* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download - Journal of Vestibular Research
Microneurography wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
Brain Rules wikipedia , lookup
Neurotransmitter wikipedia , lookup
Neural engineering wikipedia , lookup
Mirror neuron wikipedia , lookup
Artificial general intelligence wikipedia , lookup
Neural coding wikipedia , lookup
Multielectrode array wikipedia , lookup
Neuroeconomics wikipedia , lookup
Synaptogenesis wikipedia , lookup
Electrophysiology wikipedia , lookup
Haemodynamic response wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Endocannabinoid system wikipedia , lookup
NMDA receptor wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Aging brain wikipedia , lookup
Single-unit recording wikipedia , lookup
Neuroscience in space wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neural oscillation wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Hypothalamus wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Nervous system network models wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Circumventricular organs wikipedia , lookup
Neuroanatomy wikipedia , lookup
Central pattern generator wikipedia , lookup
Metastability in the brain wikipedia , lookup
Optogenetics wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Synaptic gating wikipedia , lookup
Journal of Vestibular Research, Vol. 6, No. 3, pp. 185-201, 1996
Copyright © 1996 Elsevier Science Inc.
Printed in the USA. All rights reserved
0957-4271196 $15.00 + .00
ELSEVIER
0957-4271 (95)02042-X
Original Contribution
THE RECOVERY OF STATIC VESTIBULAR FUNCTION FOLLOWING
PERIPHERAL VESTIBULAR LESIONS IN MAMMALS:
THE INTRINSIC MECHANISM HYPOTHESIS*
Cynthia L. Darlington* and Paul F. Smitht
*Department of Psychology and the Neuroscience Research Centre, University of Otago
and the tDepartment of Pharmacology, School of Medical Sciences,
University of Otago Medical School, Dunedin, New Zealand
Reprint address: Dr. Cynthia L. Darlington, Dept. of Psychology, University of Otago, Dunedin,
New Zealand. Tel: (64) (3) 479 764'1, Fax: (64) (3) 479 8335
D Abstract- This theoretical paper describes the
"intrinsic mechanism hypothesis," a new hypothesis
of vestibular compensation, the behavioral recovery
that follows unilateral deafferentation of the vestibular labyrinth (UVD). The most salient characteristic of vestibular compensation is the decrease in
the severity of the static ocular motor and p$ostural
symptoms that follow UVD, associated with a recovery of resting activity in the ipsilateral vestibular
nucleus complex (VNC). The speed of static compensation in some mammalian species (for example,
cat) has suggested that reactive synaptogenesis is an
unlikely explanation because it is too slow. Other,
more rapid mechanisms, such as denervation supersensitivity, receptor-up-regulation, or increased
neurotransmitter release, were reasonable possibilities. However, to date, each study that has addressed these possibilities has failed to find any
change that could account for the recovery of VNC
resting activity. The search for such "substitutive"
mechanisms was based on the hypothesis that something other than the VNC neurons themsefives would
activit: that
have to "replace'' the
the ipsilateral vestibular nerve normaHy provides.
However, brainstem slice studies demonstrate that,
at least in vitro, VNC neurons do not need the vestHndar nerve in order to generate resting activity.
On the basis of these and other considerations,
*This paper is dedicated to the memory of Professor John
I. Hubbard, our mentor and friend, who died October
1, 1995.
RECEIVED
20 June 1995;
AccEPTED
we suggest that following a brief calcium-induced
diaschisis, VN C neurons ipsilateral to the UVD reactivate the intrinsic membrane properties that normally contribute to their resting activity in vivo, and
that this recovery of resting activity accounts for
static vestibular compensation.
D Keywords- vestibular compensation;
unilateral labyrinthectomy; vestibular nerve
transection; vestibular nucleus.
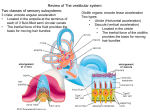
Introduction
"Vestibular compensation~' is a process of behavioral recovery that occurs following theremoval of afferent input from the vestibular
labyrinth, either by surgical removal of the
vestibular receptors or by transsection of the
vestibular nerve. Immediately following unilateral peripheral vestibular deafferentation
'
o:; ocmar
motor
and postural symptoms develops; these symptoms are usually divided into static and dynamic symptoms, depending upon whether
they persist in the absence of head movement
(static) or occur as a result of head movement
(dynamic) (see 1-5 for reviews). However, in
some mammalian species, within a few days,
the static symptoms have undergone a remarkable degree of recovery (compensation), even in
12 October 1995.
185
('
186
C. L. Darlington and P. F. Smith
darkness. The extent of the static compensation is generally greater in light than in darkness, because vision is used to reduce symptoms
like spontaneous ocular nystagmus (SN) (Table 1). However, among lower mammalian
species (for example, rat, guinea pig, cat), even
in darkness, static symptoms such asSN have
usually compensated to less than 1007o of their
initial values by 3 to 4 days
ble
For reasons that are not clear, SN compensation in dar!<.ness appears to take
sus monkey
0 to 20 days in humans
(1
Compensation of the static symptoms
is correlated with a recovery of resting activity in the ipsilateral vestibular nucleus complex
(VNC), although the extent of the recovery of
resting activity is controversial and varies between the different subnuclei (Table 2).
Despite the fact that vestibular compensation research began in the 1800s with the work
of Flourens and Bechterew (see 1 for a review)
and that an enormous volume of research has
been published on the subject since, the neurophysiological and neurochemical bases of vestibular compensation are still not completely
understood. In this theoretical paper, we propose a new hypothesis, called the "intrinsic
mechanism hypothesis," which we believe ac-
counts for most of the published data in the
area of vestibular compensation of static vestibular function in mammals. The intrinsic mechanism hypothesis proposes that vestibular
compensation of the static symptoms of UVD
is directly related to the recovery of resting activity in the ipsilateral VNC, which is largely a
result of changes in the intrinsic 1nembrane
of VNC neurons (22). This
~zddresses static vestibular
is not intended to
include submammalian species such as frogs:
1)
do not exhibit SN, which in lower
mammals compensates more quickly than
most other static and dynamic symptoms (see
1-5 for reviews); 2) in frogs, compensation of
static symptoms such as roll head tilt occurs
over a period of months and has not been
demonstrated to be tightly correlated with the
recovery of resting activity of type I VNC neurons ipsilateral to the UVD (see 1-5 for reviews); 3) in frogs, vestibular compensation is
associated with an enhancement of the efficacy of the excitatory brainstem commissural
input to the ipsilateral VNC, whereas in mammalian species no such change in efficacy has
been demonstrated and these commissural fibres form part of a functionally inhibitory
commissural system (see 1-5 for reviews).
Table 1. Examples of Studies of Different Mammalian Species That Demonstrate
Rapid Compensation of Spontaneous Nystagmus
Author(s)
Species
Sirkin et al. (6)
Smith et al. (7)
rat
guinea pig
Newlands and Perachio (8)
Yamanaka et al. (9)
Haddad et al. ( i 0)
gerbil
rabbit
cat
Days to
Compensation
Min Value
Post-UVD
3-4 (light)
2 (light)
2 (red light)
1 -2 (light)
4 (light)
3 (light and dark)
2 b/1 5 s
1 . 6 b/1 5 s
1 b/15 s
N/A
2.5 b/i 0 s
2 °/S
%Max Value
Post-UVD
2
8
5
N/A
7
2
Examples of studies of 4 species in which compensation of spontaneous nystagmus (SN) develops rapidly. "Light" and "dark" refer
to the conditions under' which the measurements were made; "b" refers to beats of spontaneous nystagmus; s: second; N/ A: data
not available. % max value: %of maximal SN measured at that time post-UVD. 'red light' in (7) refers to measurements made in red
light, to which guinea pigs are blind; before this the animals were maintained in total darkness for 50 h post-UVO. All values tor these
studies are approximate and are estimated from the authors' graphs. Note that these particular studies have been selected as examples because the majority are systematic studies, that is, measurements made on a regular basis so that "time-to-compensation"
can be estimated.
The Intrinsic Mechanism Hypothesis
187
Table 2. Examples of Studies of Mammalian Species That Demonstrate Recovery
of Resting Activity in the Ipsilateral VNC
Author(s)
Hamann and Lannou (14)
Smith and Curthoys (1 5)
Newlands and Perachio (8)
Precht et al. (16)
Ried et al. (17)
Pompeiano et al. (1 8)
Lacour et al. (i 9)
Zennou-Azogui et al. (20)
Waespe et al. (21)
Species
Preparation
Subnucleus
rat
guinea pig
gerbil
cat
cat
cat
cat
cat
monkey
anesthetized
anesthetized
decerebrate
decerebrate
anesthetized
decerebrate
alert
alert
alert
MVN
MVN
MVN
MVN
MVN
LVN
LVN
LVN
MVN
MVN medial vestibular nucleus. LVN lateral vestibular nucleus.
For the purposes of our hypothesis, which
specifically concerns static compensation in
mammals, we propose that the dynamic aspects of vestibular compensation are largely
dependent on the recovery of resting activity
in the ipsilateral VNC and, in addition, the
substitution of other sensory inputs for the
missing labyrinthine input (see 2-5,23 for reviews). Hence, the intrinsic mechanism hypothesis in not a mono-causal hypothesis of
vestibular compensation: it proposes a specific
mechanism for one aspect of vestibular compensation, but fully acknowledges that other,
more complex mechanisms, involving other
parts of the CNS, are responsible for dynamic
compensation. We acknowledge that much of
our hypothesis is speculative; however, we
suggest that whether or not it is entirely correct, it is completely testable. As Robinson
(24, p. 519) said in discussing his early models of oculomotor function, "These hypothetical schemes attempt to anticipate what must
eventually be discovered by ... experimentation and are offered here
the
Drovoking debate and further investigation.''
Our hypothesis is based on a number of assumptions regarding vestibular compensation,
which we will address in turn. We emphasize
that the following is not intended to be an
exhaustive review of the literature (for literature reviews on vestibular compensation in
mammals, see 1-5, 25-28); we cite papers only
according to their direct relevance to the hypothesis we are describing.
Assumptions of the "Intrinsic
Mechanism Hypothesis"
Assumption 1: The stimulus for
vestibular compensation is the
inactivation of the vestibular
afferents, not their degeneration.
A comprehensive theory of any form of
plasticity must address the question of what,
precisely, is the stimulus for the adaptive or
maladaptive neural change in question. In the
case of vestibular compensation, there is considerable evidence that surgical removal of the
vestibular receptor cells (unilateral labyrinthectomy, UL) and surgical transsection of the
vestibular nerve result in similar behavioral
symptoms, a similar asymmetry in neuronal
activity between the bilateral vestibular nucleus complexes (VNC), and similar patterns
of vestibular compensation (for example,
compare 6,15,29,30). However, it is clear that
the vestibular nerve degenerates much more
tion than following a UL
example 6,30).
This suggests that it is the inactivation of the
vestibular nerve, or some event associated \vith
it, that is the stimulus for vestibular compensation, not the structural degeneration of the
vestibular nerve itself. This hypothesis has not
been tested directly in mammalian species;
however, it has been tested in frogs. Kunkel
and Dieringer (31) reported that the electrophysiological changes that occur in the frog
188
VNC following UVD are similar following preor post-ganglionic vestibular nerve transsection (see also 32). In the cochlear nucleus,
blockade of VIIIth nerve activity by tetrodotoxin produces a rapid glial reaction in the absence of degeneration, suggesting that the
cessation of presynaptic activity may be a sufficient stimulus for the activation of "recovery" processes (33). That inactivation of the
vestibular nerve is the stimulus for vestibular
compensation is also supported by the observation that
celeraceci
the vestibular nerve ipsilateral to the labyrinthectomy (34).
Assumption 2: Vestibular compensation
is not due to any form of recovery in
the peripheral vestibular system.
Although evidence has been reported recently that demonstrates regeneration of
vestibular receptor hair cells following aminoglycoside toxicity (35-38), there is no evidence
to suggest that vestibular receptor cells can regenerate following surgical UL or vestibular
nerve transsection (for example, 6,39,40). Only
a few studies have examined the function of
neurons in Scarpa's ganglion following UVD:
all of these studies have shown that the number of neurons with remaining resting activity is very small and that the discharge of these
few neurons is erratic and of low frequency
(6, 15,29).
Taken together, these studies suggest that
vestibular compensation is not due to any
form of recovery in the peripheral vestibular
labyrinth.
Assumption 3: Static compensation is
correlated with a recovery of resting ·
activity in ipsilateral vestibular nucleus
complex (VNC) neurons.
There is little question that vestibular compensation of static ocular motor symptoms
such as SN is correlated with a recovery of
resting activity in type I neurons of the ipsilat-
C. L. Darlington and P. F. Smith
eral medial vestibular nucleus (MVN). What
is debatable is the extent of the recovery,
which seems to vary in different studies according to the type of preparation used (see
41, 42 for discussion of this point; Table 2).
Recently, Waespe et al. (21) have demonstrated, in the alert monkey, that a substantial degree of resting activity has recovered in
type I MVN neurons at 1 month following a
bilateral vestibular neurectomy. This result
demonstrates quite dearly that the recovery of
lYfVN neurons cannot be attributed to the anesthetic
used to record them, or to the use of decerebration or spinal transsection. Furthermore,
because the neurectomy was bilateral, therecovery of type I resting activity cannot be attributed to the contralateral MVN or to the
vestibular commissures (for example, 15,43,
44). Lacour et al. (19) and Zennou-Azogui
et al. (20,45) have also reported a significant
recovery of resting activity in the ipsilateral
lateral vestibular nucleus (LVN) following
unilateral vestibular neurectomy in the alert
cat; however, Pompeiano and colleagues (eg
18) have reported a limited recovery of resting activity in small LVN neurons with cervical spinal projections. The recovery of resting
activity in the LVN may be more limited than
in the MVN, which may explain the slower
and less complete compensation of some static
postural symptoms (for example, roll head tilt
in guinea pigs; see 2 for a review).
The general consensus that static compensation is correlated with a recovery of resting
activity in the ipsilateral VNC is supported by
metabolic studies using 2-deoxyglucose (for
example, 46,47 ,48) and cytochrome oxidase
(49). However, these same studies demonstrate
changes in widespread areas of the CNS,
which may be related to the compensation of
persistent static postural symptoms and dynamic ocular motor and postural symptoms.
According to morphological studies, there
is little cell loss in the ipsilateral VNC following UVD (18,50-52). In the ipsilateral and
contralateral LVN following UVD, the presence of glial fibrillary acidic protein (GFAP)
has been reported, which may be related to
some form of structural reorganization in the
The Intrinsic Mechanism Hypothesis
189
LVN in addition to phagocytosis of primary
vestibular terminals (53).
Although the electrophysiological and metabolic studies described are correlational, it is
not unreasonable to suggest that the partial recovery of resting activity in neurons of the ipsilateral VNC may have a causal role in static
compensation, since lesions of the ipsilateral
VNC have been shown to prevent compensation or to cause a loss of compensation (decompensation) (54,55).
Assun1ption 4: Some aspects of
mam1nalian static compensation
(for example, compensation of
SN) are not dependent on reactive
synaptogenesis, denervation
supersensitivity, receptor
up-regulation, or increased
neurotransmitter release within
the ipsilateral VNC.
Since Spiegel and Demetriades (54), numerous researchers have entertained possible explanations for static compensation in terms
of changes within the ipsilateral VNC, for example, reactive synaptogenesis, denervation
supersensitivity, receptor up-regulation, or increased neurotransmitter release.
To date, none of these explanations can adequately account for static compensation in all
mammalian species (Table 3). Reactive synaptogenesis has often been suggested as a possible explanation for vestibular compensation;
although there is evidence to support its occurrence in frog (for example, 66,67), the evidence from lower mammalian species (for
example, 5 ,68) suggests that these changes
develop too slowly to be the primary cause
of the compensation of SN, which has been
shown to occur within 3 to 4 days, even in
darkness, in guinea pig and cat (68; Table 1).
It has been demonstrated in many studies
that vestibular compensation in frog is associated with an increase in the efficacy of excitatory brainstem commissural input to ipsilateral
VNC neurons (for example, 31 ,69, 70; see 2 for
a review). However, studies in mammalian
species have failed to find any corresponding
change in commissural efficacy (for example,
8,15,16,17,71,72) and, in any case, in mammals the vestibular commissures are part of a
functionally inhibitory system between horizontal canal-related 2nd-order MVN neurons
(for example, 73; see 44 for a discussion).
Table 3. Studies That Have Not Demonstrated Changes in the Ipsilateral VNC That
Can Account for the Recovery of Resting Activity Following UVD
Author(s)
Species
Cochran et al. (56)
frog
Knopfel and Dieringer (57)
frog
de Waele eta!. (58)
Raymond et al. (59)
Li et ai. (60)
Calza et a!. (61)
Smith and Curthoys (I 5)
de Waele et al. (62)
Smith and Darlington (63)
Darlington et al. (64)
Newlands and Perachio (8)
Precht et al. (16)
Reid et al. (17)
Korte and Friedrich (51)
Gacek et al. (52)
Thompson et al. (65)
rat
ral
Finding
no evidence for increased NMDA receptor mediation of commissural
input
no evidence for increased NMDA receptor mediation of commissural
input
no increase in NMDJ\ receptor mRI\I.t,
no increase 1r: giuta:1atE receptor~
~a;
rat
guinea pig
guinea pig
guinea pig
guinea pig
gerbil
cat
cat
cat
cat
monkey
no evidence for up-regulation of acetylcholine or GABA receptors
no increase in efficacy of commissural input to ipsilateral VNC
no evidence for increased NMD/\ receptor function in ipsilateral VNC
no increase in NMDA receptor sensitivity
no increase in ACTH-(4-1 0) receptor sensitivity
no increase in efficacy of commissural input to ipsilateral VNC
no increase in efficacy of commissural input to ipsilateral VNC
no increase in efficacy of commissural input to ipsilateral VNC
morphological changes slow
morphological changes slow
increased GABA levels in ipsilateral VNC, presumed to increase
inhibition
NMDA: N-methyl-o-asparate. "VNC ": vestibular nucleus complex. 'ACTH-( 4-1 0)': adrenocorticotrophic hormone, fragment 4-1 0.
190
We and others have suggested that static
compensation might be related to an increase
in the affinity, efficacy, or number of Nmethyl-n-aspartate (Nl\IIDA) receptors on the
ipsilateral VNC neurons (62, 74-78). However,
electrophysiological (57 ,63), pharmacological
(59), and biochemical studies (58) do not support an increase in the number or sensitivity
of NlVfDA receptors in the ipsilateral VNC.
Other studies of inhibitory amino acid and
acetylcholine receptors also do not suooort
,,:oanges
might be rei a ted w the recovery of resting activity (61).
Some studies have examined whether therelease of amino acid neurotransmitters changes
during vestibular compensation. Thompson
and colleagues (65) reported that GABA levels
increase in the ipsilateral LVN and decrease in
contralateral LVN at 3 to 6 days post-UVD.
Li and colleagues (60) have reported that glutamate concentrations gradually decrease in
the ipsilateral VNC following UVD and that
they do not recover to normal levels within
7 days post-UVD. However, Henley and
Igarashi (79) have reported that at 10 months
post-UVD, normal glutamate levels have been
re-established in the ipsilateral VNC of the
squirrel monkey. These studies suggest that,
at least with respect to amino acids, it is unlikely that increased neurotransmitter release
can explain the recovery of resting activity in
ipsilateral VNC neurons.
One possibility that has not been systematically investigated in mammals is that static
compensation may be due to a rapid alteration
of the intrinsic membrane properties of ipsilateral VNC neurons (22,80,81). Darlington
and colleagues, using extracellular recording
from 1\!IVN neurons in guinea pig brainstem
slices ipsilateral to a previous
have reported a trend toward higher resting discharge
rates compared to MVN neurons in slices from
labyrinthine-intact animals (22,63,64). However, to date, no intracellular studies of the
membrane properties of MVN neurons ipsilateral to a chronic UVD, have been conducted
(except in frog, where the resting potentials
and input resistances of ipsilateral VNC neu-
C. L. Darlington and P. F. Smith
rons were found to be similar to those in
labyrinthine-intact frogs (70)).
Although many lesion studies have been
conducted in the search for an explanation of
vestibular compensation (see 2 for a review),
one area of the CNS in which lesions have consistently been shown to disrupt compensation
is the inferior olive. Llinas and colleagues (82)
reported that inferior olive lesions in rat prevented compensation or caused decompensation of the static oc:1lar '11otor and postural
symptoms, a result that has been replicated
Azzena and colleagues
using guinea pig.
Other studies have shown that in labyrinthineintact rats, inactivation of the inferior olive by
chemical lesions or reversible cooling causes
a decrease (approximately 33 OJo) in the resting
activity of contralateral MVN neurons, which
recovers over time (84). It is interesting that
inferior olivary neurons are themselves endowed with numerous intrinsic membrane
properties, some of which allow them to maintain pacemaker activity in vitro (for example,
85). It is possible that, under normal circumstances, the contralateral inferior olive contributes to the resting activity of type I MVN
neurons and that, following UVD, synaptic input from inferior olivary neurons, driven by
their intrinsic properties, is used to "recalibrate" pacemaker activity in the deafferented
MVN.
Assumption 5: In the labyrinthineintact animal, the intrinsic properties
of VNC neurons contribute to their
resting activity_, along with synaptic
input.
The hypothesis that the recovery of resting
activity in the ipsilateral VNC during vestibular compensation is due to a "substitutive"
process that provides the missing resting activity (for example, reactive synaptogenesis or
denervation supersensitivity) is based on the
assumption that resting activity is reduced in
the ipsilateral VNC following UVD because
the ipsilateral vestibular nerve usually supplies
VNC neurons with all of their tonic excitation.
The Intrinsic Mechanism Hypothesis
However, this assumption is difficult to test
because it is difficult to obstruct vestibular
nerve input to the ipsilateral VNC without
producing a UVD and thereby activating the
changes that lead to vestibular compensation.
There are several lines of evidence that suggest that the vestibular nerve may not be solely
responsible for VNC neuron resting discharge.
First, Raymond and colleagues (68) have estimated that approximately 35!1Jo of immunoreactive synaptic
in the
JvfVI\f are due to vestibular nerve input, suggesting that, for many IVIVN neuronsj another
65% of synaptic inputs derive from other
sources. Second, Li and colleagues (60) have
reported that following UVD, the loss of glutamate across the various subnuclei of the
ipsilateral VNC is variable and, at 2 days postUVD, reaches a maximum of only about 21 OJo
in the ipsilateral MVN. Since there is convincing evidence that the transmitter used by the
vestibular nerve is glutamate (see 86,87 for reviews), these results are also consistent with
the view that the vestibular nerve is only partially responsible for the resting activity of
VNC neurons and that a substantial amount
of glutamatergic excitation may derive from
other sources. Third, many LVN neurons do
not show large decreases in resting activity following UVD, suggesting that they do not rely
on the vestibular nerve for the majority of
their resting activity (for example, 18). Fourth,
a large number of in vitro brainstem slice studies have shown that the resting discharge of
MVN neurons persists in brainstem slices
maintained in vitro, in the absence of
from the vestibular nerves and mos1 other
Sf; fer·
191
88,124 for reviews). However, at present,
there is no evidence that such intrinsic membrane properties contribute to the resting activity of VNC neurons in vivo; this will be an
important area of investigation for future
studies. It is unlikely that intrinsic properties
would account for all, or even most, of the
resting activity that is observed in VNC neurons in vivo 0 25,
there will be an in1portant contribution made·
the: vestibular nerve
ex~·L.,_,.,~ . inferior
ne:urons;
, including other VNC neurons. Hovvever, the in vitro
data suggest that intrinsic properties n1ay,
nonetheless, make a contribution to VNC neuron resting activity.
Assumption 5 may offer a partial solution
to a persistent problem that has confronted
modellers of oculomotor function. In order to
obtain an eye position signal from a head velocity signal, the head velocity signal must be
mathematically integrated. If, however, the
head velocity signal (that is, vestibular nerve
input) is superimposed upon a background
signal (that is, resting activity also supplied by
the vestibular nerve), then both signals would
be integrated, resulting in errors of eye position (see 127, 128). If, however, the resting activity of VNC neurons were provided partially
by intrinsic membrane properties that are independent of synaptic input, this problem
would be partially overcome because the major function of the vestibular nerve would be
to deliver head movement infonnation that
modulates this resting
2
or in the presence of
81,1 3, 4;see88fo:·a
neurons demonstrate
In vitro
studies suggest that a persistent
conductance may be at least partly responsible for this
resting activity (1 02,113, 114; Table 4; see also
122, 123). This evidence strongly suggests that
VNC neurons have the capacity, at least in
vitro, to generate resting activity as a result of
their intrinsic membrane properties (see 22,81,
1\1any vestibular compensation studies have
failed to find neurotransmitter, receptor, or
other changes within the ipsilateral VNC that
account for the recovery of resting activity (see
Table 3). We propose that this is because the
C. L. Darlington and P. F. Smith
192
Table 4. Studies That Have Demonstrated Resting Activity in the Mammalian VNC In Vitro
Species
Subnucleus
Recording
rat
rat
rat
rat
rat
rat
guinea pig
rat
guinea pig
rat
explant incl. VNC
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
extracellular
extracellular
extra/intracellular
intracellular
extra/intracellular
extracellular
extracellular
intracellular
intracellular
extracellular
'ntracellular
extracellular
extracellular
intracellular
intracellular
extracellular
intracellular
extra/intracellular
intracellular
extracellular (FP)
extracellular
extracellular
intracellular
extracellular
extracellular
intracellular
extra/intracellular
extra/intracellular
extracellular
patch clamp
patch clamp
intracellular
intracellular
extracellular
extracellular
extracellular
intracellular
extracellular
Author(s)
Fukuda and Loeschcke (89)
Kobayashi and Murakami (90)
Gallagher et al. (9 i)
Lewis et al. (92)
Ujihara et al. (93)
Ujihara et al. (94)
Darlington et al. (22)
Lewis et al. (95)
Serafin et al. (96)
Doi et al. (97)
Phelan et 81. 1·~8\
Smith et al. 199)
Darlington et al. ( 1 00)
Serafin et al. ( 1 0 1 )
Serafin et al. ( 1 02)
Smith et al. (1 03)
Phelan and Gallagher (1 04)
Carpenter and Hori (1 05)
Gallagher et al. (1 06)
Capocchi et al. (1 07)
Smith and Darlington (63)
Dutia et al. (80)
Serafin et al. (1 08)
Darlington et al. (64)
Johnston et al. (1 09)
Serafin et al. (11 0)
de Waele et al. ( 1 11 )
Lin and Carpenter (81 )
Lin and Carpenter (112)
Kinney et al. (113)
Takahashi et al. (114)
Johnston et al. (115)
Vibert et al. (116)
Hutchinson et al. (117)
Hutchinson et al. ( 11 8)
Darlington and Smith (119)
Vibert et al. (120)
Lapeyre and De Waele ( 1 21 )
~
,-.. .!.
:;u1nea p1g
guinea pig
guinea pig
guinea pig
guinea pig
rat
rat
rat
rat
guinea pig
rat
guinea pig
guinea pig
rat
guinea pig
guinea pig
rat
rat
rat
rat
rat
guinea pig
guinea pig
guinea pig
guinea pig
guinea pig
guinea pig
~;iVl\1
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVn
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
MVN
Abbreviations as in previous tables. FP: field potential recording.
resting activity that reappears during vestibular
c01npensation was never completely dependent
upon vestibular nerve input in the first place.
It was partially due to intrinsic membrane
properties that also contribute to VNC neuron
resting activity in the labyrinthine-intact animal. At present, the best evidence in support
of this assumption is that when the MVN ipsilateral to the UVD is removed from a compensated animal and maintained in vitro,
resting activity recovers within a few hours in
many MVN neurons, even in the presence of
synaptic blockade within the slice (22,63 ,64).
A number of in vivo electrophysiological stud-
ies have provided evidence that is consistent
with these in vitro results: the recovery of resting activity in VNC neurons ipsilateral to a
UVD has been found to persist following transsection ofbrainstem and cerebellar commissural inputs (for example, 15,16), decerebration
(for example, 8, 16,18, 129) or spinal transsection (42,130), although the amount of resting
activity remaining may be reduced in some
cases (for example, spinal transsection; 42,130).
We do not exclude the possibility that intrinsic membrane properties (for example, persistent Na + conductances) in ipsilateral VNC
neurons are in some way up-regulated during
193
The Intrinsic Mechanism Hypothesis
vestibular compensation in order to compensate for the loss of the contribution that
vestibular nerve input makes to VNC neuron
resting activity (for example, 22, 115). However, our hypothesis places the main responsibility for the recovery of resting activity in
ipsilateral VNC neurons on intrinsic properties
that are already present in the normal VNC.
If the resting activity that returns during
vestibular compensation is provided by intrinsic
properties that are present under normaL.
labyrinthine-intact circumstances, why does it
disappear immediately following UVD? One
very likely possibility is "neural shock" or
"diaschisis" (see 131 for a review). It is well
known that following deafferentation due to
physical trauma or hypoxia/ischemia, neurons
at the center of the damage (the so-called
"core") die quickly, whereas those that received synaptic input from the neurons in
the core (the "penumbral region") undergo
secondary pathological changes, sometimes referred to as the "secondary injury" phenomenon (see 131 for a review). In many cases, a
reduction in electrical and metabolic activity
and, sometimes, cell death in the penumbra is
due to excitotoxicity caused by an increased
release of glutamate from dying neurons in the
core. At first, the increased glutamate release
causes injury discharges, but gradually intracellular calcium increases as N-methyl-Daspartate (NMDA) receptor /channels and
voltage-dependent calcium channels are opened
(for example, 132; see 133 for a review).
Recent high-performance liquid chromatography (HPLC) studies by Lj et al. (60) demonstrate that the levels of glutamate within the
ipsilateral VNC do not decrease
'"'·'·n
even
the reduction in glutamate concentrations
in the ipsilateral I'v1VN is only about 12 to 21 GJo.
This means that high glutamate concentrations
remain in the ipsilateral VNC following
possibly without normally functioning presynaptic mechanisms for metabolising the glutamate once it is released. This may create a
situation in which VNC neurons are overstimulated by glutamate released by the dying vestibular nerve; those VNC neurons that do not
received direct input from the vestibular nerve
i.ULAU._, ........
may receive increased glutamatergic input from
other VNC neurons.
Since the original brainstem slice studies in
mammalian species (91; see 88 for a review),
researchers have been surprised by the degree
of resting activity present in the deafferented
VNC and how quickly this resting activity recovers following a brief incubation period
(that is, 1 to 2 h) in vitro. Why is it that the
resting activity of MVN neurons in vivo gradually recovers over 2 to 3 days following
whereas the resting activity of I'vfVN neurons
in brainstem slices n1aintained in vitro can recover following 1 to 2 h of incubation in artificial cerebrospinal fluid (ACSF)? One possible
explanation is that, in the latter case, superfusion with ACSF washes out the glutamate released by the vestibular nerve following UVD,
thus short-circuiting the diaschisis that normally follows the deafferentation.
Assumption 7: Peripheral vestibular
deafferentation causes biochemical
changes in the ipsilateral VNC
that are consistent with
diaschisis, especially those
relating to calcium.
We propose that following UVD, the glutamate concentrations within the ipsilateral
VNC, which are sustained during the first
24 hours, result in a form of calcium-induced
diaschisis: glutamate overstimulates AMPA/
kainate and NMDA receptors on VNC neurons,
resulting in increased calcium influx, leading
to increased
and perhaps the
calcimn chanrurtner
nol"\f'rlf"c>Al"li·
glutamate into the
There is no direct evidence to support this
assumption, although there are now a great
many findings that are consistent with it. The
induction of immediate early genes (lEGs) has
been demonstrated to be a marker for cell
damage in the penumbral regions of a stroke
or a surgical lesion (132; see 134 for a review).
194
The lEG protein, fos, in particular, is induced
in many cases of neural damage, and its induction is often correlated with increased intracellular calcium concentrations ( 132; see 134,
135 for reviews). Kaufman and colleagues
(136) reported the induction of c fos in the bilateral l\IIVN at 24 h
a chemical
UVD in rats. Elsewhere
t'os induction was transient and
and Perachio
c fos and zif/268
a lesser
are induced by anodal stimulation of the vestibular
nerve.
At present, there is no direct evidence to
support the assun1ption that UVD or anodal
stimulation of the vestibular nerve results in
an increased calcium influx in the ipsilateral
VNC. However, it has recently been reported
that depolarization of the vestibular nerve
causes an increased calcium influx in ipsilateral
MVN neurons, measured using rhod 2 fluorescence (140). This increased calcium influx
could be blocked by an NMDA receptor antagonist or reduced by the L-type calcium
channel antagonist, nifedipine. If UVD causes
injury discharges in the vestibular nerve at the
time of the deafferentation, then this might result in increased calcium influx in ipsilateral
VNC neurons. One result that is consistent
with the possibility of injury discharge is that
administration of procaine to the round window prior to UVD results in a reduction in the
severity of UVD
) . The VHUJH.,.u.-~.<.A'V•U
that the
·;es:ibular
disat the time of the UVD and reducing
calcium inf1ux 1n ipsilateral VI'IC neurons
see also 132).
To date, the available protein phosphorylation studies also support calcium-related
changes in the VNC following UVD. Flohr
and colleagues have found a number of phosphorylation changes in whole brain homogenates from different stages of vestibular
C. L. Darlington and P. F. Smith
compensation in the frog; some of the protein
substrates are phosphorylated by calciumcalmodulin-dependent protein kinases (142),
another appeared to be immunologically similar to the GAP-43/B-50 protein, which is
phosphorylated
the calcium-diacylglycerolactivated
see also
One
is why cell death
VNC in the
presence of increased intracellular calcium. It
has :Jeen demonstrated ~hat some populations
aeurons the increase in intracellular calcium
is reversible
; see 146 for a review). Ourthe development of hindbrain ischemia,
the lVIVN was found to be one of the most resistant brainstem areas to cell death (145). One
possible explanation for the survival of VNC
neurons is the availability of calcium-binding
proteins within the cytoplasm of the neurons,
which can bind and therefore inactivate free
calcium ions. It has been reported that neurons that are immunoreactive for the calciumbinding protein, calretinin, are resistant to the
neurotoxicity induced by $-amyloid protein,
w~ich is presumably calcium related (147). A
recent study by Sans and colleagues (148) has
demonstrated the presence of mRNA for calretinin in the VNC and that following UVD,
the concentration of calretinin mRNA does
not decrease in the ipsilateral VNC during the
first 3 days post-UVD (see also 149). It may
be that the return of resting activity to the
VNC following UVD is the recovery from
calcium-induced diaschisis, not simply a replacement of resting activity previously supplied by the vestibular nerve.
If the accumulation of intracellular calcium
is the cause of a
which accounts for
the loss of resting
in the ipsilateral
VNC immediately following UVD, then it
would be expected that drugs that reduce this
calcium influx would reduce the extent of the
diaschisis and therefore the severity of the
UVD symptoms. A number of behavioral
studies have demonstrated that a pre-UVD systemic injection of a voltage-sensitive calcium
channel antagonist or an NMDA receptor I
calcium channel antagonist can reduce the
The Intrinsic Mechanism Hypothesis
195
UVD syn1ptoms (see Table 5; see 159 for contrary evidence regarding flunarizine). In the
most compelling of these studies, a series of
injections of the calcium-dependent enzyme
inhibitor, calmidazolium chloride, into the ipsilateral VNC or IVth ventricle resulted in a
large reduction in the severity of the UVD
symptoms (154).
Assumption 8: The contribution of
intrinsic properties to the resting
activity of V_NC neurons enhances
sensitivity to dynan1ic vestibular
inputs in both the labyrinthine-intact
and compensated states.
Mathematical models of the vestibular
compensation process have indicated that it is
difficult, if not impossible, for the mechanism
that is responsible for the recovery of resting
activity in ipsilateral VNC neurons to also
bring about a recovery of the dynamic response of those neurons to head movement
(except insofar as recovery of resting activity
contributes to dynamic recovery) (compare 43
and 72,160,161). The "intrinsic mechanism"
hypothesis that we propose entails that the recovery of resting activity is partially independent of synaptic modulation of that resting
activity by remaining vestibular, visual, proprioceptive, or cutaneous afferent inputs.
Since "pacemaker" neurons are very sensitive
to synaptic modulation (see 81 for a discussion), the provision of resting activity by intrinsic properties would increase the sensitivity
of VNC neurons to synaptic inputs. This
would be especially important in the compensated animal where the only remaining vestibular input is that communicated via the
commissural fibers from the contralateral VNC.
Conclusions
The hypothesis described in this paper was
inspired both by the rapid progress made by
in vitro studies of the mammalian VNC (see
81,88,92 for reviews) and the slow progress
made in the attempt to identify the cause of
the return of resting activity to the ipsilateral
VNC following UVD (see 2, 25 for reviews).
We believe that the most salient characteristic of vestibular compensation is the reduction
in the severity of the static ocular motor and
postural symptoms following UVD, correlated
with a recovery of resting activity in the ipsilateral VNC, particularly type I neurons in the
MVN. The slower and less complete dynamic
compensation depends on this recovery of
symmetrical vestibular tone in order to modulate VNC neuron activity during head movement by signals from the remaining labyrinth
(via the vestibular commissures) and other sensory inputs (see 2,4 for reviews). The speed of
static compensation in lower mammals has al-
Table 5. Studies That Support the Hypothesis That Reducing Calcium
Influx Facilitates Vestibular Compensation
Author(s)
Tolu et al. (150)
Darlington and Smith (I 51 )
Sansom et al. (I 52)
Leinhos and Flohr (1 53)
Sansom et al. ( 1 54)
Sansom et al. (155)
Darlington et al. ( 1 56)
Jerram et al. (I 57)
Yamanaka et al. (9)
Maclennan et al. (158)
Specie;:
guinea
guinea
guinea
frog
guinea
guinea
guinea
guinea
rabbit
guinea
pig
pig
pig
pig
pig
pig
pig
pig
flunarizine
verapamil
MK-801
flunarizine
calmidazolium chloride
CGS 19755
MK-801
methylprednisolone
dexamethasone
ginkgolide B
blocks VSCC
blocks VSCC
blocks NMDA/CC (noncompetitive)
blocks VSCC
blocks calcium-dependent enzymes
blocks NMDA/CC (competitive)
blocks NMDA/CC (noncompetitive)
synthetic steroid- may block calcium influx
glucocorticoid may block calcium influx
platelet-activating factor antagonist that
reduces calcium influx
VSCC: voltage-sensitive calcium channels. NMDA/CC: N-methyl-o-aspartate receptor-mediated calcium channels. competitive: competitive antagonist. noncompetitive: noncompetitive antagonist.
C. L. Darlington and P. F. Smith
196
ways suggested that reactive synaptogenesis is
an unlikely explanation with respect to those
species, because it is too slow. Other, more
rapid mechanisms, such as denervation supersensitivity, receptor-up-regulation or increased
neurotransmitter release, were reasonable possibilities. However, to date, each study that
has addressed these possibilities has failed to
find any change that could account for therecovery of l\IIVN resting activity that correlates
with static compensation. The search for such
''substitutive" :nechanisms was based 0n 1.he
VNC
neurons themselves would have to "replace))
the missing resting activity, which the ipsilateral vestibular nerve normally provides. However, this hypothesis has been challenged by
the wealth of in vitro brainstem slice studies
that demonstrate that, at least in vitro, MVN
neurons do not need the vestibular nerve in order to generate resting activity. On the basis
of these considerations, we suggest that the
most parsimonious explanation of static compensation in mammals is that, following a
brief diaschisis, VNC neurons ipsilateral to the
UVD reactivate the intrinsic membrane properties that normally contribute to their resting
activity in vivo. His possible that such properties are in some way up-regulated
examS ) ; ho\'v ever, this is not a necessary
of our
Acknowledgment- This research was supported by
a Project Grant from the Health Research Council of New Zealand (to CD and PS).
REFERENCES
l. Schaefer KP, Meyer DL. Compensation of vestibu11. Igarashi M, Ishikawa K. Post-labyrinthectomy ballar lesions. In: Kornhuber HH, ed. Handbook of senance compensation with preplacement of cerebellar
sory physiology, vol. 6/2, Berlin: Springer; 1974:
vermis lesion. Acta Otolaryngol (Stockh). 1985;99:
463-90.
452-8.
2. Smith PF, Curthoys IS. Mechanisms of recovery fol12. Fetter M, Zee DS, Proctor LR. Effect of lack of vilowing unilateral labyrinthectomy: a review. Brain Res
sion and of occipital lobectomy upon recovery from
Brain Res Rev. 1989;14:155-80.
unilateral labyrinthectomy in rhesus monkey. J Neuro3. Lacour M, Toupet M, Denise P, Christen Y, eds. Yes- '
physiol. 1988;59:394-407.
tibular compensation: facts, theories and clinical per13. Fetter M, Dichgans J. Adaptive mechanisms of VOR
compensation after unilateral peripheral vestibular lespectives. Paris: Elsevier; 1989.
4. Curthoys IS, Halmagyi GM. Behavioural and neusions in humans. J Vestib Res. 1990;1:9-22.
ral correlates of vestibular compensation. Baillieres
14. Hamann KF, Lannou J. Dynamic characteristics of
Clin Neurol. 19~2; 1 (2):345-72.
vestibular nuclear neurons responses to vestibular and
5. Dieringer N. 'Vestibular compensation': neural plasoptokinetic stimulation during vestibular compensaticity and its relations to functional recovery after labtion in the rat. Acta Otolaryngol (Stockh). 1988;Suppl
yrinthine lesions in frogs and other vertebrates. Prog
455:1-19.
Neurobiol. 1995;46:97-129.
15. Smith PF, Curthoys IS. Neuronal activity in the ipsi6. Sirkin DW, Precht W, Courjon JH. Initial, rapid
lateral medial vestibular nucleus of the guinea pig folphase of recovery from unilateral vestibular lesion in
lowing unilateral labyrinthectomy. Brain Res. 1988;
rat not dependent on survival of central portion of
44:308-19.
vestibular nerve. Brain Res. 1984;302:245-56.
16. Precht W, Shimazu H, Markham CH. A mechanism
7. Smith PF, Darlington CL, Cunhoys IS. The effect
of central compensation of vestibular function followof visual deprivation on vestibular compensation in
ing hemilabyrinthectomy. J Neurophysiol. 1966;29:
the guinea pig. Brain Res. !986;364:195-8.
996-1010.
8. Newlands SD, Perachio /\A. Compensation (Jf horil7. Ried S, Maioli C, Precht W. Vestibular nuclear neuzontal canal related activity in the medial vestibular
ronal activity in chronically labyrinthectomized cats.
nucleus following unilateral labyrinth ablation in the
Acta Otolaryngol (Stockh). 1984;98: 1-13.
decerebrate gerbil; 1: Type neurons. Exp Brain Res.
18. Pompeiano 0, Xerri C, Gianni S, Manzoni D. Cen1990;82:359-72.
tral compensation of vestibular deficits; 2: Influences
9. Yamanaka T, Sasa M, Amano T, Miyahara H, Matof roll tilt on different size lateral vestibular neurons
sunaga T. Role of glucocorticoid in vestibular comafter ipsilateral labyrinth deafferentation. J Neuropensation in relation to activation of vestibular
physiol. 1984;52: 18-38.
nucleus neurons. Acta Otolaryngol (Stockh). 1995;
19. Lacour M, Ez-Zaher L, Raymond J. Plasticity mechSuppl 519:168-172.
anisms in vestibular compensation in the cat are im10. Haddad GM, Friendlich AR, Robinson DA. Comproved by an extract of Ginkgo bi!oba (EGb 761).
pensation of nystagmus after Vlllth nerve lesions in
Pharmacal Biochem Behav. 1991;40:367-79.
20. Zennou-Azogui Y, BorelL, Lacour M, Ez-Zaher L,
vestibulo-cerebellectomized cats. Brain Res. 1977; 135:
192-6.
Ouaknine M. Recovery of head postural control fol-
The Intrinsic Mechanism Hypothesis
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
!owing unilateral vestibular neuroectomy in the cat.
Acta Otolaryngol (Stockh). 1993;Suppl 509:1-19.
Waespe W, Schwarz U, Wolfensberger M. Firing characteristics of vestibular nuclei neurons in the alert
monkey after bilateral vestibular neurectomy. Exp
Brain Res. 1992;89:311-22.
Darlington CL, Smith PF, Hubbard JI. Neuronal activity in the guinea pig medial vestibular nucleus in
vitro following chronic unilateral labyrinthectomy.
Neurosci Lett. 1989;105:143-8.
Berthoz A. The role of gaze in compensation of vestibular dysfunction: the gaze substitution hypothesis.
In: Pompeiano 0, Allum JHJ, eds. Progress in brain
research, vol. 76, Amsterdam: Elsevier; 1988:4-20.
Robinson DA. Models of oculomotor neural organization. ln: Bach-y-Rita P, Colins, CC, eds. Control of eye movements. New York: Academic Press;
1971:519-35.
Smith PF, Darlington CL. Neurochemical mechanisms of recovery from peripheral vestibular lesions
(vestibular compensation). Brain Res Brain Res Rev.
1991;16:117-33.
Darlington CL, Flohr H, Smith PF. Molecular mechanisms of brainstem plasticity. the vestibular compensation model. Mol Neurobiol. 1991;5:355-68.
Smith PF, de Waele C, Vidal PP, Darlington CL. Excitatory amino acid receptors in normal and abnormal
vestibular function. Mol Neurobiol. 1991;5:369-87.
Smith PF, Darlington CL. Can vestibular compensation be enhanced by drug treatment? A review of
recent evidence. J Vestib Res. 1994;4:169-79.
Jensen DW. Survival of function in the deafferented
vestibular nerve. Brain Res. 1983;273: 175-8.
Cass SP, Goshgarian HG. Vestibular compensation
after labyrinthectomy and vestibular neurectomy in
cats. Otolaryngol Head Neck Surg. 1991;104:14-9.
Kunkel AW, Dieringer N. Morphological and electrophysiological consequences of unilateral pre- versus
post-ganglionic vestibular lesions in the frog. J Comp
Physiol A. 1994;171:621-32.
Straka H, Dieringer N. Spinal plasticity after hemilabyrinthectomy and its relation to postural recovery
in the frog. J Neurophysiol. 1995;73: 1617-31.
Canady KS, Rubel EW. Rapid and reversible astrocytic reaction to afferent activity blockade in chick
cochlear nucleus. J Neurosci. 1992;12:1001-9.
Matsumitsu Y, Sekitani T. Effect of electric stimulation on vestibular compensation in guinea pigs. Acta
Otolaryngol (Stockh). 1991;111:807-12.
Forge A, Li L, Corwin JT, Nevill G. Ultrastructural
evidence for hair cell regeneration in the mammalian
inner ear. Science. 1993;259:1616-19.
Warchol ML Lambert PR, Goldstein BJ, Forge A,
Corwin JT. Regenerative proliferation in inner ear
sensory epithelia from adult guinea pigs and humans.
Science. 1993;259:1619-22.
Lambert PR. Inner ear hair cell regeneration in a
mammal: identification of a triggering factor. Laryngoscope. 1994;104:701-18.
Rubel EW, Dew LA, Roberson DW. Mammalian vestibular hair cell regeneration. Science. 1995 ;267:
701-3.
Igarashi M, Watanabe T, Maxian PS. Dynamic equilibrium in squirrel monkeys after unilateral and bilateral labyrinthectomy. Acta Otolaryngol (Stock h).
1970;69:24 7-53.
197
40. Favre, D, Sans A. Dedifferentiation phenomena after denervation of mammalian adult vestibular receptors. NeuroReport. 1991;2:501-4.
41. Smith PF, Curthoys IS. Comments to: S.D. Newlands
and A. A. Perachio: Neuronal activity in the medial
vestibular nuclei following unilateral labyrinthectomy.
Exp Brain Res. 1991;86:679-82.
42. Newlands SD, Perachio AA. Effect of T2 spinal transection on compensation of horizontal canal related
activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil.
Brain Res. 1991 ;541: 129-33.
43. Galiana HL, Flohr H, Melvill Jones G. A reevaluation of intervestibular nuclear coupling: its role
in vestibular compensation. J Neurophysiol. 1984;51:
258-75.
44. Curthoys IS, Harris RA, Smith PF. The effect of unilateral labyrinthectomy on neural activity in the guinea
pig vestibular nuclei. In: Graham MD, Kemink JL,
eds. The vestibular system: neurophysiologic and clinical research. New York: Raven Press; 1987:677-87.
45. Zennou-Azogui Y, Xerri C, Harlay F. Visual sensory
substitution in vestibular compensation: neuronal
substrates in the alert cat. Exp Brain Res. 1994;98:
457-73.
46. Llinas R, Walton K. Vestibular compensation: a distributed property of the central nervous system. In:
Asanuma H, Wilson VJ, eds. Integration in the nervous system. Tokyo: Igaku-Shon; 1979:145-66.
47. Flohr H, Bienhold H, Abeln W, Macskovics I. Concepts of vestibular compensation. In: Flohr H, Precht
W, eds. Lesion-induced neuronal plasticity in sensorimotor systems. Amsterdam: Springer; 1981:153-72.
48. Luyten WHML, Sharp FR, Ryan AF. Regional differences of brain glucose metabolic compensation
after unilateral labyrinthectomy in rats: a[14C]2-deoxyglucose study. Brain Res. 1986;373:68-80.
49. Kevetter GA, Perachio AA. Cytochrome oxidase histochemistry in Scarpa's ganglion after hemilabyrinthectomy. Neurosci Lett. 1994;175:141-4.
50. Schwarz DWF, Schwarz IE, Frederickson JM. Fine
structure of the medial and descending vestibular nuclei in normal rats after unilateral transsection of the
vestibular nerve. Acta Otolaryngol (Stockh). 1977;
84:80-90.
51. Korte GE, Friedrich VL. The fine structure of the feline superior vestibular nucleus: identification and
synaptology of the primary vestibular afferents. Brain
Res. 1979;176:3-32.
52. Gacek RR, Lyon MJ, Schoonmaker .J. Ultrastructural
changes in vestibula-ocular neurons following vestib-·
ular neurectomy in the cat. Anr Otol Rhino] ~.aryn
gol. 1988 ;97 :42-51.
53. Cass SP, Goshgarian HG. Increased glial fibrillar)'
acidic protein immunoreactivity in astrocytes within
the lateral vestibular nucleus of the cat following labyrinthectomy and vestibular neurectomy. Ann Otol
Rhino! Laryngol. 1990;99:221-7.
54. Spiegel EA, Demetriades TD. Die zentrale compensation des labyrinthverlustes. Pflug Arch Ges Physiol. 1925;210:215-22.
55. Uemura T, Cohen B. Vestibula-ocular reflexes: effects
of vestibular nuclear lesions. In: Broda! A, Pompeiano 0, eds. Basic aspects of central vestibular
mechanisms. Progress in Brain Research; vol. 37. Amsterdam: Elsevier; 1972:515-28.
C. L. Darlington and P. F. Smith
198
56. Cochran SL, Kasik P, Precht W. Pharmacological aspects of excitatory synaptic transmission to second
order vestibular neurons in the frog. Synapse. 1987; 1:
102-23.
57. Knopfel T, Dieringer N. Lesion-induced vestibular
plasticity in the frog: are N-methyl-D-aspartate receptors involved? Exp Brain Res. 1988;72: 129-34.
58. de Waele C, Abitbol M, Chat M, Menini, C, Mallet
J, Vidal PP. Distribution of glutamatergic receptors
and GAD mRNA-containing neurons in the vestibular nuclei of normal and hemilabyrinthectomized rats.
Eur J Neurosci. 1994;6:565-76.
59. Raymond J, Touati J, Dememes D. Changes in the
glutamate binding sites in the rat vestibular nuclei fol~o\ving
!989; :.:5 us.
60. Li H, Godfrey TG, Rubin AM. Changes of amino
acid distributions in rat vestibular complex after unilateral removal of Scarpa's ganglion. Soc Neurosci
Abstr. 1993; 19:136.
61. Calza L, Giardino L, Zanni M, Galetti R, Parchi P,
Galetti G. Involvement of cholinergic and GABAergic systems in vestibular compensation. In: Lacour
M, Toupet M, Denise P, Christen Y, eds. Vestibular
compensation: facts, theories and clinical perspectives. Paris: Elsevier; 1989:189-99.
62. de Waele C, Vibert N, Baudrimont M, Vidal PP.
NMDA receptors contribute to the resting discharge
of vestibular neurons in the normal and hemilabyrinthectomized guinea pig. Exp Brain Res. 1990; 81:
125-33.
63. Smith PF, Darlington CL. Comparison of the effects
of NMDA antagonists on medial vestibular nucleus
neurons in brainstem slices from labyrinthine-intact
and chronically labyrinthectornized guinea pigs. Brain
Res. 1992;590:345-9.
64. Darlington CL, Smith PF, Gilchrist DPD. Comparison of the effects of ACTH-(4-10) on medial vestibular nucleus neurons in brainstem slices from
labyrinthine-intact and compensated guinea pigs.
Neurosci Lett. 1992;145:97-9.
65. Thompson GC, Igarashi M, Cortez AM. GABA
imbalance in squirrel monkey after unilateral vestibular end-organ ablation. Brain Res. 1986;370:
182-5.
66. Dieringer N, Kunzie H, Precht W. Increased projection of ascending dorsal root fibers to vestibular nuclei after hemilabyrinthectomy in the frog. Exp Brain
Res. 1984;55:574-8.
67. Will U, Kortmann H, Flohr H. HRP study on structural changes in the commissural fibre system of Rana
temporaria following labyrinthectomy. In: Flohr H,
ed. Post-lesion neural plasticity. Berlin: Springer;
1988:345-56.
68. Raymond J, Ez-Zaher L, Dememes D, Lacour M.
Quantification of synaptic density changes in the
medial vestibular nucleus of the cat following vestibular neurectomy. Rest Neurol Neurosci. 1991;3:197203.
69. Dieringer N, Precht W. Modification of synaptic input
following unilateral labyrinthectomy. Nature. 1977;
269:431-3.
70. Dieringer N, Precht W. Mechanism of compensation
for vestibular deficits in the frog. 1: Modification of
the excitatory commissural system. Exp Brain Res.
1979;36:311-28.
71. Smith PF, Darlington CL, Curthoys IS. Vestibular
compensation without brainstem commissures in the
guinea pig. Neurosci Lett. 1986;65:209-13.
72. Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular
nucleus following unilateral labyrinth ablation in the
decerebrate gerbil; 2: Type II neurons. Exp Brain Res.
1990;82:373-83.
73. Shimazu H, Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its
mediating pathway. J Neurophysiol. 1966;29:467-92.
74. Smith PF, Darlington CL. The NMDA receptor antagonists MK-801 and CPP disrupt compensation for
unilateral labyrinthectomy in the guinea pig. Neurosci
;_c:tt. 1988;94:J09-l3.
Darlington CL, Smith PF. NMDA receptor antagonists disrupt the development of vestibular compensation in the guinea pig. Eur J Pharmacal. 1989; 174:
273-8.
76. Sansom AJ, Darlington CL, Smith PF. Intraventricular administration of an N-methyl-D-aspartate receptor antagonist disrupts vestibular compensation.
Neur.opharmacol. 1990;29:83-4.
77. Pettorossi VE, Della Torre G, Grassi S, Zampolini
M, Capocchi G, Errico P. Role of NMDA receptors
in the compensation of ocular nystagmus induced by
hemilabyrinthectomy in the guinea pig. Arch !tal Bioi.
1992;130:303-13.
78. Flohr H, Luneburg U. Role of NMDA receptors in
lesion-induced plasticity. Arch Ita! Bioi. 1993;131:
173-90.
79. Henley CM, Igarashi M. Amino acid assay of vestibular nuclei 10 months after unilateral labyrinthectomy in squirrel monkeys. Acta Otolaryngol (Stockh)
1991;Suppl. 481:407-10.
80. Dutia MB, Johnston AR, McQueen DS. Tonic activity of rat medial vestibular nucleus neurones in vitro
and its inhibition by GABA. Exp Brain Res. 1992;88:
466~72.
81. Lin Y, Carpenter DO. Medial vestibular neurons are
endogenous pacemakers whose discharge is modulated by neurotransmitters. Cell Mol Neurobiol. 1993;
13:601-13.
82. Llinas R, Walton K, Hillman DE, Sotelo C. Inferior
olive: its role in motor learning. Science. 1975; 190:
1230-1.
83. Azzena GB, Tolu E, Mameli 0. The lateral reticular
nucleus: role in vestibular compensation. In: Flohr
H, Precht W, eds. Lesion-induced neuronal plasticity
in sensori-motor systems. Berlin: Springer; 1981:
254-64.
84. De'Sperati C, Montarolo PG, Strata P. Effects of inferior olive inactivation and lesion on the activity of
medial vestibular neurons in the rat. Neurosci. 1993;
53:139-47.
85. Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types
of voltage-dependent ionic conductances. J Physiol
(Lond). 1981;315:549-67.
86. Smith PF, Darlington CL. The pharmacology of the
vestibular system. In: Baloh R, ed. Baillieres Clin
Neurol Neurotol. 1994; 3(3) 467-84.
87. de Waele C, Muhlethaler M, Vidal PP. Neurochemistry of the central vestibular pathways. Brain Res
Rev. 1995;20:24-46.
88. Darlington CL, Gallagher JP, Smith PF. In vitro elec-
199
The Intrinsic Mechanism Hypothesis
89.
90.
91.
9'1
93.
94.
95.
96.
97.
98.
99.
100.
101.
102.
103.
104.
electrophysiological studies of the vestibular nucleus
complex. Prog Neurobiol. 1995;45:335-46.
Fukuda Y, Loeschcke HH. Effect of H+ on spontaneous neuronal activity in the surface layer of the
rat medulla oblongata. Pflugers Arch. 1977;371:
125-34.
Kobayashi S, Murakami N. Thermosensitive neurons in slice preparations of rat medulla oblongata.
Brain Res Bull. 1982;8:721-6.
Gallagher JP, Lewis MR, Shinnick-Gallagher P. An
electrophysiological investigation of the rat medial
vestibular nucleus in vitro. In: Correia MJ, Perachio
AA, eds. Contemporary sensory neurobiology. New
York: AR Liss; 1985:293-304.
Lewis MR, Gallagher JP, Shinnick-Gallagher P. An
in vitro brain slice preparation to study the pharmacology of central vestibular neurons. J Pharmacal
Meth. 1987;18:267-73.
Ujihara H, Akaike A, Sasa M, Takaori S. Electrophysiological evidence for cholinoceptive neurons
in the medial vestibular nucleus: studies on rat brainstem in vitro. Neurosci Lett. 1988;93:231-5.
Ujihara H, Akaike A, Sasa M, Takaori S. Muscarinic regulation of spontaneously active medial vestibular neurons in vitro. Neurosci Lett. 1989;106:
205-10.
Lewis MR, Phelan KD, Shinnick-Gallagher P, Gallagher JP. Primary afferent excitatory transmission
recorded intracellularly in vitro from rat medial vestibular neurons. Synapse. 1989;3:149-53.
Serafin M, Khateb A, de Waele C, Vidal PB, Muhlethaler M. Low threshold calcium spikes in medial
vestibular nuclei neurones in vitro: a role in the generation of the vestibular nystagmus quick phase in
vivo? Exp Brain Res. 1990;82: 187-90.
Doi K, Tsumoto T, Matsunaga T. Actions of excitatory amino acid antagonists on synaptic inputs to
the rat medial vestibular nucleus: an electrophysiological study in vitro. Exp Brain Res. 1990;82:
254-62.
Phelan KD, Nakamura J, Gallagher JP. Histamine
depolarizes rat medial vestibular nucleus neurons recorded intracellularly in vitro. Neurosci Lett. 1990;
109:287-92.
Smith PF, Darlington CL, Hubbard JI. Evidence
that NMDA receptors contribute to synaptic function in the guinea pig medial vestibular nucleus.
Brain Res. 1990;513:149-51.
Darlington CL, Smith PF, Hubbard .TI. Guinea pig
medial vestibular nucleus neuron~ in vitro respond
to ACTH-(4-10! at picomoia: concentratiom..
Brain Res. 1990;82:637-40.
Serafin M, de Waele C, I.;::hateb A, Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea
pig; 1: Intrinsic membrane properties in brainstem
slices. Exp Brain Res. 1991;84:417-25.
Serafin M, de Waele C, Khateb A, Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea
pig; 2: Ionic basis of the intrinsic membrane properties in brainstem slices. Exp Brain Res. 1991 ;84:
416-33.
Smith PF, Darlington CL, Hubbard Jl. Evidence for
inhibitory amino acid receptors on guinea pig medial vestibular nucleus neurons in vitro. Neurosci
Lett. 1991 ;121 :244-6.
Phelan KD, Gallagher JP. Direct muscarinic and nic-
105.
106.
107.
108.
109.
110.
111.
112.
113.
114.
115.
116.
otinic receptor-mediated excitation of rat medial vestibular nucleus neurons in vitro. Synapse. 1992; 10:
349-58.
Carpenter DO, Hori N. Neurotransmitter and peptide receptors on medial vestibular nucleus neurons.
Ann N Y Acad Sci. 1992;656:668-85.
Gallagher JP, Phelan KD, Shinnick-Gallagher P.
Modulation of excitatory transmission at the rat medial vestibular nucleus synapse. Ann NY Acad Sci
1992;656:630-44.
Capocchi G, Della Torre G, Grassi S, Pettorossi VE,
Zampolini M. NMDA receptor-mediated long-term
modulation of electrically evoked field potentials in
the rat medial vestibular nuclei. Exp Brain Res. 1992;
90:546-50.
Serafin M, Khateb A, de Waele C, Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea
pig: NMDA induced oscillations. Exp Brain Res.
1992;88: 187-92.
Johnston AR, Murnion B, McQueen DS, Dutia MB.
Excitation and inhibition of rat medial vestibular nucleus neurones by 5-hydroxytryptamine. Exp Brain
Res. 1993;93:293-8.
Serafin M, Khateb A, Vibert N. Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea
pig- histaminergic receptors; 1: An in vitro study.
Exp Brain Res. 1993;93:242-8.
de Waele C, Serafin M, Khateb A, Yabe T, Vidal PP,
Muhlethaler M. Medial vestibular nucleus in the
guinea pig: apamin-induced rhythmic burst firingan in vitro and in vivo study. Exp Brain Res. 1993;
95:213-22.
Lin Y, Carpenter DO. Direct excitatory opiate effects mediated by non-synaptic actions on rat medial vestibular neurons. Eur J Pharmacol. 1994;262:
99-106.
Kinney GA, Peterson BW, Slater NT. The synaptic
activation of N-methyl-D-aspartate receptors in the
rat medial vestibular nucleus. J Neurophysiol. 1994;
72:1588-95.
Takahashi Y, Tsumoto T, Kubo T. NMDA receptors contribute to afferent synaptic transmission in
the medial vestibular nucleus of young rats. Brain
Res. 1994;659:287-91.
Johnston AR, MacLeod NK, Dutia MB. Ionic conductances contributing to spike repolarization and
after-potentials in rat medial vestibular nucleus neurones. J Physiol (Lond). 1994;481:61-77.
Vibert N, Serafin M, Vidal PP, Muhlethaler M. Effects of baclofer1 on medial vestibular nucleus neurons in g:uine2 oig b:-ainsten- slices. Neurosci Lett.
l995;l83:l9~i-7.
117. Hutchinson M, Smith PF, Darlington CL. Further
evidence on the contribution of GABAA receptors
to the GABA-mediated inhibition of medial vestibular nucleus neurons. NeuroReport. 1995;6:1649-52.
118. Hutchinson M, Darlington CL, Smith PF. The effects of long-term, low dose, diazepam administration on guinea pig righting reflex latency and medial
vestibular nucleus neuronal activity. Pharmacal Biochem. Behav. 1995;50:665-9.
119. Darlington CL, Smith PF. Metabotropic glutamate
receptors in the guinea pig medial vestibular nucleus
in vitro. NeuroReport. 1995;6: 1799-802.
120. Vibert N, Serafin M, Vidal PP, Muhlethaler M. Direct and indirect effects of muscimol on medial ves-
C. L. Darlington and P. F. Smith
200
121.
122.
123.
124.
125.
126.
127.
128.
129.
130.
131.
132.
133.
134.
135.
136.
137.
138.
tibular nucleus neurones in guinea pig brainstem
slices. Exp Brain Res. 1995;104:351-6.
Lapeyre PNM, de Waele C. Glycinergic inhibition
of spontaneously active guinea pig medial vestibular nucleus neurons in vitro. Neurosci Lett. 1995;
188:155-8.
Bobker DH, Williams JT. Serotonin-mediated inhibitory post-synaptic potentials in guinea pig
prepositus hypoglossi and feedback inhibition by serotonin. J. Physiol. 1990;422:447-62.
Rekling JC, Laursen AM. Evidence for a persistent
sodium conductance in neurons from the nucleus
prepositus hypoglossi. Brain Res. 1989;500:276-86.
Llinas R. The intrinsic electrophysiological properties of mammalian neurons: insights into central ner''0Us o:;vstem function. 'Science. 1 ORR:2d2: 1654-{;4.
.v!inor LB. Goldberg .. vf. Vestibular-nerve inputs to
the vestibula-ocular rer'lex: a functional-ablation
study in the squirrel monkey. 1 Neurosci. 1991; 11:
1636-48.
Angelaki DE, Perachio AA, Mustari MJ, Strunk
CL. Role of irregular otolith afferents in the steadystate nystagmus during off-vertical axis rotation.
J Neurophysiol. 1992;68:1895-900.
Cannon SC, Robinson DA, Sharmma S. A proposed
neural network for the integrator of the oculomotor
system. Bioi. Cybern. 1983;49:127-36.
Cannon SC, Robinson DA. An improved neuralnetwork model for the neural integrator of the oculomotor system: more realistic neuron behavior.
Bioi Cybern. 1985;53:93-108.
Xerri C, Gianni S, Manzoni D, Pompeiano 0. Compensation of central vestibular deficits; 1: Response
characteristics of lateral vestibular nucleus neurons
to roll tilt after ipsilateral labyrinth deafferentation.
J Neurophysiol. 1983;50:428-48.
Azzena GB, Mameli 0, Tolu E. Vestibular units during decompensation. Experientia. 1977;33:234-5.
Feeney DM, Baron J-C. Diaschisis. Stroke. 1986;17:
817-30.
Chi S-I, Levine JD, Basbaum AI. Effects of injury
discharge on the persistent expression of spinal cord
fos-like immunoreactivity produced by sciatic nerve
transsection in the rat. Brain Res. 1993;617:220-4.
Choi DW, Rothman SM. The role of glutamate
neurotoxicity in hypoxic-ischemic neuronal death.
Annu Rev Neurosci. 1990;13: 171-82.
Hughes P, Dragunow M. Immediate early genes and
the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacal Rev.
199 5 ;47 : 13 3-7 5.
Morgan JI, CurranT. Stimulus-transcription coupling in the nervous system: involvement of the
inducible proto-oncogenes fos and jun. Annu Rev
Neurosci. 1991; i4:421-51.
Kaufman GO, Anderson JH, Beitz AJ. Brainstem
fos expression following unilateral labyrinthectomy
in rat. NeuroReport. 1993;3:829-32.
Kaufman GD, Anderson JH, Beitz AJ. Otolithbrainstem connectivity- evidence for differential
neural activation by vestibular hair cells based on
quantification of fos expression in unilaterallabyrinthectomized rats. J Neurophysiol. 1993;70: 117-27.
Kitahara T, Takeda N, Kubo T, Kiyama H. Role of
NMDA receptor in vestibular compensation in rats.
Neurosci Res. 1993;Suppl. 19:S159.
139. Kaufman GD, Perachio AA. Translabyrinthine electrical stimulation for the induction of immediateearly genes in the gerbil brainstem. Brain Res. 1994;
646:345-50.
140. Takahashi Y, Takahashi MP, Tsumoto T, Doi K,
Matsunaga T. Synaptic input-induced increase in intraneuronal calcium in the medial vestibular nucleus
of young rats. Neurosci Res. 1994;21 :59-69.
141. Darlington CL, Smith PF. Middle ear procaine injection before surgical labyrinthectomy reduces nystagmus. NeuroReport. 1993;4:1353-5.
142. Janssen U, Richter-Landsberg C, Flohr H. Vestibular compensation affects endogenous phosphorylation of frog brain proteins. J Neurochem. 1992;58:
65-71.
\.13 ..fansse~1 t}, ~ichter-Landsberg C. Oestreicher AB,
De Graaf ?!\IE, Gispen WH, Flohr H. Identification of a B-50-like protein in frog brain synaptosomes. Brain Res. 1992;570:21-6.
144. Li H, Dokas LA, Godfrey DA, Rubin AM. Changes
of GAP-43 immunoreactivity during vestibular compensation in rat. Otolaryngol Head Neck Surg. 1994;
111:P153.
145. Hata R, Matsumoto M, Hatakeyama T, Ohtsuki T,
Handa N, Niinobe M, Mikoshiba K, Sakaki S,
Nishimura T, Yanagihara T, Kamada T. Differential vulnerability in the hindbrain neurons and local
cerebral blood flow during bilateral vertebral occlusion in gerbils. Neurosci. 1993;56:423-39.
146. Fineman I, Houda DA, Smith M, Yoshino A, Becker
DP. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45 Ca autoradiographic study. Brain Res. 1993;624:94-102.
147. Pike CJ, Cotman CW. Calretinin-immunoreactive
neurons are resistant to beta-amyloid toxicity in
vitro. Brain Res. 1995;671 :293-8.
148: Sans N, Moniot B, Raymond J. Distribution of calretinin mRNA in the vestibular nuclei of rat and
guinea pig and the effects of unilateral labyrinthectomy: a non-radioactive in situ hybridization study.
Mol Brain Res. 1995;28:1-11.
149. Rickman M, Wolff JR, Meyer DL. Expression of
S 100 protein in the vestibular nuclei during compensation of unilateral labyrinthectomy symptoms.
Brain Res. 1995;688:8-14.
150. Tolu E, Mameli 0, Caria MA, Melis F. Improvement
of vestibular plasticity in the guinea pig with a calcium entry blocker. Acta Otolaryngol (Stockh) 1988;
Suppl 460:72-9.
151. Darlington CL, Smith PF. Pre-treatment with a calcium channel antagonist facilitates vestibular compensation. NeuroReport. 1992;3: 143-5.
152. Sansom AJ, Darlington CL, Smith PF. Pretreatment and MK-801 reduces spontaneous nystagmus following unilateral labyrinthectomy. Eur J
PharmacaL 1992;220: 123-9.
153. Leinhos P, Flohr H. Calcium antagonists and lesioninduced neural plasticity. Eur J Neurosci. 1992;
Suppl 5:74.
154. Sansom AJ, Darlington CL, Smith PF, Gilchrist
DPD, Keenan CJ, Kenyon R. Injections of calmidazolium chloride into the ipsilateral medial vestibular nucleus or .fourth ventricle reduce spontaneous
ocular nystagmus following unilateral labyrinthectomy in guinea pigs. Exp Brain Res. 1993;93:271-8.
155. Sansom AJ, Darlington CL, Smith PF. Comparison
The Intrinsic Mechanism Hypothesis
of the effects of pre-treatment with competitive or
non-competitive NMDA receptor antagonists on vestibular compensation. Pharmacal Biochem Behav.
1993;46:807-11.
156. Darlington CL, Smith PF, Gilchrist DPD. The effects of combined pre-treatment with an NMDA receptor antagonist and post-treatment with a synthetic
ACTH-(4-9) fragment on spontaneous nystagmus
compensation following vestibular deafferentation.
Neurosci Res Commun. 1994;14:133-40.
157. Jerram A, Darlington CL, Smith PF. Methylprednisolone enhances vestibular compensation of spontaneous ocular nystagmus following unilateral
labyrinthectomy in guinea pig. Eur J Pharmacol.
1995;275:291-3.
158. Maclennan K, Smith PF. Darlington CL. Ginkgo-
201
lide B accelerates vestibular compensation of spontaneous ocular nystagmus following unilateral
labyrinthectomy in guinea pig. Exp Neurol. 1995;
131:273-8.
159. Gilchrist DPD, Darlington CL, Smith PF. The effects of flunarizine on ocular motor and postural
compensation following peripheral vestibular deafferentation. Pharmacal Biochem Behav. 1993 ;44:99105.
160. Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol. 1988;
59:370-93.
161. Anastasio TJ. Simulating vestibular compensation
using recurrent back-propagation. Bioi Cybern. 1992;
66:389-97.
Note added in proof: Since the submission of this manuscript, a number of important papers
have been published which are consistent with the hypothesis which we have advanced. Ris
et al. (1) have reported a complete recovery of ipsilateral type I neuron resting activity in the
alert guinea pig, 1 week following unilateral labyrinthectomy (UL). Several papers have been
published which confirm the induction of c-fos (2-4) in the vestibular nuclei following UL.
In one study, Kitihara et al. (4) reported that decompensation induced by the administration
of an NMDA receptor/channel antagonist, caused a reappearance of c-fos expression in the
vestibular nuclei. Finally, in a recent study by Zirpel et al. (5), it has been reported that unilateral
cochlear removal causes an increased calcium influx in the ipsilateral nucleus magnocellularis
(the avian cochlear nucleus).
1. Ris L, de Waele C, Serafin M, Vidal PP, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol. 1995; 74:2087-2099.
2. Cirelli C, Pompeiano M, D'Ascanio P, Arrighi P, Pompeiano 0. C-fos expression in the rat brain after unilateral
labyrinthectomy and its relation to the uncompensated and compensated stages. Neurosci. 1996; 70:515-546.
3. Kitihara T, Saika T, Takeda N, Kiyama H, Kubo T. Changes in Fos and Jun expression in the rat brainstem in
the process of vestibular compensation. Acta Otolaryngol. (Stockh.) 1995; Suppl. 520:401-404.
4. Kitihara T, Takeda N, Saika T, Kubo T, Kiyama H. Effects of MK-801 on Fos expression in the rat brainstem
after unilateral labyrinthectomy. Brain Res. 1995; 700:182-190.
5. Zirpel L, Lachica EA, Lippe WR. Deafferentation increases the intracellular calcium of cochlear nucleus neurons
in the embryonic chick. J Neurophysiol. 1995; 74:1355-1357.