* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lester-Lect to CaltechAssociates-Nov
Survey
Document related concepts
Feature detection (nervous system) wikipedia , lookup
Axon guidance wikipedia , lookup
End-plate potential wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Optogenetics wikipedia , lookup
Synaptic gating wikipedia , lookup
Aging brain wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neurotransmitter wikipedia , lookup
NMDA receptor wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Signal transduction wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Substantia nigra wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Transcript
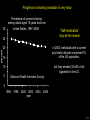
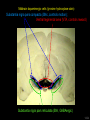
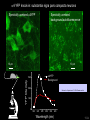
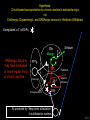
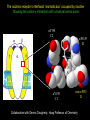
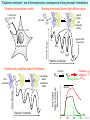
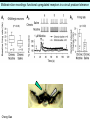
Three responses to chronic nicotine exposure: Studies on genes, proteins, drugs, cells, circuits, and behavior Henry Lester November, 2007 1/24 1. How does one explain nicotine addiction? Does it matter? Won’t everyone stop smoking soon? Smokeless tobacco? 2. Nicotine as an imperfect therapeutic drug Best example: Parkinson’s disease 3. Cellular / molecular approaches to better therapies 2/24 Progress on smoking cessation is very slow 30 Prevalence of current smoking among adults aged 18 years and over United States, 1997–2006 percent 25 In 2002, individuals with a current psychiatric disorder comprised 7% of the US population, 20 15 but they smoked 34-46% of all cigarettes in the US. 10 5 “Self-medication” may be the reason (National Health Interview Survey) 0 1996 1998 2000 2002 year 2004 2006 3/24 The nicotine video Produced for Pfizer to explain varenicline (Chantix) to physicians This summarizes knowledge in ~ 2004. “ligand” is a molecule that binds to another. “physical” addiction vs “psychological” addiction. “Desensitization“ and “Upregulation” receptors become “bored” 1 million channels Some abbreviations on future slides: ACh, acetylcholine nAChR, nicotinic acetylcholine receptor DA, dopamine nicotine 20 seconds 4/24 Focus on a4β2 receptors Conclusions from knockout and hypersensitive mice (2005): Activation of a4b2-containing (a4b2*) receptors by nicotine Is necessary and sufficient for sensitization, tolerance, reward, (but withdrawal?) What are the mechanisms? 5/24 Dialysate DA (nM) 1. Chronic nicotine exposure causes tolerance of dopamine release The “yoked self-administration” experiment Yoked animal Master animal 4.0 3.5 Yoked saline Yoked nicotine 3.0 2.5 2.0 1.5 1.0 0.5 0.0 Saline -40 0 Nicotine 40 80 120 160 Time (min) Rahman, Zhang, Engleman, & Corrigall, 2004 6/24 2. Chronic nicotine exposure causes cognitive sensitization In the human context, cognitive sensitization is epitomized by smokers’ reports that they think better when they smoke; this anecdotal observation is confirmed by data that smokers who smoke nicotine cigarettes (but not nicotine-free cigarettes) display several cognitive enhancements. In the rodent context, rats show more contextual fear conditioning if, one day after withdrawal from chronic nicotine, they receive an acute nicotine dose; also chronic nicotine produces better spatial working memory performance in the radial arm maze. 7/24 3. Inverse correlation between long-term tobacco smoking and Parkinson’s disease In identical twins discordant for both Parkinson’s disease & smoking, the unaffected twin smoked at a significantly higher rate. In those twins where one or both smoked, The unaffected twin smoked 12 pack-years more. There are good indications that nicotine itself is a protective agent. Clinical trials of nicotine patches have given mixed results because of side effects Beneficial results of short-term nicotine exposure: Pain reduction. Increased concentration: ADHD, Schizophrenia. Alzheimer (Aricept = donepezil, a cholinesterase inhibitor; Reminyl = galantamine) Decreased inflammation. Antidepressant actions. 8/24 Possible mechanism 1: The “Molecular Relay Race”: Signal transduction triggered by a ligand-gated channel receptor G protein i q s t effector channel enzyme nAChRs are highly permeable to Ca2+ as well as to Na+. intracellular messenger Ca2+ cAMP kinase phosphorylated protein 9/24 Possible Mechanisms 2a, 2b: a. The “Bored Receptor” (desensitized) versus b. The “Exuberant Receptor” (upregulated) Chronic exposure to nicotine induces more nicotinic receptors The “Receptor Dilemma”: How (if at all) do changed receptors contribute to . . . nicotine addiction? neuroprotection? If the upregulated receptors are Active (“exuberant”), If the upregulated receptors are Desensitized (“bored”), If the upregulated receptors are active, upregulation might cause better synaptic transmission and excitation, leading to cognitive sensitization. this might cause decreased synaptic transmission and excitation, leading to tolerance. excitotoxicity might exacerbate Parkinson’s disease. But this does not explain tolerance. But this does not explain cognitive sensitization. If the upregulated receptors are desensitized, this might be neuroprotective. 10/24 Strategy to choose between the “bored” or “exuberant” receptors in the response to chronic nicotine exposure 1. Generate mice with fully functional, fluorescent a4* receptors. (Why mice?) 2. Chronically expose the mice to nicotine (2 weeks). 3. Find the brain regions and cell types with changed fluorescence. 4. Perform experiments on these regions and cells to decide whether the new receptors are “bored” or “exuberant”. 5. Model the cellular and circuit changes 11/24 Chronic nicotine increases a4 fluorescence ~ 2-fold in hippocampus --a brain area that provides a good model for cognition. Alveus Py Or Functional studies show: the new receptors are “exuberant”, not “bored” Rad LMol V 200 mm Medial Perforant Path Temperoammonic Path 12/24 Midbrain dopaminergic cells (tyrosine hydroxylase stain) Substantia nigra pars compacta (SNc, controls motion); Ventral tegmental area (VTA, controls reward) Substantia nigra pars reticulata (SNr, GABAergic) 14/24 a4-YFP knock-in: substantia nigra pars compacta neurons Spectrally unmixed a4YFP Spectrally unmixed background autofluorescence 10 mm 10 mm a4YFP YFP Intensity 1500 Background 1000 Shortcut to Projections of 32-32-LS5unmix.avi.lnk 500 0 500 520 540 560 580 Wavelength (nm) 600 15/24 Substantia nigra data also support the “exuberant receptor” idea Chronic nicotine does not change a4 levels in dopaminergic neurons . . . Cumulative Percentage 100 80 60 Substantia Nigra Pars Compacta 40 20 0 0 500 1000 1500 2000 2500 3000 α4 intensity per TH+ neuron . . . but does upregulate a4 levels in GABAergic inhibitory neurons. Cumulative Percentage 100 80 60 Substantia Nigra Pars Reticulata 40 20 0 0 500 1000 1500 α4 intensity per GAD+ neuron 16/24 Chronic nicotine cell-specifically upregulates a4* receptors: Basis for circuit-based tolerance in midbrain via “exuberant inhibition” Chronic Saline Endogenous ACh VTA LDT Cholinergic Dialysate DA (nM) 4.0 3.5 Upregulated a4* nAChRs Craving NAc DAergic Chronic Nicotine Tolerance 2A 1A Endogenous ACh Endogenous ACh GABAergic Yoked saline Yoked nicotine 2B 1B 1B Reward Decreased Reward 3.0 2.5 2.0 1A Plus Acute Nicotine (1st expsoure) 1.5 1.0 0.5 0.0 Plus Acute Nicotine (repeated exposure) 2B 2A Saline Nicotine -40 -20 0 20 40 60 80 100 120140 160180 Time (min) Rahman et al, 2004 + acute nicotine 17/24 Hypothesis: Circuit-based neuroprotection by chronic nicotine in substantia nigra via Cholinergic, Dopaminergic, and GABAergic neurons in Hindbrain & Midbrain Upregulated a4* nAChRs Striatum SNc DAergic GABAergic neurons may have increased or more regular firing in chronic nicotine. . . PPTg Thalamus, superior colliculus Cholinergic GABAergic Endogenous ACh SNr . . . As produced by “deep brain stimulation” in subthalamic nucleus 18/24 Conclusions from hypersensitive & fluorescent mice When a4* nicotinic receptors are repeatedly occupied/activated these receptors become “exuberant” in specific neurons. This produces improved cognition via forebrain synapses, but tolerance occurs via changes in a GABA-dopamine circuit. How do we develop better therapeutics based on these ideas? 19/24 The nicotinic receptor’s interfacial “aromatic box” occupied by nicotine Showing the cation-p interaction with unnatural amino acids aY198 C2 aW149 B aY93 A aY190 C1 non-aW55 D Collaboration with Dennis Dougherty, Hoag Professor of Chemistry 20/24 “Stolen” photons tell us which subunits are near each other . . . After 24 hours in nicotine, exuberant receptors are assembled more tightly. 17.5 15.0 439 nm12.5 FRET Efficiency (%) 439 nm 514 nm 10.0 485 nm Experiments like these may show us nm how to develop better535 therapies for Parkinson’s Disease. 7.5 5.0 485 2.5 nm 535 nm 0.0 a4-b2XFP a4-b2XFP + nicotine 21/24 The ultimate reductionist approach, studying nAChR traffic/regulation at the single molecule level. TIRF microscopy of nAChR geGFP in oocytes 1 3 2 2 3 1 4 4 12 μm 22/24 Caltech Bruce Cohen, Ryan Drenan, Purnima Deshpande, Carlos Fonck, “Alpha Club” Sheri McKinney, Raad Nashmi, Qi Huang, Rigo Pantoja, Johannes Schwarz, Cagdas Son, Andrew Tapper, Larry Wade, Cheng Xiao “Unnatural Amino Joanne Xiu, Nyssa Puskar, Jai Shanata, Shawna Frazier, Acid Club” Dennis A. Dougherty Univ of Cambridge Sarah Lummis Univ Queensland Stephan Pless, Joseph Lynch Univ of Colorado, Sharon Grady, Al Collins, Mike Marks, Jeremy Owens, Boulder Tristan McClure-Begley, Paul Whiteaker UCLA Jim Boulter, Istvan Mody, Oliver Dorigo, Arnie Berk, Max Shao, Jack Feldman Univ. Pennsylvania Jon Lindstrom Rockefeller Univ Julie Miwa, Nathaniel Heintz Institut Pasteur Uwe Maskos, Jean-Pierre Changeux 23/24 More pontifications about upregulation (“exuberance”) Increased nAChR due to chronic nicotine exposure probably confers no selective advantage . . . could be a thermodynamic necessity. A substantial, regulated pool of unassembled or cytoplasmic high-sensitivity nAChRs receptors may confer a selective advantage. If so, the selective advantage may involve responding to circadian rhythms in ACh levels. If so, is there a disease caused by faulty nAChR regulation? Autosomal dominant nocturnal frontal lobe epilepsy? 24/24 “Exuberant receptors” are a thermodynamic consequence of durg-receptor Interactions Nicotine accumulates in cells 1 mM Nicotine+ (pKa = 7.9) Binding eventually favors high-affinity states + unbound 0 mV Free Energy -70 mV Bound states with increasing affinity 20 mM Nicotine+ Highest affinity bound state C AC A2C A2O A2D Reaction Coordinate + + Free Energy Free subunits Increasingly stable assembled states + nicotine RLS Covalently stabilized RHS Degradation AR*HS ? Nicotine Increased High-Sensitivity Receptors Nicotine may stabilize subunit interfaces Reaction Coordinate 0 20 40 60 hr 25/24 “Upregulation” Chronic exposure to nicotine causes upregulation of nicotinic receptor binding (1983: Marks & Collins; Schwartz and Kellar); Upregulation 1) Involves no change in receptor mRNA level; 2) Depends on subunit composition (Lindstrom, Kellar, Perry). Shown in experiments on clonal cell lines transfected with nAChR subunits: Nicotine seems to act as a “pharmacological chaperone” (Lukas, Lindstrom) or “maturational enhancer” (Sallette & Corringer, Heinemann) or “Novel slow stabilizer” (Green). Upregulation is “cell autonomous” and “receptor autonomous” (Henry). 26/24 Midbrain slice recordings: functional upregulated receptors in a circuit produce tolerance Cheng Xiao 27/24 Substantia nigra data also support the “exuberant receptor” idea Substantia Nigra Pars Compacta Number of Neurons Chronic nicotine does not change a4 levels in dopaminergic neurons ... 40 30 20 10 0 0 500 1000 1500 2000 100 Cumulative Percentage Saline (n=612) Nicotine (n=581) 50 80 60 40 20 0 2500 0 Mean a4YFP Intensity per TH+ Neuron 500 1000 1500 2000 2500 3000 Substantia Nigra Pars Reticulata Saline (n=256) Nicotine (n=237) 40 30 20 10 0 100 Cumulative Percentage 50 Number of Neurons . . . but does upregulate a4 levels in GABAergic inhibitory neurons 80 60 40 20 0 0 250 500 750 1000 1250 1500 Mean a4YFP Intensity per GAD67+ Neuron 0 500 1000 1500 28/24 Simple model for cognitive sensitization: Acute Nicotine Chronic Saline 10 min Acute Saline 80 min Chronic Saline 0.5 mV 0.5 mV 10 ms 10 ms 1.5 10 ms Chronic Acute Nicotine Nicotine Saline Nicotine 160 150 140 130 120 110 100 90 80 1 mV 0.5 0 20 10 ms 40 80 30 Stimulus Strength (mA) 120 110 100 90 100 0 20 40 40 40 Acute Saline P < 0.001 20 10 0 Saline Nicotine Chronic 80 100 Chronic Nicotine 40 40 30 60 Time (min) LTP Induction (% increase) 20 130 80 60 Acute Nicotine 10 Chronic Acute Nicotine Saline Saline Saline Time (min) 0.0 0 140 1 mM Nicotine 1.0 LTP Induction (% increase) Slope (-mV/ms) 2.0 Nicotine 1mV 30 20 10 0 Saline Nicotine Chronic LTP Induction (% increase) 1 mV Nicotine fEPSP Slope (%) 2.5 5 ms fEPSP Slope (%) chronic nicotine + acute nicotine lowers the threshold for perforant pathway LTP 80 min 10 min 30 20 10 Acute Saline Mecamylamine 0 -10 -20 p < 0.001 29/24