* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 5 Action Potential.key
Multielectrode array wikipedia , lookup
Synaptic gating wikipedia , lookup
Chemical synapse wikipedia , lookup
Nervous system network models wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Patch clamp wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Node of Ranvier wikipedia , lookup
Single-unit recording wikipedia , lookup
Biological neuron model wikipedia , lookup
Resting potential wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Action potential wikipedia , lookup
Membrane potential wikipedia , lookup
G protein-gated ion channel wikipedia , lookup
Electrophysiology wikipedia , lookup
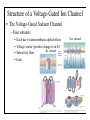
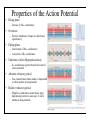
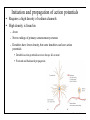
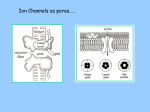
Brain Science Fundamentals Christopher Fiorillo BiS 328, Fall 2011 042 350 4326, [email protected] Part 5: A Neuron’s Membrane Voltage: Active Properties Reading: Bear, Connors, and Paradiso, Chapter 4. Assistant: Sora Yun <[email protected]> Summary of a Neuron’s Inputs and Output • Output corresponds to voltage across the neuron’s membrane • Inputs correspond to current flowing through ion channels • Ion channels are small pores in the membrane. They only allow current to flow when they are open. • The opening of ion channels depends on the concentration of chemical neurotransmitters, or the voltage across the membrane. Passive versus Active Membrane Properties • “Passive” refers to the flow of current and changes in voltage that do not involve voltage-dependent opening or closing of ion channels • “Active” refers to voltage changes that depend on channels that open or close in response to changes in membrane voltage – This depends on a feedback process • The membrane voltage in neurons depends on both active and passive membrane properties The Action Potential • Graded changes in current cause graded changes in voltage. • If voltage is depolarized beyond a threshold (about -50 mV), an action potential is triggered. An Action Potential is “All-or-None” • At a brief moment in time (~1 ms), an action potential either occurs or it does not • The shape of an action potential is always the same (almost) • The magnitude of the current and depolarization that triggered the action potential do not matter (but the depolarization must reach threshold) Firing Rate Depends on Current Magnitude • The frequency of action potentials (“firing rate”) depends on the magnitude of depolarizing current (assuming that the current lasts long enough). Why do neurons have action potentials? • Current leaks out of the membrane • Therefore, a large change in voltage at one location will produce only a small change at another location • An action potential is regenerative. It does not decline in its voltage amplitude as it moves through a neuron Why Do Neurons Have Action Potentials? • Current that enters the cell at one point will spread passively through the cytosol of a dendrite. • Because of membrane permeability, current leaks out of the neuron as it travels along a dendrite. • Thus information cannot be conveyed over long distances through passive spread of current. • An action potential is “regenerative” and therefore it can reliably carry information over long distances. • Trade-off: Digital (action potential) versus Analog (membrane voltage) • Most neurons have action potentials. • Many neurons that do not need to transmit information over long distances do not have action potentials. These include: – Most retinal neurons – All neurons in C. elegans (a small worm) Types of Electrophysioligical Recordings from Single Neurons • Intracellular – Records voltage or current across the membrane – Sharp microelectrodes penetrate inside the cell – Patch electrodes are attached to the membrane • Extracellular – Record only the times of action potentials – Small and slow changes in membrane voltage cannot be measured An experiment in my lab with two glass pipettes. One is for patch recording of membrane voltage. The other is to deliver a neurotransmitter. Haram Kim, a student in my lab, made this image, and she named it “genesis.” A neuron can be seen in the center, but it is out of focus. A photo of the stage of the microscope. A living brain slice from a rat is under the objective of the microscope, between the two pipettes. A very nice painting by Haram. When Haram is not working in the lab, she makes art like this. Recording Methods: Current and Voltage Clamp • “Current Clamp” – Measures voltage across cell’s membrane – A constant current is injected through the electrode • The current can be manually adjusted by the experimenter • “Voltage Clamp” – Measures current passing through the cell’s membrane – Clamps voltage across the cell’s membrane • A feedback circuit calculates the amount of current that is necessary to keep the voltage constant, and then injects that amount of current through the electrode. Current versus Voltage Clamp • Advantages of current clamp – More physiological. Voltage is never clamped under physiological conditions – Fast and accurate voltage clamp can be difficult to achieve • Especially in the dendrites of a large neuron • Advantages of voltage clamp – Greater experimental control: it eliminates voltage as a variable • Keeps driving force constant • Better for studying voltage-gated channels • Better temporal resolution for fast channels, because it removes the effect of membrane capacitance • In general, voltage-clamp is better for studying the properties of ion channels. Current clamp is better for studying the properties of neurons. The Patch Clamp Method • Developed by Erwin Neher • Very useful for electrophysiology • Enables the recording of single channels Structure of a Voltage-Gated Ion Channel • The Voltage-Gated Sodium Channel – Four subunits • • • • Each has 6 transmembrane alpha-helices Voltage sensor (positive charges) on S4 K+ channel Selectivity filter Gate Na+ channel An Ion Channel Has Many States (Conformations) • States of a glutamate-gated ion channel (4 subunits) are shown • Open states require that glutamate is bound • There are many desensitized (inactivated) states • Gray states are seldom visited • This is typical of many ion channels, including voltagegated ion channels • This is probably more simplistic than reality Recordings of Single Na+ Channels • Channels exist in discrete states: Open or closed • The channels “behavior” will not be the same, even under identical conditions. It is “stochastic.” • Inactivation usually occurs after the channel opens • A large number of sodium channels like this can produce an action potential Phases of the Action Potential • • • • Rising phase Overshoot Falling phase Undershoot (after-hyperpolarization, or AHP) Properties of the Action Potential • Rising phase – Increase in Na+ conductance • Overshoot – Positive membrane voltage (no functional significance) • Falling phase – Inactivation of Na+ conductance – Activation of K+ conductance • Undershoot (After-Hyperpolarization) – K+ conductance greater than before start of action potential • Absolute refractory period – Na+ channel inactivation makes it impossible to elicit another action potential • Relative refractory period – High K+ conductance means that a large depolarizing current is necessary to elicit another action potential The Threshold of the Action Potential • The threshold is between -50 to -40 mV in most cells • The threshold is caused by positive feedback between sodium channels – When a few sodium channels open, sodium flows into the cell and causes a small depolarization – The depolarization causes more sodium channels to open, which causes a larger depolarization, and so on • The threshold depends on the density of sodium channels, and their voltagedependence • The threshold is a property of the population of ion channels • Single channels do not have a threshold Relationship between single channel and cellular currents • Macroscopic currents in the cell result from the summation of many microscopic single channel currents • Although single channel currents are stochastic, currents within the cell are not. They are highly reproducible. • Single sodium channels do not have a threshold voltage at which they open • The action potential threshold depends on positive feedback between many sodium channels • Action potentials require a high density of sodium channels – A sufficient number of channels must be deinactivated (ready to be opened) • • • • • The Hodgkin-Huxley Model Hodgkin and Huxley described the ionic basis of the action potential (1952) This is considered the most important single achievement in cellular neurophysiology. Their approach: Experiments on the squid giant axon – Two electrode voltage clamp (1 mm diameter axon) – Measured the voltage-dependence and kinetics of sodium and potassium currents – Ion substitution experiments They derived a relatively simple but detailed mathematical and biophysical model of the action potential Their model is still the “textbook” model • The utility of their model extends beyond the action potential. It is useful for understanding all voltage-dependent ion channels • • Their model predicted some of the key properties of ion channels It was about 30 years later (~1980) that scientists were able to identify and record single ion channels • It was about 10 years after that (~1990) that people began to clone ion channels (to discover their amino acid sequence) It was about 10 years after that (~2000) that the 3-dimensional structure and function of ion channels began to be understood • Three Key Properties of Voltagegated Ion Channels • Ion selectivity • Voltage-dependence • Time-dependence (Kinetics) Voltage-Dependence of Ion Channels in HH model • ‘n-infinity’ is the steady-state probability that a K+ channel subunit “gate” is in the “open” conformation • The channel is only open when all four subunit gates are in open conformation – Thus, the steady-state probability that a channel is open: • Po = ninfinity4 • The Na+ channel has three activation gates (m) and one inactivation gate (h) – Thus, the steady-state probability that a channel is open: • Po = minfinity3h Summary of Voltage-Dependence of Parameters in HH model Kinetics Steady-state VoltageDependence Kinetics of Ion Channels Underlying the Action Potential • Sodium channels activate and inactivate quickly • Potassium channels activate more slowly Initiation and propagation of action potentials • Requires a high density of sodium channels • High density is found in: – Axon – Nerve endings of primary somatosensory neurons – Dendrites have lower density, but some dendrites can have action potentials • Dendritic action potentials are not always all-or-none • Forward and backward propagation Action Potential Conduction • Propagation of the action potential – It is an active, regenerative process, but it still relies upon the passive spread of current – Orthodromic: Action potential travels from soma to terminal – Antidromic: Backward propagation (towards soma) • This is artificial and only happens in experiments Conduction Velocity • Conduction velocity (0.5-80 m/s) • Two means of increasing velocity – Diameter of axon (or dendrite) • Increases speed by decreasing axial resistance • Squid Giant Axon: 1 mm – Insulation • Myelination by glia • Increases membrane resistance and decreases membrane capacitance • Some information needs to be transmitted quickly, some does not • Large axons and myelination are each costly • Some axons are small and unmyelinated Conduction of Action Potentials through Myelinated Axons • The myelinated part of the axon has few or no channels – Very little current leaks out • The action potential’s voltage amplitude gets smaller as it spreads – But it spreads over a long distance with little decay, because of the low conductance of the membrane • At the node of Ranvier, there is no myelin, and there is a high density of sodium and potassium channels • Thus the A.P. is regenerated at nodes of Ranvier • This is called “saltatory” conduction, which means that the A.P. jumps from one node to the next Beyond the Action Potential • We have focused on action potentials, but the HH model can be extended (by changing parameter values) to include many other types of voltage-gated ion channels • Voltage-gated ion channels do not only mediate the action potential. They also influence the pattern of action potentials. • Different neurons express different sets of voltageregulated ion channels – Therefore, different neurons have different firing patterns in response to the same excitatory input