence of neurotrophins during the stage axons are growing to their targets in vivo (Davies and Lumsden, 1984). Recent studies of avian cranial sensory neurons grown in low-density dissociated cultures show that these neurons survive independently of neurotrophins when cultured before innervating their targets and that the duration of neurotrophin independence correlates with the distance and time that it takes their axons to grow to their targets in vivo (Vogel and Davies, 1991). Here we show that trigeminal neurons cultured in low-density at E10 and E11, when the earliest axons are growing to their peripheral targets, survive for 24 hours without neurotrophins. A substantial number of neurons also survive for 24 hours without neurotrophins in cultures set up at E12 and E13 (Fig. 1). Because axons are recruited to the trigeminal nerve from E9.5 to E13 in vivo (Davies and Lumsden, 1984), the neurons that survive independently of neurotrophins in culture are probably those whose axons are normally growing to their targets at the time when the cultures were set up. By E15, when virtually all trigeminal axons have reached their targets in vivo, none of the neurons survive independently of neurotrophins in vitro. Before trigeminal neurons become responsive to NGF, their survival is promoted by either BDNF or NT-3. In E10 cultures, virtually all neurons die by 48 hours unless the medium is supplemented with BDNF or NT-3, but not NGF. By following the fates of individual neurons from 6 to 48 hours in these cultures we demonstrated that BDNF and NT3 exert a direct survival-promoting effect on early trigeminal neurons. The earliest trigeminal axons come into proximity with the peripheral target epithelia of the mandibular process by E10.5 and the maxillary process by E11 (Davies and Lumsden, 1984). The finding that the early survival response of trigeminal neurons to BDNF and NT-3 begins in E10 cultures between 24 and 48 hours in vitro, immediately after the phase of neurotrophin independence, suggests that the onset of this response in vivo is closely related to the arrival of the earliest axons in the peripheral target field. Over the next few days of development the proportions of neurons responding to BDNF and NT-3 decrease whereas the proportion responding to NGF increases. The loss of the BDNF and NT-3 survival responses could be due to the death of a short-lived population of BDNF- and NT-3-responsive neurons present in the early trigeminal ganglion. Alternatively, responsiveness to BDNF and NT-3 may be a stage through which trigeminal neurons pass before becoming dependent on NGF for survival. The former possibility is unlikely because the BDNF survival response is over by the time naturally occurring neuronal death begins in the 998 V. L. Buchman and A. M. Davies A B C Fig. 11. Regional differences in NT-3 mRNA levels in cutaneous epithelia. (A,B) Autoradiograms of a northern blot of E13 and E14 total RNA extracted from maxillary (Mx), mandibular (Md) and ophthalmic (Op) epithelia hybridized sequentially with the 32P-labelled NT-3 riboprobe (A) and the 32P-labelled nicktranslated actin DNA probe (B). The 1.4 kb NT-3 mRNA doublet band is indicated by the double arrows in A. High levels of NT-3 mRNA (relative to actin mRNA) are present in the mandibular epithelium at E13 and in the maxillary epithelium at E14. The ophthalmic epithelium has much lower levels of NT-3 mRNA at both ages. The faint band below the main actin mRNA band observed in B is due to a minor shorter actin mRNA transcript. (C) Levels of NT-3 mRNA relative to actin mRNA in the maxillary (Mx), mandibular (Md) and ophthalmic (Op) epithelia at E13 and E14. ganglion at E13 (Davies and Lumsden, 1984) and the NT-3 response is waning at this time. The demonstration that there is no additional neuronal survival in cultures containing NGF plus either BDNF or NT-3 in E11 and E12 cultures indicates that the population of neurons that responds to NGF overlaps with those that respond to BDNF or NT-3. These findings suggest that there is indeed a switch in the neurotrophin requirements of developing trigeminal neurons from BDNF and NT-3 to NGF during the early stages of target field innervation (Fig. 12). Not all neurons become refractory to BDNF and NT-3. Each of these factors promotes the survival of approximately 5% of trigeminal neurons at E14 and at stages throughout the phase of naturally occurring neuronal death (data not shown). The observation that these neurons are distinctly larger than those surviving with NGF suggests that they are distinct from those supported by NGF at this stage. Work on the neurons of the embryonic chicken trigeminal ganglion has clearly shown that during the phase of naturally occurring neuronal death there are separate populations of BDNF-responsive and NGF-responsive neurons. The ventrolateral part of the ganglion contains a small number of large-diameter, placode-derived, BDNF-responsive neurons (Davies et al., 1986b) and the dorsomedial part of the ganglion is composed of numerous small-diameter, neuralcrest-derived, NGF-responsive neurons (Davies and Lindsay, 1985; Davies et al., 1986b). The absence of a similar anatomical segregation of placode- and neural-crestderived neurons in the embryonic rodent trigeminal ganglion (Altman and Bayer, 1982; Davies and Lumsden, 1983) precludes similar definitive studies of the existence of separate NGF- and BDNF-responsive neurons in the midembryonic mouse trigeminal ganglion. The onset of the NGF survival response lags behind the BDNF and NT-3 responses. In E10 cultures after 48 hours incubation, NGF supports less than 5% of neurons that survive in the presence of BDNF or NT-3. If neuronal development proceeds at the same rate in vitro, then only a very minor proportion of the neurons in the E12 ganglion in vivo are capable of responding to NGF. This is some 1.5 days after the earliest trigeminal axons come into proximity with target field epithelium in the mandibular process. There is an increase in the proportion of NGF responsive neurons in cultures set up at E10 to those set up at E11, but this only amounts to 25% NGF-responsive neurons in E11 cultures after 48 hours incubation. At the developmentally equivalent stage in vivo, E13, a prominent dermal plexus of nerve fibres has formed and the innervation of developing whisker follicles is well under way. Thus, contrary to the previous assertion that NGF responsiveness commences in trigeminal neurons with the arrival of their axons in the peripheral target field (Davies and Lumsden, 1984), our present findings suggest that NGF responsiveness begins in these neurons shortly after their axons reach the peripheral target field. A striking feature of the NGF dose response in embryonic trigeminal neurons is that it undergoes a marked developmental shift (Fig. 4). In E12 cultures, when most if not all neurons have started to respond to NGF, the concentration of NGF that promotes half-maximal survival is 1.8×10-13 M. In E15 cultures, however, the concentration of NGF that Changing neuronal response to neurotrophins 999 Fig. 12. Summary of the developmental changes in neurotrophin responsiveness through which trigeminal ganglion neurons pass. Time in the life history of a typical trigeminal ganglion neuron is represented from left to right in the diagram (not necessarily drawn to scale). The onset of axon outgrowth and the approximate times when the peripheral axon reaches the vicinity of its target and the neuron is susceptible to being eliminated during the phase of naturally occurring neuronal death are shown along the lower part of the diagram. From the onset of axon outgrowth to the time the axon comes into proximity with its peripheral target, the neuron survives independently of neurotrophins (upper box). As the axon reaches its target the neurons become responsive to both BDNF and NT-3. There is then a transitional period during which the neurons respond to NGF as well as BDNF and NT-3. The BDNF response is lost first followed by the loss of the NT-3 response, leaving the neuron dependent on NGF for survival during the time when it is competing with other neurons for the limiting supply of NGF. By analogy with in vitro studies on sensory neurons from adult dorsal root ganglia (Lindsay, 1988), the trigeminal neuron loses its survival dependence on NGF at some later stage of development. promotes half-maximal survival has increased by over an order of magnitude to 2×10-12 M. This shift in the NGF dose response takes place during the phase of naturally occurring neuronal death in the trigeminal ganglion, which extends from E13 to E18 in vivo Thus, the number of neurons that survive this phase of development in vivo is not only limited by the supply of NGF but is limited by the increasing requirement of individual trigeminal neurons for NGF. In summary, our findings suggest that the neurotrophin requirements of sensory neurons change during development (Fig. 12). Initially, when their axons are growing to their targets, the neurons survive independently of neurotrophins. When their axons come into proximity with their peripheral targets, the neurons become responsive to both BDNF and NT-3. Shortly afterwards, the neurons additionally become responsive to NGF. With the onset of naturally occurring neuronal death, the neurons lose their response first to BDNF and then to NT-3. The NGF survival response is maintained throughout the phase of naturally occurring neuronal death but there is a marked shift in the NGF dose response during this period. Developmental significance of changing neurotrophin requirements It is unlikely that the early survival responses of developing trigeminal neurons to BDNF and NT-3 play a direct role in regulating the final number of neurons that survive in the trigeminal ganglion because these responses are largely over by the time naturally occurring neuronal death begins. It is possible, however, that the function of these early survival responses to BDNF and NT-3 may be to sustain the survival of the neurons whose axons reach the target field during the early stages of its innervation. This would delay the onset of neuronal death in the trigeminal ganglion until most of the neurons have started to innervate the target field, thereby ensuring that the majority of neurons compete for a supply of NGF during the same period of development. If, in the absence of the early BDNF and NT-3 survival responses, the supply of NGF were limiting from the earliest stages of target field innervation, it is possible that too many neurons would be eliminated before the capacity of the growing target field has increased to support the required number of neurons. It may also be advantageous for most of the neurons that innervate a given target field to compete for survival at the same time because this would maximise the choice for selectively maintaining neurons on the basis of the appropriateness of their axon terminations in the target field. Changing patterns of neurotrophin gene expression during development We have shown that BDNF mRNA and NT-3 mRNA are expressed in presumptive maxillary tissue as early as E9.5. This is approximately 1.5 days before the arrival of the earliest trigeminal axons and the onset of NGF mRNA expression in the maxillary process (Davies and Lumsden, 1984; Davies et al., 1987). Studies of neurite outgrowth from trigeminal ganglia co-cultured with appropriate and inappropriate cutaneous target tissues have shown that the uninnervated maxillary process produces a diffusible substance that promotes and attracts the growth of early trigeminal neurites (Lumsden and Davies, 1983). It is unlikely, however, that either BDNF or NT-3 is the trigeminal chemotropic factor because BDNF and NT-3 are expressed in both epithelium and mesenchyme whereas the chemotropic factor is released by the epithelium alone (Lumsden and Davies, 1986). Furthermore, the production of the chemotropic factor is apparently restricted to the 1000 V. L. Buchman and A. M. Davies trigeminal territory whereas BDNF mRNA and NT-3 mRNA are present in both the hyoid process and forelimb bud during the earliest stages of target field innervation at E11. The level of BDNF mRNA remains low in the maxillary target field at E9.5 and E10, then increases over ten-fold to reach a peak at E12 followed by a fall to reach a lower level by E13 that is maintained through later ages. NT-3 mRNA also shows a peak of expression in the maxillary target field. However, relative to BDNF mRNA, the increase begins a day later (during E11), the peak occurs a day later than the BDNF mRNA peak and the level of NT-3 mRNA does not fall to comparable BDNF mRNA levels until E16. These findings together with previous work on the time-course of NGF mRNA expression in the developing maxillary target field (Davies et al., 1987) indicate that developmental changes in neurotrophin mRNA expression in the peripheral trigeminal territory are correlated with the changing responsiveness of trigeminal neurons to neurotrophins. The early expression of BDNF mRNA and NT-3 mRNA is related to the early response of trigeminal neurons to these factors, and the subsequent expression of NGF mRNA correlates with the onset of NGF responsiveness. The early peak and decline in BDNF mRNA relative to NT-3 mRNA accords with the loss of BDNF responsiveness preceding the loss of NT-3 responsiveness. The expression of BDNF and NT-3 mRNAs at lower levels after the majority of neurons have lost their response to BDNF and NT-3 may be required to promote the survival of the small proportion of trigeminal neurons that remain dependent on these factors for survival. In contrast to the early peaks of BDNF mRNA and NT3 mRNA expression in the peripheral trigeminal territory, the level of BDNF mRNA is very low and NT-3 mRNA is barely detectable in the hindbrain over the same period of development. This suggests that early trigeminal neurons obtain their supply of BDNF and NT-3 mainly, if not exclusively, from their peripheral targets. Although there is no early peak of BDNF mRNA expression in the hindbrain, the level increases slowly throughout the early period of naturally occurring neuronal death in the trigeminal ganglion. Thus, the hindbrain may be an important source of BDNF for the small proportion of trigeminal neurons that remains dependent on this factor for survival later in development. The hindbrain may also be a source of BDNF for the BDNF-dependent trigeminal mesencephalic neurons (Davies et al., 1986a, 1987) and placode-derived cranial sensory neurons (Lindsay et al., 1985; Davies et al., 1986b) whose central axons innervate various groups of neurons in the hindbrain. In addition to showing that each neurotrophin mRNA has a distinct temporal pattern of expression in the developing maxillary target field, we have shown that there are regional differences in the expression of these mRNAs within and among developing cutaneous territories. Throughout the early stages of maxillary target field innervation up to the onset of neuronal death, the level of NGF mRNA (relative to tissue protein content) in the epithelium is 5-fold higher than in the mesenchyme (Davies et al., 1987). During the earliest stages of target field innervation, the levels of both BDNF mRNA and NT-3 mRNA (relative to either actin mRNA or L27 mRNA) are higher in mesenchyme than in epithelium. At later stages, however, the overall levels of each of these mRNAs are expressed at approximately similar levels in both tissues. A recent in situ hybridization study has also shown that NGF, BDNF and NT-3 mRNAs are expressed in the epidermal and dermal tissues of the embryonic mouse maxillary target field from E11.5 (the earliest stage studied) to E17.5 (Schecterson and Bothwell, 1992). NGF mRNA was expressed mainly in the epithelium, in agreement with previous studies (Davies et al., 1987), whereas BDNF and NT-3 mRNAs were expressed primarily in dermal mesenchyme. Although this latter observation appears to conflict with our finding that the overall levels of BDNF mRNA or NT-3 mRNA are similar in these tissues in the later stages of innervation, northern blotting and in situ hybridization provide complementary information on mRNA expression. Whereas northern blotting provides valuable quantitative data on the mean level of neurotrophin mRNA expression in different tissues, this technique does not provide information about any regional differences in mRNA expression within tissus. Thus, both epithelium and mesenchyme may express similar mean levels of NT-3 mRNA by northern blotting, yet within the mesenchyme NT-3 mRNA could be preferentially expressed in the presumptive dermal region and give a stronger signal in this part of the mesenchyme compared with the overlying epithelium in in situ hybridization. The significance of the different regional patterns of neurotrophin mRNA expression within developing cutaneous tissues is unclear. It is likely, however, that expression of BDNF and NT-3 mRNAs at relatively high levels in the early target field mesenchyme results in BDNF and NT-3 being made available to growing axons from the earliest stages of reaching the target field. The predominant expression NGF mRNA with the epithelium and subjacent mesenchyme indicates that NGF is synthesized in the region where the great majority of sensory axons terminate and as such is more likely to play a major role in selectively supporting the survival of neurons that make appropriate axon terminations in the target field. We have demonstrated that there are differences in neurotrophin mRNA levels in the epithelia of different cutaneous territories. Previous work has shown that during the earliest stages of naturally occurring neuronal death in the trigeminal ganglion at E13 and E14, there are marked differences in the level of NGF mRNA in the epithelia of the maxillary, mandibular and ophthalmic innervation territories; the level is highest in the epithelium of the densely innervated maxillary territory and lowest in the epithelium of the sparsely innervated ophthalmic territory (Harper and Davies, 1990). This suggests that regional differences in NGF production govern the number of neurons that survive to innervate each territory. Likewise, in the present study, we have shown that the levels of NT-3 mRNA in the epithelia of the maxillary, mandibular and ophthalmic territories during the early stages of naturally occurring neuronal death at E14 are also correlated with final innervation density. Thus, regional differences in NT-3 production may also govern regional differences in innervation density by the small proportion of trigeminal neurons that retain dependence on NT-3 throughout the phase of naturally occurring neuronal death. Changing neuronal response to neurotrophins 1001 In summary, we have demonstrated a sequence of neurotrophin gene expression in developing cutaneous targets and corresponding changes in the response of the innervating sensory neurons to neurotrophins. How these temporal patterns of gene expression and neuronal responsivenss are controlled are important questions that need to be resolved in future studies in order to provide a more complete understanding of the trophic interactions between neurons and their targets that govern the formation and maintenance of appropriate neural connections. We thank John Winslow and Gene Burton of Genentech for the purified recombinant BDNF and NT-3, Bill Mobley for the NGF, Marc Tessier-Lavigne for the rat NT-3 cDNA, Patrick Ernfors for the mouse BDNF cDNA, Rolf Heuman for the mouse 18S rRNA cDNA and Tom Jessell for the 2H3 monoclonal antibody. We thank Sarah Harper for carrying out some preliminary studies of NT-3 mRNA expression in the trigeminal system. This work was supported by grants from the Wellcome Trust, Action Research, Medical Research Council and The Royal Society. REFERENCES Altman, J. and Bayer, S. A. (1982). Development of the cranial nerve ganglia and related nuclei in the rat. Adv. Anat. Embryol. Cell Biol. 74, 190. Barde, Y. A. (1989). Trophic factors and neuronal survival. Neuron 2, 1525-1534. Berkemeier, L. R., Winslow, J. W., Kaplan, D. R., Nikolics, K., Goeddel, D. V. and Rosenthal, A. (1991). Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron 7, 857-866. Chizgwin, J. M., Przybyla, A. E., McDonald, R. J. and Rutter, W. J. (1979). Isolation of biologically active ribonucleic acid from sources enriched in ribonucleases. Biochemistry 18, 5294-5299. Davies, A. M. (1987a). The growth rate of sensory nerve fibres in the mammalian embryo. Development 100, 307-311. Davies, A. M. (1988). Role of neurotrophic factors in development. Trends Genet. 4, 139-143. Davies, A. M., Bandtlow, C., Heumann, R., Korsching, S., Rohrer, H. and Thoenen, H. (1987). Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature 326, 353-358. Davies, A. M. and Lindsay, R. M. (1985). The cranial sensory ganglia in culture: Differences in the response of placode-derived and neural crestderived neurons to nerve growth factor. Dev. Biol. 111, 62-72. Davies, A. M. and Lumsden, A. (1983). Influence of nerve growth factor on developing dorso-medial and ventro-lateral neurons of the chick and mouse trigeminal ganglia. Int. J. Devl. Neurosci. 1, 171-177. Davies, A. M. and Lumsden, A. (1984). Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J. Comp. Neurol. 223, 124-137. Davies, A. M. and Lumsden, A. G. (1986). Fasciculation in the early mouse trigeminal nerve is not ordered in relation to the emerging pattern of whisker follicles. J. Comp. Neurol. 253, 13-24. Davies, A. M., Lumsden, A. G. and Rohrer, H. (1987). Neural crestderived proprioceptive neurons express nerve growth factor receptors but are not supported by nerve growth factor in culture. Neuroscience 20, 3746. Davies, A. M., Thoenen, H. and Barde, Y. A. (1986a). Different factors from the central nervous system and periphery regulate the survival of sensory neurones. Nature 319, 497-499. Davies, A. M., Thoenen, H. and Barde, Y. A. (1986b). The response of chick sensory neurons to brain-derived neurotrophic factor. J. Neurosci. 6, 1897-1904. Ernfors, P., Ibanez, C. F., Ebendal, T., Olson, L. and Persson, H. (1990). Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc. Natl. Acad. Sci. USA 87, 5454-5458. Hallbook, F., Ibanez, C. F. and Persson, H. (1991). Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron 6, 845-858. Harper, S. and Davies, A. M. (1990). NGF mRNA expression in developing cutaneous epithelium related to innervation density. Development 110, 515-519. Heumann, R., Korsching, S., Scott, J. and Thoenen, H. (1984). Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J. 3, 3183-3189. Hohn, A., Leibrock, J., Bailey, K. and Barde, Y. A. (1990). Identification and characterization of a novel member of the nerve growth factor/brainderived neurotrophic factor family. Nature 344, 339-341. Ip, N. Y., Ibanez, C. F., Nye, S. H., McClain, J., Jones, P. F., Gies, D. R., Belluscio, L., Le, B. M., Espinosa, R. III., Squinto, S. P., Person, H. and Yancopoulos, G. D. (1992). Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl. Acad. Sci. USA 89, 3060-3064. Jones, K. R. and Reichardt, L. F. (1990). Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc. Natl. Acad. Sci. USA 87, 8060-8064. Korsching, S. and Thoenen, H. (1988). Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev. Biol. 126, 40-46. Leibrock, J., Lottspeich, F., Hohn, A., Hofer, M., Hengerer, B., Masiakowski, P., Thoenen, H. and Barde, Y. A. (1989). Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149-152. Lindsay, R. M. (1988). Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J. Neurosci. 8, 2394-2405. Lindsay, R. M., Thoenen, H. and Barde, Y. A. (1985). Placode and neural crest-derived sensory neurons are responsive at early developmental stages to brain-derived neurotrophic factor. Dev. Biol. 112, 319-328. Lowry, O.H., Rosenbrough, N. J., Farr, N. J. and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265-275. Lumsden, A. G. and Davies, A. M. (1983). Earliest sensory nerve fibres are guided to peripheral targets by attractants other than nerve growth factor. Nature 306, 786-788. Lumsden, A. G. and Davies, A. M. (1986). Chemotropic effect of specific target epithelium in the developing mammalian nervous system. Nature 323, 538-539. Maisonpierre, P. C., Belluscio, L., Squinto, S., Ip, N. Y., Furth, M. E., Lindsay, R. M. and Yancopoulos, G. D. (1990). Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science 247, 1446-1451. Rohrer, H., Henke, F. S., el, S. T., Lux, H. D. and Thoenen, H. (1985). Progenitor cells from embryonic chick dorsal root ganglia differentiate in vitro to neurons: biochemical and electrophysiological evidence. EMBO J. 4, 1709-1714. Rosenthal, A., Goeddel, D. V., Nguyen, T., Lewis, M., Shih, A., Laramee, G. R., Nikolics, K. and Winslow, J. W. (1990). Primary structure and biological activity of a novel human neurotrophic factor. Neuron 4, 767-773. Schecterson, L. C. and Bothwell, M. (1992). Novel roles for neurotrophins are suggested by BDNF and NT-t mRNA expression in developing neurons. Neuron 9, 449-463. Sieber, B. M. (1991). Role of the neurotrophic factors BDNF and NGF in the commitment of pluripotent neural crest cells. Neuron 6, 949-55. Tanaka, T., Kuwano, Y., Ishikawa, K. and Ogata, K. (1988). Eur. J. Biochem. 173, 53-56. Theiler, K. (1972). Development and normal stages from fertilization to 4 weeks. In The House Mouse Berlin: Springer-Verlag. Vogel, K. S. and Davies, A. M. (1991). The duration of neurotrophic factor independence in early sensory neurons is matched to the time course of target field innervation. Neuron 7, 819-830. Wright, E., Vogel, K. S. and Davies, A. M. (1992). Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron 9, 139-150. Wyatt, S., Shooter, E. M. and Davies, A. M. (1990). Expression of the NGF receptor gene in sensory neurons and their cutaneous targets prior to and during innervation. Neuron 4, 421-427. (Accepted 30 March 1993)