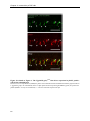
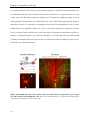
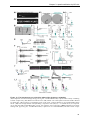
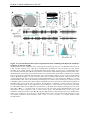
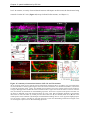
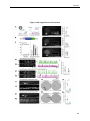
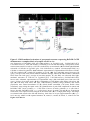
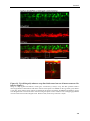
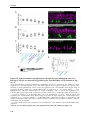
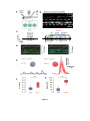
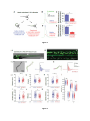
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Modulation of premotor circuits controlling locomotor activity by
Nonsynaptic plasticity wikipedia , lookup
Apical dendrite wikipedia , lookup
Biological neuron model wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Electrophysiology wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Single-unit recording wikipedia , lookup
Neural engineering wikipedia , lookup
Synaptogenesis wikipedia , lookup
Metastability in the brain wikipedia , lookup
Embodied language processing wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Axon guidance wikipedia , lookup
Mirror neuron wikipedia , lookup
Multielectrode array wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neural coding wikipedia , lookup
Evoked potential wikipedia , lookup
Neural oscillation wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Circumventricular organs wikipedia , lookup
Nervous system network models wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroanatomy wikipedia , lookup
Synaptic gating wikipedia , lookup
Development of the nervous system wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Optogenetics wikipedia , lookup
Modulation of premotor circuits controlling locomotor activity by spinal GABAergic sensory neurons in zebrafish : connectivity mapping of an intraspinal sensory feedback circuit Kevin Fidelin To cite this version: Kevin Fidelin. Modulation of premotor circuits controlling locomotor activity by spinal GABAergic sensory neurons in zebrafish : connectivity mapping of an intraspinal sensory feedback circuit. Neurons and Cognition [q-bio.NC]. Université Pierre et Marie Curie - Paris VI, 2016. English. . HAL Id: tel-01426126 https://tel.archives-ouvertes.fr/tel-01426126 Submitted on 4 Jan 2017 HAL is a multi-disciplinary open access archive for the deposit and dissemination of scientific research documents, whether they are published or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers. L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés. Université Pierre et Marie Curie École doctorale Cerveau-Cognition-Comportement (ED 158) Institut du Cerveau et de la Moelle épinière (ICM) Laboratoire Dissection Optogénétique des Circuits Spinaux Sous-Tendant la Locomotion Modulation of premotor circuits controlling locomotor activity by spinal GABAergic sensory neurons in zebrafish Connectivity mapping of an intraspinal sensory feedback circuit Kevin Fidelin Thèse de doctorat - Sciences de la vie, spécialité Neurosciences Présentée et soutenue publiquement le 30 Septembre 2016 devant un jury composé de Dr. Claire Wyart Directrice de thèse Prof. Abdel El Manira Rapporteur Dr. Alessandra Pierani Rapporteuse Dr. Daniel Zytnicki Examinateur Dr. Philippe Faure Examinateur Dr. Elena Dreosti Examinatrice This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License © 2016 – Kevin Fidelin All rights reserved -I dedicate this thesis to my teachers, all of them, from middle school to college, and to my previous mentors. Thank you for everything. Table of contents Acknowledgements ............................................................................................................................................ i Abstract .............................................................................................................................................................ii Résumé ............................................................................................................................................................ iii List of abbreviations ......................................................................................................................................... iv Introduction ........................................................................................................................................................ 1 I. Contribution of sensory feedback during locomotion in vertebrates ......................................................... 4 I.1. Sensory feedback as a driver of locomotor activity: spinal reflexes vs. half-center models ................... 4 I.2. Integration of sensory inputs in spinal circuits ........................................................................................ 5 I.2.a. Descending pathways reconfigure the output of sensory afferents .................................................. 7 I.2.b. State-dependent modulation of motor output by sensory feedback ................................................. 9 I.2.c. Modulation of sensory signaling by presynaptic inhibition ........................................................... 10 I.3. Connectivity between primary afferent neurons and spinal premotor interneurons ............................. 11 I.4. Cellular and molecular investigation of proprioception in mammals ................................................... 13 I.5. Sensory feedback in fish and amphibians ............................................................................................. 13 Key concepts of part I .................................................................................................................................. 16 Transition ..................................................................................................................................................... 16 II. Anatomical, genetic, and functional organization of locomotor central pattern generators ................... 17 II.1. Basic organization of spinal circuits controlling the pattern and rhythm of locomotion .................... 17 II.2. Inductive signals control the fate of spinal neuron specification during development ....................... 19 II.3. Contribution of genetically identified spinal neurons to locomotion ................................................. 19 II.3.a. V0 neurons .................................................................................................................................... 19 II.3.b. V1 neurons .................................................................................................................................... 22 II.3.c. V2a neurons .................................................................................................................................. 23 II.3.c. V3 neurons .................................................................................................................................... 24 II.3.d. Sensory-motor interactions between locomotor CPGs and sensory afferents .................................. 24 Key concepts of part II ................................................................................................................................ 26 Transition ..................................................................................................................................................... 27 III. Cerebrospinal fluid contacting neurons are polymodal sensory neurons in the ventral spinal cord ...... 28 III.1. Identification and characterization of sensory neurons lining the central canal in the spinal cord .... 28 III.2. Molecular analysis of CSF-cNs across species reveals specific markers and a double developmental origin ........................................................................................................................................................... 30 III.3. Sensory modalities underlying the recruitment of CSF-cNs .............................................................. 32 III.4. Experimental strategies and optogenetic approaches to unravel the function of CSF-cNs ............... 34 Key concepts of part III ............................................................................................................................... 35 IV. Aims of the thesis .................................................................................................................................. 36 Chapter 1 - Functional connectivity mapping between CSF-cNs and spinal premotor neurons controlling slow swimming ........................................................................................................................................................ 37 Predictions regarding the connectivity of CSF-cNs .................................................................................... 37 Building the experimental paradigm ........................................................................................................... 37 Highlights of the findings described in this chapter .................................................................................... 38 Graphical abstract of the results .................................................................................................................. 38 Published article: State-Dependent Modulation of Locomotion by GABAergic Spinal Sensory Neurons .......................................................................................................................................... 39 Figures and Supplemental Information ....................................................................................................... 54 Discussion and perspectives ............................................................................................................................ 65 On the specificity of CSF-cNs connectivity ................................................................................................ 65 CSF-cNs target V3 interneurons, a second class of ventral glutamatergic interneurons ............................. 69 On the modulation of bout generation ......................................................................................................... 71 The problem of chloride homeostasis in developing neuronal networks .................................................... 71 On the role of CSF-contacting neurons during slow swimming ................................................................. 73 Can CSF-cNs modulate distinct locomotor behaviors using circuit-specific mechanisms? ....................... 75 Analysis of the contribution of CSF-cNs to evoked fast locomotion .......................................................... 75 Chapter 2- Modulation of spinal circuits controlling the frequency of locomotion by cerebrospinal fluid-contacting neurons ............................................................................................................................................................ 77 BoTxBLC-mediated silencing of V2a interneurons confirms their critical role in fast locomotion ........... 78 Silencing of V2a interneurons decreases the locomotor frequency during spontaneous slow swimming ............................................................................................................................................ 81 Topographic organization of CSF-cNs inputs onto V2a interneurons ......................................................... 83 Discussion and perspectives ............................................................................................................................ 85 Experimental strategy to probe the modulation of V2a neurons by CSF-cNs ............................................ 85 Some assembly required: building a global picture of CSF-cNs-mediated modulation of spinal central pattern generators .................................................................................................................. 86 Are V2a interneurons at the core of a global mechanosensory feedback loop? .......................................... 86 Conclusions and future directions ................................................................................................................... 89 Experimental procedures ................................................................................................................................. 90 References ....................................................................................................................................................... 96 Annex 1 ......................................................................................................................................................... 111 Annex 2 ......................................................................................................................................................... 139 Annex 3 ......................................................................................................................................................... 159 Acknowledgements This is it. The PhD is almost over! I would like to thank here all the people who made this journey a unique human and scientific experience. I hope to highlight each of your precious contributions as best as I can. First and foremost, I wish to thank my thesis committee for evaluating my work and for taking the time to read this manuscript. Thank you Abdel for your support all along the PhD, your feedback at every step of the way has been precious to me. Thank you Philippe for asking the difficult questions when I was defending my project four years ago. It really helped me grasp the key concepts in my field of research (and thank you for the beer in Banyuls!). Thank you Daniel for your support and for gathering our labs together on multiple occasions, there is so much to share and it’s always a pleasure to brainstorm together. Thank you Alessandra for your advices regarding the postdoc decision, our discussion was very helpful. Thank you Elena for crossing the Channel and for your support and implication during TENSS 2014. Claire, I will be brief even if I have a lot to say. Thank you for accepting me in your lab. Giving a neuroscience project to someone who barely knew what the brain was about (as of November 2011) was a risky choice, but we made it work. I am super proud of what we have accomplished as a team. Thank your for your constant enthusiasm, trust, support, and passion. You are an example to follow on many different levels and I hope we will continue to share our love for science in the future. Perhaps you will teach me how to ride a mechanical bull someday….? My dear fantastic three, I remember being worried when I learned we would be so many students in the lab, the same year. It turned out to be the best thing ever. Djenoune, thanks for EVERYTHING, you’ve been the best lab buddy/friend I could dream of. Thank you for supporting me when all I could do was failing my recordings. Thanks for the food and for dealing with my shitty mood. AH OUI C’EST MOI! Thank you Ginna for being a super inspiring colleague and friend. You taught me never to give up and that’s a pretty good skill in science. Thank you Urs for being the smartest and the coolest. I wish I have learned more from you, especially when it comes to building stuff. See you in Boston! Thank you Kris for being the kindest, can I say that? (and sometimes the craziest). You are the type of person who makes a lab a happy place and who is always available to help. Thank you for all the good cakes and for your energy. I do not thank you for converting me to GoT though… Thank you Laura for being the coolest handball player ever and for being supportive all the time. Thank you Andy for being the super-powered postdoc who is always there to help. May the chauffage concept live forever. Thank you Steven for cheating and ruining i my killer game during the first lab retreat. If we were to compete on Survivor together, I can’t tell whether you would be my best ally or my worst enemy ;). Thank you Brian and Charlie for your sense of humor and your dedication. Thank you Pierre for the good work and for the good spirit, it’s been a pleasure to work with you. Thank you PLuc for always being the wise one and for our many discussions. Thank you Jeff for bringing this unique Californian vibe to the lab. Thank you Sophie-la-sagesse for being our best lab and facility manager ever. Thank you Johanna for being the most dedicated lab engineer I have ever met. The lab still misses you a lot. Thank you Natalia and Bogdan for taking good care of our beloved fish, you are the best, I hope you know that. Thank you Audrey for being my nerdy friend and for mentioning the lab to me back in 2011, I owe you much. Thanks to the rest of the lab, past and present members, for being part of this adventure. Thank you Beyonce, for everything. You made it to the defense and you’re definitely a survivor, how cool is that? Thank you Caleb for teaching me how to perform VNR recordings, you’re an incredible teacher and I wish we had more time to work together. Jean, my dear Jean, you have no idea how important your support was to me. Thank you very much for sitting with me and taking the time to review my patch clamp protocols. Thank you Charlotte and Caroline for our many discussions. Big thank you to you Alberto for your support during the PhD. Thank you Richard for evaluating my project proposal early on and for believing in me. Thank you Patricia and Philippe for your help. Thank you to my friends from the AJITES. William, Morwena, Maria-Belen, Morgane, Alizee, Elise, Aysegul, Sean, Typhaine, and everyone. It’s been super fun (and sometimes stressful) to run the association with you guys. It will probably sound pretentious but I think we’ve done a very good job together. Merci à mes amis du lycée, du BTS, du Master et du CEA pour votre soutien sans faille tout au long de la thèse, et pour certain(e)s, bien avant qu’il ne soit question d’une thèse. Je veux vous dire combien je suis fier de vous avoir dans ma vie. Merci à Marelly, Fredo, Emy, Alex, Marcus et la famille Dubus pour votre soutien indéfectible durant toutes ces années. Et pour ces nombreux weekends qui m’ont permis de décompresser, autour d’un bon Uby. Merci à mes frères, Julien et Valentin, Nathalih, mon petit Kael et mes parents chéris. Je pourrais écrire des lignes et des lignes mais je préfère simplement vous dire merci, pour tout. Je vous aime. Enfin merci à toi mon amour. Merci pour ton infinie patience, pour ton écoute, pour ton soutien. La vie est tellement belle à tes côtés. Je t’aime. Abstract Locomotion is one of the most vivid expressions of the central nervous system in action. Looking at people walking in the street or the ballet dancer on the stage, motion seems effortless to the point that many movements are almost executed unconsciously. Indeed, the generation of sophisticated motor behaviors relies on the complex interplay between supraspinal brain structures and circuits in the spinal cord. Understanding how the central nervous system generates a large repertoire of motor sequences, coordinate limbs and body orientation in an ever-changing environment while adapting to a myriad of sensory cues remains a central question in the field of systems neuroscience. The work presented here aims to understand how local sensory neurons in the spinal cord contribute to the production and/ or the modulation of locomotor activity. We focused our attention on dissecting the circuit architecture and function of a conserved class of spinal sensory neurons termed cerebrospinal fluid contacting neurons (CSF-cNs). These neurons lie at the interface between the CSF and spinal interneurons controlling motor output and represent an interesting yet poorly understood sensorimotor loop in the vertebrate spinal cord. Recent studies have revealed that CSF-cNs are responsive to pH variations and bending of the spinal cord, suggesting that CSFcNs are polymodal sensory neurons able to carry information from distinct sensory cues in a context dependent manner. Furthermore, their remote activation using optogenetics in zebrafish was shown to induce slow swimming, demonstrating the ability of CSF-cNs to modulate spinal circuits controlling locomotion. However, the connectivity of CSF-cNs remains completely uncharacterized. To understand how CSF-cNs modulate locomotion in vertebrates, we combined genetics, imaging, optogenetics, electrophysiology, and behavior analysis to map the functional connectivity of these sensory neurons and test their function in the zebrafish larva. Our results demonstrate that CSF-cNs project onto several elements thought to be part of the locomotor central pattern generator in zebrafish, including glutamatergic spinal interneurons involved in slow and fast swimming. We show that CSFcNs can modulate the duration and occurrence of spontaneous locomotor events in a state dependent manner and tune the frequency of evoked fast escape responses. Altogether our work dissecting sensorimotor integration in the spinal cord bridged single cell function to behavior in zebrafish in vivo and should contribute to a better understanding of the role of sensory feedback during locomotion in vertebrates. ii Résumé La capacité à se mouvoir est sans doute l’expression la plus évidente du système nerveux en action. À observer les passants marcher dans la rue ou un spectacle de danse classique, le mouvement semble si fluide et rapide qu’il parait être exécuté de manière inconsciente. Il n’en est rien. La locomotion résulte d’interactions complexes entre de nombreuses structures supra-spinales et les circuits de la moelle épinière. Comprendre les mécanismes mis en place au sein du système nerveux pour générer des répertoires locomoteurs complexes, coordonner les membres et l’orientation du corps dans l’espace, dans un environnement qui évolue à chaque instant tout en intégrant des entrées sensorielles variées, reste l’un des grands défis des neurosciences systémiques. Le travail présenté dans ce manuscrit vise à comprendre comment les neurones de la moelle épinière contribuent à la production et à la modulation de l’activité locomotrice. Pour répondre à ce problème, nous utilisons le poisson-zèbre comme organisme modèle et avons développé de nouvelles approches génétiques et optiques afin de disséquer l’architecture du circuit formé par une classe de neurones sensoriels de la moelle et qui est conservée chez tous les vertébrés. Ces neurones sont appelés les neurones au contact du liquide céphalo-rachidien (Nc-LCR) et nous proposons de sonder leur(s) fonction(s) in vivo. Ces neurones sensoriels forment une interface unique entre le liquide céphalo-rachidien et le réseau de neurones impliqué dans le contrôle du mouvement dans la moelle épinière. Nous formulons donc l’hypothèse selon laquelle ces neurones participent à l’intégration sensorimotrice dans la moelle épinière des vertébrés. Des études récentes ont démontré la capacité de ces cellules à répondre aux variations locales de pH ainsi qu’à la torsion de la moelle épinière durant le mouvement ce qui suggère que ces cellules sont polymodales, avec de potentielles fonctions qui diffèrent selon le contexte dans lequel elles sont recrutées. De plus, leur activation par des méthodes optogénétiques a révélé leur capacité à induire la nage lente chez la larve du poisson, ce qui démontre que ces cellules peuvent moduler l’activité des neurones de la moelle épinière. Cependant, leur diagramme de connectivité demeure complètement inconnu. Afin de comprendre comment ces « Nc-LCR ou CSF-cNs » modulent la locomotion chez les vertébrés, nous avons développé un projet combinant des approches génétiques, électrophysiologiques, d’imagerie, et d’analyse du comportement, afin de cartographier le circuit qu’elles forment avec les neurones de la moelle épinière. Nos résultats montrent que les CSF-cNs projettent leurs axones sur de nombreux éléments du centre générateur de rythme de la moelle, et en particulier sur les neurones excitateurs glutamatergiques impliqués dans la locomotion lente et rapide. Notre approche révèle également la capacité des CSF-cNs à moduler la locomotion selon l’état dans lequel se trouve l’animal, une propriété caractéristique des circuits proprioceptifs dans la moelle épinière. Dans l’ensemble, ce travail de dissection des circuits impliqués dans l’intégration sensorimotrice de la moelle épinière fait le lien entre la fonction de neurones à l’échelle cellulaire, du réseau et du comportement, au sein d’un animal intact. Ces résultats devraient apporter de nouveaux éléments permettant d’appréhender et de mieux comprendre de rôle du retour sensoriel durant la locomotion chez les vertébrés. iii List of abbreviations ASIC Acid-sensing ion channel 5-HT 5-hydroxytryptamine 5-HTP 5-hydroxy-tryptophan aIN Ascending interneuron BMP Bone Morphogenetic Protein Btx-LC or BTXB-LC Botulinum toxin light chain CaP Caudal Primay motor neuron Chx10 Ceh-10 homeodomain-containing homolog ChR2 Channelrhodopsin cIN Commissural interneuron CiA Circumferential Ascending interneuron CiD Circumferential Descending interneuron CoBL Commissural Bifurcating Longitudinal interneuron CoLA Commissural Longitudinal Ascending interneuron CoPA Commissural Primary Ascending interneuron CoSA Commissural Secondary Ascending interneuron CPG Central pattern generator CNS Central nervous system CSF-cNs Cerebrospinal-fluid contacting neurons dpf Day post fertilization Dbx1 Developing brain homeobox 1 DRG Dorsal root ganglia DsRed Discosoma sp. red fluorescent protein DTA Diphteria toxin A DV or D-V Dorso-ventral eIN Excitatory interneuron EM Electron microscopy EMG Electromyogram EPSP Excitatory post-synaptic potential En1 Engrailed-1 iv Egr3 Early growth response protein 3 Evx1 Even-skipped homeobox 1 FRA Flexor reflex afferents GABA gamma-Aminobutyric acid GAD Glutamic acid decarboxylase GFP Green fluorescent protein Hz Hertz IN Interneuron IPSP Inhibitory post-synaptic potential KCC2 Potassium-Chloride co-transporter LCR Liquide céphalo-rachidien LiGluR Light-gated glutamate receptor L-DOPA L-3,4-dihydroxyphenyl-alanine MAG-1 Maleimide-azobenzene-glutamate 1 MCoD Multipolar Commissural Descending interneuron MN Motor neuron ms millisecond nAchR Nicotinic acetylcholine receptor Nc-LCR Neurones au contact du liquide céphalo-rachidien NKCC1 Sodium-Potassium-Chloride co-transporter Nkx2.2 NK2 Homeobox 2 NpHR Halorhodopsin NTR Nitroreductase pH hydrogen potential PAD Primary afferent depolarization Pax Paired box PIR Post-inhibitory rebound Pkd2l1 Polycystic kidney disease-2 like-1 Pitx2 Paired like homeodomain trans 2 RB Rohon-Beard RC Renshaw cell Sim1 Single-minded homolog 1 Sp8 Sp8 trans-acting transcription factor 8 Shh Sonic hedgehog TRP Transient Receptor Potential UCoD Unipolar Commissural Descending interneuron UAS Upstream Activating Sequence VeMe Ventro-medial interneuron Vgat Vesicular GABA transporter Vglut Vesicular glutamate transporter VIP vasoactive intestinal peptide VNR Ventral nerve root YFP Yellow fluorescent protein Introduction Introduction Animals rely on locomotion to explore their environment, to feed, to find partners for reproduction, or to escape from predators. To perform these actions, the central nervous system generates elaborate goal-directed locomotor sequences that must be adapted to ever-changing environmental conditions. In particular, animals must be able to rapidly change their gait and to adapt their locomotion to various terrains while the movement is performed (Orlovsky, Deliagina, Grillner, 1999; McNeill, 2002; Bellardita and Kiehn, 2015; Kiehn, 2016). In this context, sensory information relative to the timing of movement (through the state of muscle contraction, referred to as proprioception, Dietz, 2002; Windhorst, 2007) and sensory inputs from the external world (transmitted by cutaneous afferents mediating touch or by nociceptors and referred to as exteroception, Edin, 2001; Panek et al., 2014) are integrated by central circuits during movement execution (Figure I.1). This process, referred to as sensorimotor integration, contributes to optimizing or correcting the pattern of motor output to produce smooth movements (Pearson, 1995; Büschges and El Manira, 1998, McCrea, 2001; Rossignol et al., 2006). Neuronal circuits controlling the generation of movement and receiving sensory feedback from the periphery are located in the spinal cord where they also receive descending inputs from supraspinal structures (Figures I.1, I.2, Shik et al., 1966; Armstrong, 1986; Jordan, 1998). The spinal cord can then be viewed as a neuronal hub, a site of intense neuronal processing as well as a structure driving the activation of muscles in complex and sequential manners (Figure I.2). Spinal cord injuries or diseases associated with motor or somatosensory processing dysfunctions affect or completely prevent forelimb, trunk, and/or hindlimb associated muscles from being activated, thus perturbing or abolishing movement generation and locomotion in particular (Sanes et al., 1985; Dietz, 2002; Holtz and Levi, 2010; Conte et al., 2013). Dissecting the architecture of spinal circuits by identifying the constituent neurons responsible for muscle activation and the nature of descending and sensory inputs to spinal neurons is a critical step to understanding the neural control of movement and its perturbation in diseases. Studies on spinal reflex 1 Introduction Figure I.1. Organization of glutamatergic sensorimotor circuits in the spinal cord. Schematic of neuronal circuits in a mouse spinal cord at embryonic day 18. The axons of sensory neurons project from the dorsal root ganglia to specific laminae in the dorsal horn and to the periphery. Pain and temperature are sensed by nociceptive neurons (red), and the messages are conveyed to laminae I and II. Touch is mediated by mechanoreceptors (green) in the periphery, which connect to laminae III, IV and V. Proprioception is mediated by the sensory neurons that project through the dorsal horn to the ventrally located motor neurons (shown in blue) The motor neurons also connect directly back to the muscle in the periphery to drive movement (pink). Roman numerals indicate the laminae of the dorsal horn. From Caspary and Anderson, 2003. Figure I.2. Anatomy of the human spinal cord. (A) Drawing of the spinal cord, the spinal roots and the corresponding vertebrates. Each spinal nerve is composed of nerve fibers that are related to the region of the muscles and skin that develops from one body somite (segment). A spinal segment is defined by dorsal roots entering and ventral roots exiting the cord, (i.e., a spinal cord section that gives rise to one spinal nerve is considered as a segment.) (B) Map of dermatomes (area of muscle and skin supplied by peripheral nerve fibers originating from a single dorsal root ganglion). The numbers refer to the spinal segments by which each nerve is named C = cervical; T = thoracic; L = lumbar; S = sacral spinal cord segments. From Dafny, Neuroscience online, an electronic textbook for neurosciences, The University of Texas Medical School at Houston. 2 Introduction pathways by means of sensory afferent stimulations and intracellular recordings have brought to light mechanisms regulating the recruitment and activity of motor neurons by sensory feedback. These studies have also uncovered the diagram of connectivity between sensory afferents, relay interneurons, and motor neurons, and ultimately identified principles governing muscle coordination in limbed vertebrates (reviewed in Burke, 1999; Hultborn 2001, Stuart and Hultborn, 2008). In parallel, the identification of interneurons responsible for the phasic activation of motor pools during locomotion and their genetic origin during development has improved our understanding of motor pattern generation by distinct subtypes of spinal neurons (reviewed in Jessell, 2000; Marder and Bucher, 2001; Grillner 2003; Goulding, 2009; Arber, 2012; Grillner and El Manira, 2015; Kiehn, 2016). Despite a century of research on the topic, the connectivity and specific contribution of sensory pathways during locomotion remains difficult to address in vivo. This is due to a lack of genetic access to desired neuronal population and because testing the dynamics of sensory integration, notably by recording single neurons in moving animals, is technically challenging (Dombeck et al., 2007; Naumann et al., 2010). As a consequence, the connectivity between sensory neurons modulating locomotor activity and spinal interneurons driving the rhythmic activation of motor pools remains poorly understood. While new tools and approaches are emerging in the field of systems neuroscience, new routes can be taken to unravel the nature and function of neuronal circuits controlling locomotion, including peripheral sensory circuits. In particular, genetic targeting of spinal neurons combined with electrophysiology and optogenetics open new paths to revisit and test some of the most challenging questions in the field (reviewed in Del Bene and Wyart, 2012; McLean 2013; Portugues et al., 2013; Fidelin and Wyart, 2014, Deisseroth, 2015; Montgomery et al., 2016). The work presented in this manuscript aims to 1/ understand how sensory and central circuits in the spinal cord interact during locomotion, and 2/ determine how inputs from sensory feedback pathways shape locomotor activity. In this introduction I will start by reviewing important concepts about the control and modulation of locomotor activity by sensory feedback in vertebrates. I will then explore the organization of spinal rhythmic circuits controlling locomotion and formulate predictions regard- 3 Introduction ing the consequences of their modulation by sensory feedback. Finally, I will describe the sensory pathway investigated in this project and explain how it constitutes a novel and interesting circuit for the study of sensorimotor integration in vertebrates. I. Contribution of sensory feedback during locomotion in vertebrates I.1. Sensory feedback as a driver of locomotor activity: spinal reflexes vs. half-center models Studies from the mid-19th century reported that spinal birds and mammals could use the body parts innervated by spinal circuits below the site of transection to produce patterns of locomotion (Flourens, 1824; Freusberg, 1874; Unzer, 1771). These observations led to further studies demonstrating that inputs from sensory afferents elicited by mechanical or electrical skin stimulations were sufficient to produce rhythmic contraction of hindlimb flexor and extensor muscles, thus recapitulating scratching in spinal cats and dogs (Sherrington, 1906a, 1910). At the time, it was suggested that the sequential activation of muscles during these evoked movements might be directly driven by the feedback from muscle contraction itself. Sherrington and his peers formulated the hypothesis that rhythmic movements such as stepping in cats were generated by a succession of these so-called “reflexes” in the absence of descending “activating” inputs from the brain (Sherrington, 1906a, 1906b; 1910; reviewed in McCrea, 2001; Hultborn, 2006). Although it was clear that inputs from cutaneous or proprioceptive afferent fibers (Table 1, Figure I.3, reviewed in Rossignol et al., 2006) could provide powerful excitation to spinal centers and enable the contraction of limb muscles, it was later shown that spinal cats with deafferented hindlimbs could still present rhythmic patterns of motor output (Brown, 1911, 1914). This observation followed by the work of many other groups suggested that sensory feedback was not necessary for the generation of rhythmic contraction of hindlimb muscles (Brown, 1911, 1914; Brown and Sherrington, 1912; reviewed in Delcomyn, 1980; Stuart and Hultborn; 2008; McCrea and Rybak, 2008). As a matter of fact, Sherrington himself had gathered similar evidence when he found that spinal dogs with deafferented limbs could still perform scratching, a movement that resembles stepping (Sherrington, 1906b). 4 Introduction Altogether this series of pioneer studies gave rise to the idea that the spinal cord contains autonomous “half-center” circuits composed of limb and muscle-specific excitatory neurons that are responsible for the rhythmic activation of limb muscles. These circuits were later defined as single-level locomotor central pattern generators by Wilson and Wyman from their work in the locust (Wilson and Wyman, 1965) and by Grillner and colleagues following their work in the lamprey (Grillner, 1969; reviewed in Grillner, 2003; McCrea and Rybak, 2008; Grillner and Jessell, 2009; Grillner and El Manira, 2015). This large body of work also led to the general understanding that locomotion, while initiated by supraspinal brain centers, is generated and maintained in the spinal cord. Even though sensory inputs are not necessary for the production of motor output, they may be important for muscle coordination and for the modulation of circuit activity, notably by interacting with descending circuits and by tuning the excitability of local spinal circuits (Grillner and Rossignol, 1978; Duysens and Pearsons, 1980; Hiebert et al., 1996; Lam and Pearson, 2001). Eliminating inputs from sensory feedback pathways has dramatic effects on locomotion and postural control (Akay, 2014; Takeoka; 2014), as depicted in the example of the disembodied lady from Oliver Sacks, a patient who may have suffered from a rare form of sensory neuritis targeting dorsal root ganglia. I.2. Integration of sensory inputs in spinal circuits The work from Sherrington, Brown, and colleagues revealed that spinal circuits underlying locomotion and sensory pathways emerging from muscles and tendons had complex interactions during movement. Because of the lack of genetic tools and techniques for recording individual neurons, it was not technically possible to probe the cellular organization of spinal circuits receiving these sensory signals. This limitation prevented them from understanding to what extent sensory and descending inputs are dynamically integrated at the spinal neuron level in order to generate different types of motor output. The development of intracellular recording techniques allowed researchers to further dissect the architecture of afferent feedback circuits and the function of proprioception during locomotion (Brette and Destexhe, 2012; reviewed in Jankoswka and Hammar, 2002; Hultborn, 2006). 5 Introduction Table 1. Proprioceptors in limbed vertebrates. Proprioceptors are located in muscles, tendons, joint ligaments and in joint capsules. There are no specialized sensory receptor cells for body proprioception. In skeletal (striated) muscle, there are two types of encapsulated proprioceptors, muscle spindles (Ia and II) and Golgi tendon organs (Ib), as well as numerous free nerve endings (III). Within the joints, there are encapsulated endings similar to those in skin, as well as numerous free nerve endings. Neuroscience online, an electronic textbook for neurosciences, The University of Texas Medical School at Houston. Figure 1.3. Location of proprioceptors in the body. (A) The Golgi tendon organ is located at the junction of muscle and tendon. Afferent terminal fibers are intertwined with collagenous fibers of the tendon and the entire organ is encapsulated in a fibrous sheath. (B) A muscle spindle with its sensory and motor innervation. The primary muscle spindle afferent terminates as annulospiral endings in the central area of the intrafusal muscles whereas the secondary muscle spindle afferent terminates as flower spray endings in more polar regions of intrafusal muscles. (C) The joint receptors are free nerve endings encapsulated in the joint capsule and joint ligaments. Adapted from Dougherty, Neuroscience online, an electronic textbook for neurosciences, The University of Texas Medical School at Houston. 6 Introduction Of particular relevance, the work of many labs, including the labs of Sir John Eccles, Anders Lundberg, and Elzbieta Jankowska revisited the concept of spinal half-centers developed a few decades earlier by T. G Brown, notably by identifying spinal interneurons forming rhythmic circuits that would also be part of reflex circuits (reviewed in Jankowska, 2001; McCrea, 2001; Hultborn, 2006; Jankowska 2008; Stuart and Hultborn, 2008). I.2.a. Descending pathways reconfigure the output of sensory afferents After the identification of monoaminergic terminals in the spinal cord (Carlsson et al., 1964), Lundberg and colleagues probed the influence of monoaminergic descending inputs on the regulation of spinal processing by performing intravenous injections of the noradrenergic precursor L-3,4dihydroxyphenyl-alanine (L-DOPA) and 5-hydroxy-tryptophan (5-HTP) in spinal cats. They found that these compounds modified the pattern of motor activity elicited by stimulation of sensory afferent fibers (Anden et al., 1963, 1964; Jankoswka, et al., 1965). Instead of measuring a short-latency depolarization of flexor motor neurons, at it is the case for typical reflex responses, Jankowska and colleagues observed delayed, long-lasting and rhythmic depolarizing volleys in flexor motor neurons, which were reminiscent of a locomotor-like state (Jankoswka, et al., 1967a, 1967b). These results suggest that inputs from sensory pathways have different effects on motor pools when supraspinal descending neurons are active compared to when they are not (Figure I.4). This observation led to the idea that inputs from descending neurons may either directly modulate the activity of sensory afferent’s target motor neurons or activate sets of spinal neurons that would in turn modify the recruitment or excitability of motor neurons in response to sensory stimulations. Alternatively, Jankowska and colleagues found that flexor motor neurons did receive direct reciprocal inhibition when contralateral flexor motor neurons were active. This pioneering work identified the first diagram of connectivity of spinal interneurons with ipsilateral excitatory interneurons recruited by the combination of descending and sensory inputs. These interneurons were proposed to be responsible for the rhythmic activation of motor neurons, while inputs from inhibitory interneurons were similarly recruited to ensure alternation of activity from one side of the body to the other (Jankowska, 1967a, 1967b, reviewed in Hultborn, 2006; McCrea and Rybak 2008). 7 Introduction Figure I.4. Schematic representation of neuronal pathways transmitting excitatory action to motoneurones (Mn) from flexor reflex afferents (FRA). Excitatory terminals are indicated by two branches, inhibitory by filled circles. Termination of inhibitory interneurones on a cell body merely indicates inhibitory action and there is no commitment as to whether the inhibition is postsynaptic on cell bodies or presynaptic on terminals of interneurones. Pathway A is activated in the acute spinal cat (without DOPA) and inhibits pathway B so that no effect is transmitted via this route. After DOPA, transmission in pathway A is partially or completely inhibited (by liberation of transmitter from a descending noradrenergic pathway) thereby removing the inhibition of pathway B through which the late longlasting EPSP is evoked in the motoneurone. From Jankowska et al., 1967a. Figure I5. Slow excitation of extensor motoneurons by extensor group I afferents. Reversal of the influence of group I afferents from plantaris (PL) on a motor neuron supplying either the lateral gastrocnemius or soleus muscle (LCS) following the administration of L-DOPA in an acute spinal cat. Before L-DOPA, the response was predominantly hyperpolarizing due to summation of disynaptic group lb IPSPs. After L-DOPA, the response was depolarizing due to the opening of an additional excitatory pathway. Adapted from Gossard et al., 1994 and from Pearson, 1995. Figure I.6. Diagrams illustrating convergence from primary afferents (I, prim aff) and descending pathways (II, desc) onto common INs projecting to MNs. Neuronal circuits shown on the left with (A) showing excitatory convergence from both sources whereas (B) shows excitation from primary afferents and inhibition from a descending pathway. INs (open circles) represent populations of these cells with similar convergence. Traces in the right-side diagrams show idealized IC records from a single MN after a test stimulus (I, prim aff), a conditioning (cond) stimulus (II, desc) and their combination (I+II). From Stuart and Hultborn, 2008. 8 Introduction Along the same line, stimulation of Ib fibers led to different outputs on motor neurons with or without L-DOPA (Gossard et al., 1994). While stimulating group Ib fibers at rest was associated with large volleys of inhibition in recorded target motor neurons, the same stimulation after addition of L-DOPA in the preparation triggered large depolarization of the same motor neurons (Figure I.5, Gossard et al., 1994; Pearson and Collins, 1993; Whelan et al., 1995; reviewed in Pearson, 1995). This phenomenon, termed reflex reversal, demonstrates that sensory afferents are fully integrated within rhythmic spinal circuits and that the effect of their activation depends on the state of spinal circuit’s activation and as a consequence the nature of active spinal neurons at the time of the stimulation. Conversely, these results also indicate that neuromodulators such as dopamine (and additionally for noradrenaline and serotonin) can dramatically modify the output of both spinal circuits and sensory feedback pathways. This body of work is important because it gave rise to some of the most fundamental concepts in the field of sensorimotor integration. First, these results showed that descending circuits interact with sensory afferents and can change the output of motor neurons following sensory stimulation. Then, it brought to light the architecture of sensorimotor connectivity by revealing the existence of premotor circuits, which are not active when L-DOPA is absent but seems nonetheless responsible for the rhythmicity observed in these experiments. Finally, it showed that descending commands can modulate the configuration of active circuits during sensory processing by adding or derecruiting spinal interneurons (Figure I.4). Altogether, these data and a series of follow-up papers (described in Lundberg, 1975 and Burke, 1985 and a long series of papers from Jankowska and colleagues) demonstrated that descending and sensory inputs converge onto spinal microcircuits with the ability to modify their output in a context-dependent manner (Stuart and Hultborn, 2008; Figure I.6). The latter concept is particularly important for the work presented in this manuscript. I.2.b. State-dependent modulation of motor output by sensory feedback Using L-DOPA to induce locomotor-like states in decerebrate and acute spinal cats allowed researchers to investigate the effects of activating sensory pathways during different contexts, either at rest or during fictive locomotion. It was demonstrated that the timing of Ia or group II afferents activation would 9 Introduction Figure I.7. Extension enhancement and resetting evoked by extensor group I afferents. The records are rectified-integrated neurograms obtained during fictive locomotion evoked by stimulation of the midbrain in a decerebrate cat and show rhythmic alternating activity in ankle flexor (TA) and extensor (MG) nerves. The intervals (ms) between subsequent discharges in the MG nerve are indicated below the MG recording. A, stimulation of the plantaris nerve (twice threshold (2T), 22 shocks, 200 Hz) during flexion initiates the extension phase of locomotion (i.e. resets to extension). B, the same stimulation delivered during extension prolongs the duration and enhances the amplitude of extensor activity. C, averaged rectified integrated neurogram of SmAB activity during fictive locomotion. The traces were aligned at the onset of stimulation and show the effects of LGS nerve stimulation (200 Hz) at different intensities on the activity of these hip extensor motoneurones. Note the persistence of activity well beyond the end of the stimulus train. Adapted from Guertin et al. (1995) and McCrea, 2001. determine whether these sensory inputs could entrain the rhythm if they are elicited at rest (without LDOPA) or reset the rhythmicity of spinal neurons in L-DOPA (Figure I.7, Conway et al., 1987; Kriellaars et al., 1994; Guertin et al.,1995). Thus, sensory feedback can participate in controlling the transition from one phase to the next during the step cycle. This work led to the concept that sensory feedback and local circuits in the spinal cord interact in a complex and state-dependent manner to control the timing of muscle coordination during complex motor sequences (McCrea, 2001). One interpretation for these findings is that sensory feedback could compensate for perturbations occurring in the locomotor environment or correct non-linear mechanics during movement (Stuart, 1999; McCrea, 2001). I.2.c. Modulation of sensory signaling by presynaptic inhibition Eccles and colleagues found that pre-stimulating group I afferents led to a decrease of amplitude of elicited EPSPs in recorded target motor neurons during subsequent afferent stimulations (Eccles et al., 1962a, 1962b, 1962c). In addition, they observed a reduction of the overall monosynaptic reflex amplitude without noticeable modification of motor neurons’ membrane resistance (Eccles et al., 1962a, 1962b, 1962c). These data revealed the existence of a tight regulation of the excitability of motor neu10 Introduction rons occurring at the premotor level, which was directly related to the timing of sensory inputs in antagonist motor pools. Recording of Ia afferents during these experiments revealed a strong depolarization at their terminals termed “primary afferent depolarization” (PAD), which was sufficient to discharge primary afferents and consequently abolish the excitation of target motor neurons (Marlinskii, 1983; Rudomin and Schmidt, 1999; Rudomin, 2009). This mechanism is crucial for movement coordination and stability because it limits the gain of proprioception and prevents undesired excitation of motor neurons during movement when these neurons are not supposed to be recruited. However, the nature of spinal neurons mediating this effect in vivo remained elusive for a long time. Combining electrophysiology, optogenetics, and behavior analysis, a recent study tackled this particular point by identifying local Gad2+ spinal neurons as the cellular substrate mediating GABAergic presynaptic inhibition during skilled reaching in mouse (Betley et al., 2009; Fink et al., 2014; Figure I.8). The authors tested the role of Gad2+ neurons by specifically eliminating this subpopulation and found that reaching tasks were altered in mice lacking Gad2+ neurons (Fink et al., 2014). I.3. Connectivity between primary afferent neurons and spinal premotor interneurons The dissection of reflex circuits have enabled researchers to unravel the organization of premotor interneurons integrating sensory inputs, and revealed how these neurons could shape the pattern of motor activity. In particular, Ia interneurons that receive monosynaptic excitation from Ia muscle spindle afferents became the center of intense investigation (reviewed in Jankowska, 1992). Work in cats and humans revealed that Ia neurons form a pathway providing monosynaptic excitation to homonymous motor neurons while also activating contralateral inhibitory interneurons responsible for the reciprocal inhibition of antagonists motor neurons, thus forming a disynaptic inhibitory circuit (Jankoswka et al., 1981; Baldissera et al., 1987). As a consequence, Ia interneurons form a relay pathway, which in addition to presynaptic inhibition, controls the alternation of antagonistic pairs of motor pools. Interestingly, Ia interneurons also integrate descending inputs including direct inputs from corticospinal neurons and from other local interneurons (Hultborn and Udo, 1972a, 1972b; Illert and Tanaka, 1978). In particular, inputs from Renshaw cells to Ia interneurons are thought to down regulate the Ia-mediated reciprocal 11 Introduction Figure I.8. Gad2-expressing neurons mediate presynaptic inhibition. (A) Proprioceptive sensory neurons (SN) convey sensory feedback signals from muscle to motor neurons (MN). Presynaptic inhibitory (GABApre) neurons contact sensory afferent terminals, whereas postsynaptic inhibitory (GABApost) neurons contact motor neurons directly. GABApre neurons express Gad2 (top schematic and left two images; far left: red Gad2ON contacts on blue VGluT1+ sensory afferent terminal, adjacent green GFP+ motor neuron labeled in Hb9GFP mice). GABApost neurons express Gad1 (bottom schematic and right two images; second from right: red Gad1ON contacts adjacent green GFP+ motor neuron). Although GABApre neurons express both Gad1 and Gad2 (second image from left: yellow Gad1ON/Gad2ON puncta adjacent VGluT1+ sensory terminal), GABApost neurons express Gad1 alone (far right image: red Gad1ON/ Gad2OFF puncta). (B) Injection of Cre-dependent virus (AAV-FLEX-ChR2-YFP) into cervical cord of adult Gad2-Cre mice labels GABApre neurons (top, YFP +, Gad2+ contact adjacent VGluT1+ sensory terminal) and not GABApost neurons (bottom, YFP negative red puncta). Viral injection marks ≈80% of GABApre and ≈1% of GABApost boutons. (C) After AAV-FLEX-ChR2-YFP injection in lumbar spinal cord of neonatal Gad2-Cre mice, recordings in isolated spinal cord from sensory afferents (dorsal root L4, extracellular) reveal primary afferent depolarization, and (D) recordings from motor neurons (whole cell patch clamp) reveal suppression of monosynaptic sensory-evoked excitatory postsynaptic currents after photostimulation (black, control; blue, 473-nm wavelength (λ) photostimulation). At early ages Gad2 is also expressed in GABApost boutons; therefore, all behavioral experiments were performed after viral injection in adult mice, when Gad2 expression is specific for GABApre neurons. Adapted from Betley et al. 2009; Azim et al., 2014b, Fink et al., 2014. inhibition in order to favor co-contractions of antagonistic muscles, which is necessary to maintain body position in space (McCrea et al., 1980; Pratt and Jordan, 1987). The connectivity of spinal circuits with either descending circuits and/or sensory pathways remains particularly challenging to map but new approaches combining genetic targeting with the use of viral approaches should drastically improving our ability to map the connectivity of sensory neurons in the spinal cord (Wickersham et al., 2015a, 2015b). 12 Introduction I.4. Cellular and molecular investigation of proprioception in mammals A recent study took advantage of the early growth response 3 (Egr3) mutant line, in which group Ia and II afferents are impaired, to investigate the contribution of sensory feedback during locomotion (Tourtellotte and Milbrandt, 1998; Akay et al., 2014). The authors found that eliminating these inputs altered stepping, precise locomotor pattern on ladders, and swimming activity, indicating that inputs from Ia, Ib and II afferents pathways act in concert to allow coordinated patterns of muscle activation during locomotion (Akay et al., 2014). A study published the same year confirmed these results and found that inputs from these sensory neurons are indeed critical for the functional recovery of spinal circuits after spinal cord hemi-sections (Takeoka et al., 2014). At the molecular level, Piezo2, a nonselective cation channel has been recently identified as the main mechanosensory channel responsible for the activation of mammalian proprioceptors. Accordingly, mice lacking this channel in DRG neurons display severe limb coordination problems (Woo et al., 2015). I.5. Sensory feedback in fish and amphibians In anamniotes such as the lamprey, the organization of sensory feedback circuits differs from mammals. Instead of relying on proprioceptive afferent pathways located at the periphery to detect muscle activation, swimming vertebrates have developed other sensory strategies that enable them to sense their environment. These systems can take the form of sensory cells on the skin that can detect hydrodynamics and pressure, or mechanoreceptor cells that can sense axial muscle contraction, body tension, or stretch. Lamprey possess intraspinal proprioceptors that are also referred to as stretch receptors or “edge cells” (Grillner et al., 1984, Hsu et al., 2013). One common strategy to investigate the contribution of sensory feedback in these animals consists in paralyzing muscles using blockers of the neuromuscular junction and analyzing the profile of fictive locomotion. In this configuration it remains possible to record the rhythmic activity of spinal neurons at the level of ventral nerve roots (VNR) but this activity does not translate into movement anymore due to the inactivation of muscle contraction (Masino and Fetcho, 2005). Comparison between the locomotor activity obtained with electromyogram (EMG) recordings during ‘active’ locomotion and the profile of ‘fictive’ locomotion revealed differences in rhythm, motor phase lags and intersegmental coordination (Wallén and Wil- 13 Introduction liams, 1984). These results suggest that sensory feedback in swimming animals is critical for setting the optimal rhythm, by modulating the timing or muscle contraction and propagation of excitation from head to tail (Mullins et al., 2011). Regarding the contribution of stretch receptor cells, a pioneering experiment from the Grillner lab demonstrated the influence of stretching the spinal cord in lamprey. Such manipulation was shown to induce rhythmic discharges of motor neurons in the fictive configuration, regardless of the side where the stimulation was applied (McClellan and Grillner, 1983). This study is important because it shows that sensory feedback, like in mammals, has the property to entrain locomotion under certain contexts in lower vertebrates. In Xenopus and zebrafish, early born Rohon-Beard (RB) cells are dorsally located mechanosensory neurons expressing piezo2b that respond to light touch on the skin and mediate touch-evoked escape responses (Clarke and Roberts, 1984b, Spitzer, 1984, Kohashi and Oda, 2008, Douglass et al., 2008, Faucherre et al., 2013). Rostral RB cells send axonal projection directly to the Mauthner cell, the main component of the escape response circuit, while more caudal RB neurons project on commissural primary ascending interneurons (CoPA) in zebrafish and dorsolateral commissural interneurons (dlc) in Xenopus, which are monosynaptically connected to contralateral primary motor neurons (Clarke and Roberts, 1984a, 1984b, Sillar and Roberts, 1988, 1992, Roberts and Sillar, 1990, Bernhardt, 1990, Li et al., 2004, Palanca et al., 2013, Knogler and Drapeau, 2014). RB neurons were thought to degenerate early during development but it is now accepted that RB neurons can be visualized in two weeks old zebrafish suggesting that their role is not restricted to the embryo (Reyes et al., 2004, Palanca et al., 2013). CoPA neurons are silenced by ascending inhibitory interneurons (aINs in Xenopus and CiA in zebrafish) during fictive swimming, a mechanisms thought to prevent the sensory activation of the escape response when animals are engaged in swimming (Li et al, 2003, Higashijima et al., 2004a, 2004b). Interestingly, Pietri et al reported putative anatomical contacts between axons of CoPA interneurons onto the soma of V2a interneurons, which are rhythmically active premotor interneurons driving slow and fast swimming (Pietri et al., 2009). Whether the RB-CoPA loop modulates spontaneous locomotion remains to be determined. Interesting experiments revealed that RB neurons could trigger different types of locomotor responses depending on the intensity of touch stimuli (Soffe et al., 1991, 14 Introduction 1997), pointing to more complex role from RB neurons during locomotion. While a brief light touch can trigger fictive swimming, repetitive stimulations of the RB pathway triggers struggling, a strong motor response where activity propagates from tail to head in Xenopus and zebrafish (Soffe et al., 1991, 1997, Liao and Fetcho, 2008). Trigeminal neurons are sensory neurons with “free” nerve endings in the head of Xenopus tadpoles that respond to mechanical inputs and relay these sensory signals to downstream hindbrain circuits and in the spinal cord (Roberts, 1980, Hayes and Roberts, 1983). Interestingly, trigeminal neurons form two independent pathways in Xenopus, one glutamatergic and one GABAergic, that can differentially modulate locomotor activity. Activation of the glutamatergic pathway can entrain locomotion while the recruitment of long projecting GABAergic neurons by the second pathway was shown to stop locomotion, an effect thought to recapitulate the “stopping” behavior of young tadpoles hitting solid structures (Boothby and Roberts, 1995; Perrins et al., 2002; Buhl and al., 2015). Altogether, these studies show that the state-dependent modulation of locomotion by sensory feedback pathways is present both in mammals and lower vertebrates. In each case, inputs from sensory neurons can entrain the activity of neurons involved in rhythm and pattern generation, again highlighting the strong interaction between circuits referred to as spinal central pattern generators and sensory feedback pathways. However, the circuit architecture underlying such interactions is not fully understood and more work is required to extract the cellular and circuit mechanisms engaged in modulatory effects from sensory feedback. 15 Introduction Key concepts of part I - The modulation of spinal circuits by sensory feedback is often complex and state-dependent. - Inputs from sensory pathways can entrain or reset the phase of locomotor activity. - Sensory pathways provide direct excitation to motor neurons and project onto spinal interneurons mediating recurrent and reciprocal inhibition. - The interaction between sensory pathways and central circuits are important for muscle coordination, patterning of locomotor activity, and movement stability. - Mapping of connectivity between descending, spinal and sensory neurons is key to understand sensorimotor integration during locomotion. Transition Altogether, the work reviewed in this section led to a basic functional connectivity diagram between central, motor, and peripheral sensory neurons thought to be at the basis of motor pattern generation in the spinal cord. These studies have also revealed that individual spinal interneurons are highly wired and that the net effect of their recruitment following sensory afferent stimulation is both state and context-dependent. However, the spinal cord is a complex structure containing many types of spinal neurons and only a small fraction of spinal neurons has been investigated. In order to understand how sensory feedback shapes locomotor activity by acting on rhythmically active spinal interneurons, it remains critical to better understand the nature of central pattern generating circuits, their molecular, physiological, and anatomical properties as well as their function in rhythm and pattern generation. The part II describes current knowledge on CPG organization and function. 16 Introduction II. Anatomical, genetic, and functional organization of locomotor central pattern generators Sensory feedback closely interacts with central circuits controlling the pattern of activity and rhythm of spinal motor neurons. Thus it is important to identify the elements forming the so-called central pattern generators. In cat preparations, the identification of interneurons was mainly done after recording their responses to sensory stimuli, after mapping their anatomy and connectivity to other interneurons and/or motor neurons (Harrison et al., 1984). Since cat spinal cords are not amenable to genetics and microscopy, it was difficult to rely on specific markers of interneurons or reconstruct the morphology and projections of recorded neurons even if they were filled with dyes. The use of smaller preparations (mainly in lampreys, tadpoles, or in genetic model organisms such as rodents and zebrafish), with the development of immunohistochemistry and molecular genetics for labeling, imaging, and manipulating the fate of spinal neurons complemented our understanding of the functional organization of locomotor CPGs. II.1. Basic organization of spinal circuits controlling the pattern and rhythm of locomotion Recordings of spinal interneurons in cats, lampreys, and tadpoles allowed the identification of the basic architecture of motor circuits by revealing monosynaptic connections between excitatory and inhibitory interneurons, and motor neurons. Monitoring the activity of spinal interneurons during induced fictive locomotion has revealed the nature of spinal neurons active during rhythmic oscillations of motor neurons (Buchanan and Grillner, 1987; Grillner, 2003, Liao and Fetcho, 2008; Berkowitz et al., 2010). Data acquired in several species allowed the identification of the core central pattern generator, which is composed of: ipsilateral descending excitatory interneurons that provide rhythmic drive to motor neurons through direct monosynaptic connections; ipsilateral ascending inhibitory interneurons that also project onto motor neurons and modulate their activity as well as modulating the response to sensory circuits, and contralateral descending and ascending glutamatergic and glycinergic interneurons that regulate the alternation of activity from one side of the body to the other (Figure I.9, Buchanan and Grillner, 1988; Buchanan et al., 1989; Ohta et al., 1991; Biro et al., 2008; 17 Introduction Figure I.9. Basic organization of locomotor CPG in swimming vertebrates. Four functional classes of neurons make up the swimming central pattern generator (CPG) in lamprey (see the figure): Segmentally organized motor neurons (MNs) that innervate each adjacent axial myotome. Glycinergic commissural interneurons (CINs) project to the opposite side of the spinal cord. During swimming, inhibitory connections provide the mid-cycle inhibition that ensures that the axial muscles on each side of the body contract out of phase with those on the opposite side. Ipsilaterally-projecting inhibitory L-interneurons (IINs) that provide inhibition to motor neurons and to CINs. Their exact role in swimming has not been defined. Excitatory glutamatergic neurons (EINs) that project to all three other CPG neuron cell types. These cells, or a proportion of these cells, are rhythmically active and provide rhythmic drive to motor neurons and other CPG neurons during swimming. Excitatory commissural neurons are also present in the lamprey cord, however their function is not known. From Goulding, 2009. Table 2. Putative phylogenetic relationship between spinal cord neurons. The table below illustrates what we know about the relationship between neurons identified in the spinal cords of different species. aINs and CiA neurons seem to be equivalent to the inhibitory L- interneurons of the lamprey and V1 neurons in the mouse. In Xenopus, the dIN glutamatergic neurons seem to be the major source of ipsilateral excitatory input in the hindbrain and ventral spinal cord. dIN neurons might be homologous to CiD neurons in zebrafish, which express the Chx10 transcription factor. CiD neurons, are rhythmically active during fictive swimming and provide the main source of on-cycle excitation to the swimming central pattern generator. Glycinergic inhibitory commissural interneurons have an essential role in generating these alternating outputs between each half of the spinal cord. In Xenopus, the commissural interneurons (CINs) that mediate reciprocal inhibition have been characterized in some detail. They typically fire in phase with ipsilateral motor neurons once per swimming cycle. There are multiple anatomically, distinct populations of CINs, including CoPA, CoSA, CoLA, UCoD and CoBL cells in the zebrafish spinal cord. Although these cells are largely characterized anatomically and to a lesser extent molecularly, their functions in locomotion have not been described. CoBL and Evx2+ MCoD neurons are both active during swimming. CoBL cells are bifurcating glycinergic neurons. The excitatory commissural MCoD neurons are preferentially recruited during slow swimming movements. They seem to be necessary for slow swimming, but are dispensable for coordinating the left–right alternation of segmental motor neurons during fast swimming movements. The inhibitory CINs, excitatory interneurons (EINs) and L-interneurons in the lamprey have not been molecularly characterized. The zebrafish homologues of V3 neurons might be VeMe and UCoD cells, but they are yet to be identified. UCoD neurons are similar to commissural V3 interneurons (glutamatergic with descending axons). From Goulding, 2009. 18 Introduction Green and Soffe, 1998; Roberts et al., 1998, 2000; Li et al., 2001; Hale et al., 2001; Liao and Fetcho, 2008; Berkowitz et al., 2010, Table 2). Studies investigating the molecular and cellular basis of CNS development combined with novel genetic strategies led to the identification of specific sets of markers labelling each class of spinal neurons and allowed probing the contribution of each of these neuronal classes to the regulation of locomotion. II.2. Inductive signals control the fate of spinal neuron specification during development Spinal neurons acquire their identity during early developmental stages, soon after the neural tube formation (Jessell, 2000; Lee and Pfaff, 2001; Arber, 2012; Gouti et al., 2015). During this intense period of cellular proliferation and differentiation, concentration gradients of morphogens dictate the identity of progenitor cells along the dorsoventral axis of the developing CNS. On the ventral side, the notochord and the floor plate cells release Sonic hedgehog (Shh) while roof plate cells on the dorsal side secrete Bone Morphogenetic Proteins (BMPs). The interplay between these signaling molecules condition the expression of homeodomain proteins and other transcription factors along the dorsoventral (DV) axis (Jessell, 2000). The given concentration of each of these signaling molecules and the combinatorial expression of transcription factors gives rise to discrete progenitor domains along the DV axis from which derive each of the five populations of ventral spinal neurons, respectively termed V0, V1, V2, MN, and V3 neurons (Figure I.10, I.11, Jessell, 2000; Grillner, 2003; Goulding and Pfaff, 2005; Alaynick et al., 2011; Goulding, 2009; Arber, 2012). The generation of mutant lines targeting single or multiple transcription factors allowed specific elimination of populations of spinal neurons and probing of their contribution to the control of locomotor activity. II.3. Contribution of genetically identified spinal interneurons to locomotion II.3.a. V0 neurons V0 neurons are commissural premotor interneurons expressing the dbx1 transcription factor and can be divided in dorsal inhibitory Evx1-/Pax7+ and ventral excitatory Evx1+/Pax7- subpopulations (Moran-Rivard, 2001; Pierani, 2001; Talpalar et al., 2013; Kiehn, 2016). Genetic elimination of Dbx1+ V0 neurons is associated with altered left-right coordination during fictive locomotion in isolated spinal 19 Introduction Figure I.10. Early development of the spinal cord. Schematic cross-sections through the developing mouse spinal cord showing the patterning and specification of early spinal cord progenitors and their neuronal progeny. At embryonic day 9 (E9), a gradient of Sonic hedgehog (red) (ventrally) and Bone Morphogenetic Proteins (BMPs) and Growth Differentiation Factor 7 (GDF-7) (yellow) (dorsally) provide instructive positional signals to dividing progenitors in the ventricular zone. This leads to the restricted activation of patterning factors in discrete dorsoventral domains, including Nkx6.1 (ventral), Pax6 (intermediate), and Pax3 and Pax7 (dorsal). At E11, eleven early classes of post-mitotic neuron are present in the embryonic spinal cord. dI1-dI5 neurons that are derived from dorsal progenitors (grey) primarily contribute to sensory spinal pathways, whereas dI6, MN and V0–V3 neurons arising from intermediate/ventral progenitors (yellow) are involved in the locomotor circuitry. Some of the post-mitotic transcription factors that serve to identify each of the eleven early generic populations are indicated. From Goulding, 2009. Figure I.11 Identified spinal interneurons in the embryonic mouse and zebrafish spinal cord. Similar neuronal cell types are present in the embryonic spinal cords of aquatic and terrestrial vertebrates. The putative zebrafish homologues of V0, V1, V2 and V3 locomotor interneurons are indicated by the same colour. These include V0 and CoSA neurons (light blue), V1 and CiA neurons (dark green), V2a and CiD neurons (orange), V2b and VeLD neurons (turquoise), and V3, UCoD and VeMe neurons (red). From Goulding, 2009. 20 Introduction cord preparations (Lanuza et al., 2004). This result suggests that V0 inputs to contralateral motor neurons are critical for the timing of activation of antagonist motor units in mammals (Lanuza et al., 2004). In this study, animals died early on because V0 neurons are also present in the brainstem and control respiration (Talpalar et al., 2013). Selective ablation of spinal V0 neurons enabled Talpalar and colleagues to look at the behavioral consequence of losing either the entire V0 population or only the ventral or the dorsal subpopulation (Talpalar et al., 2013). Mice lacking spinal V0 neurons lose the ability to alternate limbs and display rabbit-like hopping locomotor patterns regardless of the speed of locomotion. In contrast, the elimination of dorsal inhibitory dorsal V0D neurons triggers hopping at slow locomotor frequencies but alternation is conserved during fast locomotion while the phenotype reverses when ventral glutamatergic V0V neurons are selectively ablated (Talpalar et al., 2013; Bellardita and Kiehn; 2015; Kiehn, 2016). These results demonstrate that the V0 population, through commissural projections, plays a critical role in setting the basic pattern of terrestrial locomotion while also regulating the speed at which alternation between the left and the right side occur (Bellardita and Kiehn, 2015). However, it remains difficult to understand how individual V0 neurons regardless of their transmitter phenotype and projections pattern shape the activity of contralateral motor neurons. Future work should investigate the connectivity of V0 neurons with descending neurons and with spinal neurons to better understand their role in speed modulation. In zebrafish V0 neurons also express Dbx1 and Evx1 (Satou et al., 2012). The V0 population is also composed of both excitatory and inhibitory commissural interneurons that can either send ascending or descending projections. Little is known of the role of zebrafish V0 neurons during locomotion except for glycinergic commissural bifurcating longitudinal (CoBL) that are rhythmically active during slow swimming but not during rapid escape responses (Liao and Fetcho, 2008) and multipolar commissural descending (MCoD) interneurons that are premotor interneurons specifically active during slow swimming (Hale et al., 2001; Ritter et al., 2001; McLean et al., 2007, 2008; Satou et al., 2012; Fidelin et al., 2015). Interestingly, MCoD neurons are de-recruited during the transition from slow to fast fictive swimming in zebrafish suggesting that they might receive inhibitory inputs by spinal neurons specifically active during fast swimming or that dedicated pathway in the hindbrain are reconfig- 21 Introduction ured in a speed- dependent manner leading to a switch of recruitment of premotor excitatory interneurons. Given the role of V1 neurons in the regulation of speed (see below), we can hypothesize that MCoDs receive inputs from V1 homologs in zebrafish. II.3.b. V1 neurons V1 spinal neurons are more homogeneous compared to V0 in the sense that they are all inhibitory and characterized by the expression of glycine and GABA (Wenner et al., 2000; Alvarez et al., 2005). P0 progenitors express Pax6 and En1 and give rise to at least three classes of V1 cells: local ipsilateral Renshaw cells (RC) mediating recurrent inhibition of motor neurons, contralateral Ia interneurons involved in reciprocal inhibition of antagonist motor pools, and unidentified V1 neurons that are neither RC or Ia cells since they are ipsilateral and lack the expression of RC specific markers such as Calbindin, Gephyrin, and nicotinic acetylcholine receptor (nAchR), (Sapir et al., 2004). A substantial proportion of V1 cells are ascending and form monosynaptic connections with motor neurons, a connectivity profile driven by Pax6 in mice (Saueressig et al., 1999). Gosgnach, Goulding and colleagues generated Pax6-/- and En1-DTA lines and found that these mutations led to selective elimination of V1 cells in the spinal cord (Gosgnach et al., 2006). During induced fictive locomotion, Pax6-/- and En1DTA preparations required elevated concentration of 5-HT to induce rhythmic oscillations of motor pools compared to wild type preparations but the overall pattern of left-right alternation and rostrocaudal propagation of activity appeared normal. In contrast Pax6-/- and En1-DTA preparations were characterized by longer cycle periods and longer durations of motor neuron bursting episodes suggesting that the absence of V1 cells is important to regulate the speed of motor pool coordination during walking. Behavioral analysis of locomotor activity in mice performing rotarod tests revealed that animals lacking V1 cells could not sustain fast locomotion (Gosgnach et al., 2006). Interestingly, the presence of left-right alternation during fictive locomotion, in normal or hemicord preparations, and the ability of mice to walk indicate that mid-cycle inhibition was preserved in En1-DTA suggesting and that a second class of ipsilateral inhibitory neurons was probably contributing to the control of flexor-extensor alternation in walking vertebrates in the absence of V1 cells. In zebrafish, En1+ spinal neurons are known as circumferential ascending (CiA) interneurons (Higashijima et al., 2004c). CiA 22 Introduction neurons are purely ascending and form monosynaptic connections with motor neurons and glutamatergic interneurons mediating the touch-evoked escape response. In addition, CiA are rhythmically active during fictive swimming suggesting that they can modulate sensory responses during swimming (Higashijima et al., 2004c). In the tapdole, ascending inhibitory neurons (aINs) are CiA homologs, and also express En1 (Li et al., 2004; Roberts et al; 2008). aINs project onto motor neurons, contralateral interneurons and, like in zebrafish, form direct connections with components of the escape circuit. Whether CiA and aINs interneurons are important for the modulation of swimming speed remains unknown. II.3.c. V2a neurons V2a neurons originate from the p2 progenitor domain, specifically express the Chx10 transcription factor, and are glutamatergic premotor interneurons (Kimura et al., 2006; Crone et al., 2008). Most V2a neurons are ipsilateral descending interneurons but a subtype possess bifurcating ascending projections (Menelaou and McLean, 2014; Azim et al., 2014a). The precise contribution of V2a neurons has been approached by different means in mice and zebrafish and seems to differ from one species to the other. Genetic ablation of V2a in mice is associated with defects in left-right coordination of motor activity and perturbs gait transitions (Crone et al., 2008; Zhong et al., 2011). This effect is possibly mediated by a lack of excitatory drive to glutamatergic interneurons projecting on the contralateral side as suggested by the pattern of V2a synaptic connectivity in mice (Crone et al., 2008). In zebrafish, hindbrain and spinal V2a neurons are critical for the initiation of locomotion (Kimura et al., 2013; Eklof-Ljunggren et al., 2014) and are recruited in a frequency-dependent manner during fictive locomotion (McLean et al., 2007, 2008). This feature is also shared by mice V2a neurons (Zhong et al., 2011). This topographic recruitment mimics the speed-dependent recruitment of motor neurons in zebrafish and suggests the existence of preferential connections between subsets of V2a and motor neurons. Indeed V2a and motor neurons form specific microcircuits modules that seem to control speed in zebrafish (Bagnall and McLean, 2014; Ampatzis and El Manira, 2014). It has been suggested that V2a neurons are interconnected but how such connectivity control locomotion remains to be determined. 23 Introduction II.3.c. V3 neurons V3 neurons are the ventral-most interneurons in the spinal cord (Alaynick et al., 2011). They are glutamatergic and derive from Nkx2.2+ progenitor cells and express the transcription factor Sim1 (Zhang et al., 2008). V3 neurons seem important to maintain the robustness of rhythmic locomotion as suggested by observations following their specific ablation (Zhang et al., 2008). Immunostainings suggest that V3 send axonal projections both on the ipsilateral and contralateral side where they make putative connections with motor neurons and glutamatergic interneurons. This suggests that there may be two functionally distinct populations of V3 neurons. Indeed, V3 neurons can be divided into different subsets based on their electrophysiological properties in mice (Borowska et al., 2009). Even though V3 interneurons seem to receive rhythmic excitation during fictive locomotion, whether they are core elements of locomotor CPG is debated and remains to be determined. In zebrafish, putative V3 counterparts are often referred to as ventro-medial (VeMe) interneurons (Bernhardt et al., 1990; Hale et al., 2001) but their role and connectivity are currently unknown. Due to the ventro-medial position of V3 neurons in the spinal cord, this population might receive descending inputs from reticulospinal, vestibulospinal, and rubrospinal tracts that run closely together, thus participating in circuits regulating balance control. Further work should address the precise role of this poorly understood population. II.3.d. Sensory-motor interactions between locomotor CPGs and sensory afferents Despite their prominent roles in controlling the rhythm and pattern of locomotion, little is currently known on the sources of modulation of each of the “V” interneuron classes during locomotion. The V1 population is, in this regard, an interesting example. Ia interneurons are rhythmically active during fictive locomotion and control motor coordination by providing inhibition to antagonist motor neurons (Pratt and Jordan, 1987; Geertsen et al., 2011). Historically, Ia were the first spinal interneurons for which a direct connection with sensory afferents was identified (Hultborn et al., 1971; Jankowska and Roberts, 1972; Baldissera et al., 1981, Jankoswka, 1992). However, less is known on connections between sensory afferents and other V1 cells. Renshaw cells, like Ia, are rhythmically active during fictive locomotion (Nishimaru et al., 2006) and share the same function in cats and mice, they are activated by motor neurons and form a direct negative feedback loop to the same motor neurons during 24 Introduction locomotion (Renshaw, 1941, 1946; Eccles, 1954; Alvarez and Fyffe, 2007). While they do not seem to receive inputs from sensory afferents in cats, recent evidence indicates that this is not the case at embryonic and postnatal stages in mice (Mentis et al., 2006; Alvarez and Fyffe, 2007; Bikoff et al., 2016). Using an impressive array of techniques including fluorescent cell sorting, DNA microarray, electrophysiology and retrograde labelling, Bikoff and colleagues dissected the organization of V1 cells in mice and identified clades of V1 cells based on their combinatorial expression of transcription factors and position in the spinal cord (Bikoff et al., 2016). They investigated the putative connections between sensory afferent neurons and V1 groups and found that Sp8+ expressing V1 spinal neurons (V1Sp8) cells, like V1 Renshaw (V1R), received direct monosynaptic inputs from sensory afferent neurons (Figure I.12). The role of these sensorimotor interaction is currently unknown. Given the wide connectivity of RC onto other RC, Ia interneurons, motor neurons and possibly other V1 cells, it is possible that the activation of V1Sp8 and V1R by sensory feedback circuits helps regulating the balance between the activation of Ia contralateral interneurons and the inhibition of agonist motor neurons. Given the putative contribution of V1 cells in speed control, such an interaction could also help regulate the global excitability of motor circuits at all speed. In addition, the work of Bikoff and al. suggests that the pattern of connection between sensory afferents and V1 clades is joint-specific implying a regulation of the excitability of specific pools of motor neurons by distinct groups of V1 cells (Figure I.12). Among V0 neurons, a small subset was shown to co-express the Pitx2 transcription factor and cholinergic markers (V0C neurons, Zagoraiou et al., 2009). Interestingly, these neurons are rhythmically active during fictive locomotion and seem to receive inputs from Vglut1+ sensory processes indicating that could be directly modulated by sensory pathways. Their ablation alters the activity of motor neurons during swimming but not during walking suggesting that sensory inputs to V0C inputs are important to strengthen motor neurons firing in a task-dependent manner (Zagoraiou et al., 2009). Eliminating sensory feedback was also shown to affect the coordination of muscle activity during swimming specifically (Akay et al., 2014; Takeoka et al., 2014). Taken together these studies demonstrate that sensory feedback pathways project onto elements of spinal central pattern generators and suggest 25 Introduction that these interactions are critical for the execution of specific locomotor behaviors. To date it is not known if V2, V3 and non-cholinergic V0 neurons receive direct inputs from proprioceptors. Figure I.12. V1R and V1Sp8 microcircuits operating on hip, ankle, and foot motor neurons. The solid and dotted lines represent prevalent and sparse synaptic connectivity. From Bikoff et al., 2016. Key concepts of part II - Locomotor central pattern generating circuits are composed of at least four classes of spinal interneurons. - Each class of “V” cells is highly heterogeneous. - Inputs from CPG neurons onto motor neurons are critical to set the rhythm and the pattern of locomotion. - In zebrafish, CPG neurons project onto spinal motor neurons and have the ability to modulate sensory transmission. - Little is known on the connectivity between proprioceptors and most of “V” spinal interneurons. 26 Introduction Transition In the last two parts, we have seen that inputs from sensory feedback circuits are important for regulating muscle coordination during rhythmic movements and that sensory neurons can target several types of spinal neurons including premotor interneurons and motor neurons. The genetic dissection of spinal interneurons driving rhythmic movements, notably in zebrafish, offers the possibility to probe the effect of sensory feedback during ongoing oscillatory activity in the spinal cord. Based on the role of rhythmic spinal interneurons, we hypothesize that inputs from sensory feedback can differentially affect locomotion in zebrafish depending on the state of activation of rhythmic spinal interneurons and can potentially affect the frequency and pattern of locomotor activity. To study this question, we focused our attention on a population of conserved intraspinal sensory neurons termed cerebrospinal fluid-contacting neurons, which are thought to be modulators of locomotion but for which the connectivity and function is poorly understood. 27 Introduction III. Cerebrospinal fluid contacting neurons are polymodal sensory neurons in the ventral spinal cord III.1. Identification and characterization of sensory neurons lining the central canal in the spinal cord Spinal somatosensory neurons are usually found in the dorsal side of the cord where they receive and relay inputs from cutaneous and nociceptive pathways (Caspary and Anderson, 2003). Almost a century ago, Kolmer and Agduhr identified and gave their names to neurons surrounding the central canal in the ventral spinal cord of about 200 vertebrate species; these cells are referred to as Kolmer-Agduhr (KA) neurons or CSF-contacting neurons (CSF-cNs, Kolmer, 1921; Agduhr, 1922; Kolmer, 1931; Vigh and Vigh-Teichmann, 1998; Figure I.13). CSF-cNs are intermingled with ependymal and subependymal cells around the central canal in what is often referred to as a neurogenic niche because some of these ependymal neurons express markers of undifferentiated neural stem cells (Reali et al., 2011). The location and anatomy of CSF-cNs led to the idea that they might sense the content of the CSF. Interestingly, CSF-cNs are also present in other structures of the central nervous system including in the subcommissural organ of the third ventricle, the hypophysis, the pineal organ and the paraventricular organ among others, suggesting that CSF-cNs might be involved in relaying information relative to circadian rhythms or hormonal responses. As a matter of fact, factors such as hormone binding proteins, melatonin, and gonadotropin releasing hormones are transported in the CSF and could act as signal molecules for the activation of CSF-cNs. Based on their initial observations, Kolmer and Agduhr noted that all spinal CSF-cNs had an apical “bulbous” extension that could be seen within the central canal lumen. Later on, Vigh and VighTeichmann used electron microscopy (EM) on spinal cord slices to analyze the sensory morphology of CSF-cNs from different regions in the CNS and in different species (Vigh and Vigh-Teichmann, 1973). In particular, they studied the organization of the apical extension (or bud) of these cells and found that they were dendritic extensions composed of microvilli, sometimes with a primary 28 Introduction Figure I.13. Cerebrospinal fluid contacting neurons. Top panels, morphology and location of CSF-cNs in different species. Bottom panels, ascending projections of an isolated CSF-cN in zebrafish. GABAergic phenotype of CSF-cNs in zebrafish. Adapted from Wyart et al., 2009 and Vigh and Vigh-Teichmann, 1998. cilium (motile or not). EM experiments also revealed the presence of dense vesicles in the apical extension of CSF-cNs suggesting that these cells might also release compounds in the central canal (Vigh et al., 1977, 1983). CSF-cNs have basal projections originating from the cell body, projections that were clearly identified as ascending in Xenopus and zebrafish (Dale et al., 1987; Wyart et al., 2009; Figure I.13). In lamprey, CSF-cNs send ventrolateral projections that can reach mechanosensory edge cells while axons of CSFcNs in rats remain more ventro-medial where they potentially reach and modulate corticospinal tract descending projections (Brodin et al., 1990; Christenson et al., 1991; Stoeckel et al., 2003, Jalavand et al., 2014a). In other species such as in cats and macaques, CSF-cNs’ axons can project both ventromedially and ventro-laterally (LaMotte, 1987). 29 Introduction Despite an intense anatomical characterization, little is known about the role of CSF-cNs. The nature of their putative interactions with central circuits, and their anatomy could only lead to speculation. What was clear from these observations is that CSF-cNs are found in virtually all vertebrate species, represent a unique bridge between the CSF and neuronal circuits, and share the same anatomical features, suggesting that their function might be conserved across species. III.2. Molecular analysis of CSF-cNs across species reveals specific markers and a double developmental origin In all species were it has been investigated, CSF-cNs were found to express GABA or proteins involved in the synthesis of GABA and its transport such as GAD65/67 and VGAT (Brodin et al., 1990; Christenson et al., 1991; Stoeckel et al., 2003, Wyart et al., 2009; Djenoune et al., 2014) . In lampreys, subpopulations of CSF-cNs were shown to co-express GABA and glutamate or GABA and glycine suggesting that CSF-cNs may co-release several neurotransmitters or that these cells could be divided into multiple functional subpopulations (Fernandez-Lopez et al., 2012). In addition, subsets of ventral CSF-cNs are known to express catecholamines in salamander, garfish, and quail (Sims, 1977; Parent and Northcutt, 1982; Guglielmone and Panzica, 1985), tyrosine hydroxylase in Xenopus and chick (Heathcote and Chen, 1993; Wallace et al., 1987), dopamine in lamprey, pigeon, chameleon, and ray (Barreiro-Iglesias et al., 2008; Roberts and Meredith, 1987; Bennis et al., 1990; Acerbo et al., 2003), serotonin in salamander, lamprey, hagfish, and spotted gar (Sims, 1977; Ochi et al., 1979; Parent and Northcutt, 1982; Chiba and Oka, 1999), neuropeptides such as somatostatin and urotensin II in lamprey, salmon, and zebrafish (Buchanan et al., 1987, Christenson et al., 1991; Yulis and Lederis, 1988; Wyart et al., 2009) or VIP in rats and macaque monkeys (LaMotte, 1987). CSF-cNs can be divided into two classes based on their orientation and relative to their position around the central canal: the ventral CSF-cNs whose cell bodies are ventral to the central canal, and the dorsolateral cells with cell bodies either at the level of, or dorsal to the central canal (Djenoune et al., 2014). Indeed, these two groups of CSF-cNs originate from two distinct progenitor domains during development in zebrafish (Djenoune et al., 2014). 30 Introduction Figure. I.14. Summary of the development of spinal CSF-cNs in mice. (A) Schematic of E10-E12 ventral spinal cord showing progenitor domains and early-born neurons. Defined domains of progenitors in the dorsoventral axis of the neural tube give rise to motoneurons (MN) and V0-V3 interneurons. The transcription factor Foxn4, expressed in p2 progenitors, is essential for the specification of early-born Gata2+Gata3+ V2b interneurons. (B,B’) Schematic of E14-E16 ventral spinal cord showing differentiation of Gata2 +Gata3+ CSFcNs along with astrocytes and oligodendrocyte progenitor cells. CSF-cN’ cells are born from late Nkx6-1+Pax6+ progenitors in the p2 and the dorsal half of the pOL domains. CSF-cN’’cells arise from Nkx2-2+Foxa2+ p3 cells in the frontier with the floor plate (fp). The transcription factor Pax6 selectively controls the development of the CSF-cN’ subset. B’ shows the transcription factor identity of CSF-cN progenitors. (C) In the postnatal spinal cord, CSFcN’ and CSF-cN’’ cells are organized lateral and ventral to the central canal (cc), respectively. From Petracca et al., 2016. The dorsal layer of CSF-cNs originates from the pMN domain and is characterized by the expression of the transcription factor olig2 whereas the ventral layer originates from the V3 domain and express the transcription factor nkx2.2 (Djenoune et al., 2014). Interestingly, the developmental origin is different in mice with the majority of CSF-cNs being dorso-lateral to the central canal and emerging from the p2 progenitor domain (Petracca et al., 2016; Figure I.14). Whether these populations of CSF-cNs represent two functionally distinct groups remains to be characterized. 31 Introduction III.3. Sensory modalities underlying the recruitment of CSF-cNs In mouse, spinal and brainstem CSF-cNs express the non-selective cationic Transient Receptor Potential (TRP) channel Polycystic Kidney Disease 2-Like 1 (Pkd2l1, Huang et al., 2006). Pkd2l1+ CSFcNs are activated by local pH decrease in mice (Huang et al., 2006; Orts-Del’Immagine et al., 2012, 2016), a feature also found in CSF-cNs in lampreys (Jalalvand et al., 2016a, 2016b). This acidic response seems to be carried by ASIC channels (Del’Immagine et al., 2012; Jalalvand et al., 2016a). In addition, Pkd2l1 seems to drive a response to alkylation both in mice and lampreys (OrtsDel’Immagine et al., 2012; Jalalvand et al., 2016b). In each case, the decrease or increase in pH leads to an increase in the firing frequency of CSF-cNs. Work from our lab showed that pkd2l1 expression in spinal CSF-cNs is conversed in macaque and zebrafish and is maintained in adulthood, suggesting that the ability of CSF-cNs to sense pH variations in their environment may be conserved in these species (Djenoune et al., 2014). TRP channels form a family of polymodal receptors that can sense chemo-, thermo- and mechanical stimuli in vivo (Delmas, 2005; Ramsey et al., 2006). Given the sensory nature of spinal PKD2L1+ CSF-cNs, our lab recently showed that these cells could respond to mechanical inputs in larval zebrafish in vivo (Böhm, Prendergast et al., 2016). This study revealed that CSF-cNs are recruited by active bending during evoked acoustic escape responses and that activation of CSF-cNs could be recapitulated in paralyzed larvae by applying mechanical stimulation to the immobilized tail (Böhm, Prendergast et al., 2016, Figure I.15). Interestingly, the response was abolished in pkd2l1-/- mutants, suggesting that pkd2l1 drives the mechanosensory activation of spinal CSF-cNs in zebrafish (Figure I.16). In lamprey, CSF-cNs respond to fluid movements in spinal cord open book preparations and the response is blocked by ASIC channel blockers (Jalalvand et al., 2016a). These studies revealed that CSF-cNs are recruited by distinct sensory cues in vivo. Whether the mechanical response to body movement is conserved across vertebrate species remains to be addressed but the results obtained in zebrafish and lamprey open exciting possibilities to identify the role of these cells and how their activation might modulate spinal circuits underlying locomotion. 32 Introduction Figure I.15. CSF-cNs respond to active muscle contraction as well as to passive mechanical bending of the spinal cord. (a) Schematic describing 2-photon imaging experiments used to record simultaneously from CSF-cNs expressing tagRFP (magenta) and GCaMP5 (green) in head-embedded Tg(pkd2l1:GCaMP5, pkd2l1:tagRFP) larvae. Infrared illumination combined with high-speed video recording shows unidirectional tail deflections induced by a water jet to the otic vesicle. Sample traces for DF/F of tagRFP and GCaMP shown with tail deflection during escape (note: vertical scale is 10 times larger for GCaMP compared to tagRFP signals). Subtracting the tagRFP signal from the GCaMP signal removed motion artifacts; breaks in the trace arise from frames when cells escaped from the focal plane. Scale bar: 10 mm. (b) Quantification of calcium transient amplitude in response to muscle contraction (n = 11 larvae) in dorsal CSF-cNs either ipsilateral (red, 31 cells) or contralateral (yellow, 19 cells) or ventral (purple, 44 cells). Only dorsal ipsilateral cells exhibited responses greater than baseline (P = 9.51x10 -4) and all other cell types responded significantly less than dorsal ipsilateral cells (dorsal contralateral: P = 2.43x10 -3, ventral: P = 4.85x10-5). (c) Passive mechanical stimulation of CSF-cNs in paralyzed larvae (n = 5) was implemented with mechanical pressure exerted by pushing a glass probe laterally against the fish tail. Scale bar: 50 mm. (d) Response of proximal (< 100 mm) and distal (4100 mm) CSF-cNs. Inset: average calcium response of proximal (red) versus distal (blue) CSF-cNs. (e) Response of dorsal ipsilateral (red) CSF-cNs as function of distance from the probe. (f) Response of dorsal ipsilateral (red, 28 cells), dorsal contralateral (yellow, 16 cells) and ventral (purple, 36 cells) CSF-cNs relative to the location of mechanical stimulation. Inset: Average calcium response of dorsal ipsilateral versus dorsal contralateral and ventral CSF-cNs. All cell types show a response different from 0 (dorsal ipsilateral: P < 1.0x10 -8, dorsal contralateral: P = 5.95x10-5, ventral: P < 1.0x10-8) and all other cell types responded significantly less than dorsal ipsilateral cells (dorsal contralateral: P = 1.06.10 -3, ventral: P = 7.22x10-3). From Böhm, Prendergast et al., 2016. 33 Introduction III.4. Experimental strategies and optogenetic approaches to unravel the function of CSF-cNs The development of enhancer trap lines in zebrafish enabled researchers to test the function of CSFcNs in vivo for the first time (Scott et al., 2007; Wyart et al., 2009). By generating random insertion of the gal4 gene downstream of enhancer or gene promoter regions, a genetic strategy borrowed from Drosophila, the authors could create transgenic lines sometimes labelling only one or a few cell types in the spinal cord while expressing any reporter gene of interest using UAS sequences (Brand and Perrimon, 1993; Halpern et al., 2008). The work from Scott and colleagues led to the generation of a transgenic line specifically labelling CSF-cNs (Scott et al., 2007; Wyart et al., 2009). It was therefore possible to activate these cells by expressing light-gated channels such as LiGluR or ChR2 (Szobota et al., 2007; Wyart et al., 2009; Fidelin et al., 2015). Wyart and colleagues reported that activating a subset of CSF-cNs in head embedded zebrafish larvae at rest, i.e. when the animal is not engaged in locomotor behaviors, could trigger slow swimming (Wyart et al., 2009). At the time, these experiments did not permit the authors to investigate the targets of CSF-cNs in the spinal cord. In addition, the use of LiGluR required incubating animals with its MAG-1 co-factor, a glutamate analog, and it was unclear how this compound could affect the properties of spinal neurons and as a consequence the effect of CSF-cNs activation in vivo. However, this result is critical because it indicates that inputs from CSF-cNs can modulate locomotor activity in vivo. 34 Introduction Figure I.16. Mutation of pkd2l1 abolishes CSF-cN response to active and passive spinal bending. (a) The TALEN-mediated 8 nucleotide deletion in exon two of pkd2l1 leads to a frame shift and a premature stop in pkd2l1icm02. (b, c) Calcium transients in dorsal ipsilateral CSF-cNs are abolished in pkd2l1 mutant after mechanical stimulation during active (b) and passive (c) tail movement (same stimulation paradigms as used in Figure I.15). Mutants (10 fish, 29 cells) in b show no response different from 0 (P = 0.13) and are different from wildtypes (11 fish, 31 cells, P = 1.84x10 -2). Mutants (7 fish, 30 cells) in c show no response different from 0 (P = 0.22) and are different from wildtypes (5 fish, 28 cells, P = 2.79x10 -4). From Böhm, Prendergast et al., 2016. Key concepts of part III - CSF-cNs are conserved polymodal sensory neurons in the spinal cord of vertebrates. - CSF-cNs are recruited by tail contraction during movement in zebrafish. - CSF-cNs can modulate slow locomotion. - The connectivity of CSF-cNs within spinal circuits and the underlying mechanisms of circuit modulation are unknown. 35 Introduction IV. Aims of the thesis We have seen that CSF-cNs are unique sensory neurons in the ventral spinal cord that can sense axial bending of zebrafish larvae during locomotion and modulate the excitability of spinal circuits in return. These observations suggest that CSF-cNs form a novel yet poorly characterized proprioceptive pathway able to provide feedback to spinal circuits during locomotion in swimming vertebrates. However, the connectivity of these sensory neurons as well as the cellular mechanisms by which these cells can modulate locomotor activity remains unknown. The work presented here will aim to: 1- unravel the connectivity diagram of CSF-cNs in the zebrafish spinal cord. 2- model and test the effect of their recruitment during locomotion at the cellular and circuit level. 3- understand if the dynamics of CSF-cNs are circuit- and context-dependent. 36 Chapter 1: connectivity of CSF-cNs Chapter 1 - Functional connectivity mapping between CSF-cNs and spinal premotor neurons controlling slow swimming Predictions regarding the connectivity of CSF-cNs The observation that slow swimming could be triggered following the optogenetic activation of CSFcNs at rest brought several important insights regarding their putative diagram of connectivity and function in the spinal cord. The first possibility is that CSF-cNs modulate the activity of spinal motor neurons. While motor neurons driving slow swimming display burst firing properties during fictive swimming or when they are depolarized during current injections experiments, these cells are not able to maintain this oscillatory activity by themselves, suggesting that motor neurons need constant excitatory drive (McLean et al., 2007, Menelaou and McLean, 2012). In their pilot optogenetic experiments, Wyart and colleagues found that the tail of larval zebrafish was beating for several hundreds milliseconds, long after the end of the 50 ms UV light pulse, indicating that the effect was probably not mediated by the direct activation of motor neurons (Wyart et al., 2009). Instead, we hypothesized that inputs from CSF-cNs have an effect on spinal interneurons that can drive rhythmic locomotion, notably those who directly project onto motor neurons. Whether CSF-cNs could directly act on these premotor interneurons or upstream of this pathway remained unknown at that point. Building the experimental paradigm Understanding how CSF-cNs modulate locomotor activity relies on the following steps: 1- Identify the physiological inputs driving the recruitment of CSF-cNs, their cellular and developmental properties (addressed in Huang et al., 2006; Ort’s del Imagine 2012 & 2016, Böhm, Prendergast et al., 2016, Jalalvand et al., 2016a & 2016b, see the third part of the introduction), 2- Map the projections and functional connectivity of CSF-cNs in the spinal cord (addressed here), 3- Probe the effect of their recruitment during different states and different locomotor behaviors (addressed here and in Böhm, Prendergast et al., 2016). 37 Chapter 1: connectivity of CSF-cNs The work presented here reveals for the first time the identity of neurons modulated by CSF-cNs and point to mechanisms deployed by these sensory neurons to tune the excitability and recruitment of motor circuits in the spinal cord. To do so, we employed a large combination of techniques and approaches including the generation of transgenic lines, imaging, patch clamp and extracellular electrophysiology, pharmacology, optogenetic activation and genetic silencing strategies, and behavior analysis. Highlights of the findings described in this chapter - Spinal CSF-cNs project onto glutamatergic premotor interneurons - CSF-cNs exert a state-dependent modulation of spinal premotor circuits - CSF-cNs modulate the duration and frequency of locomotor events - CSF-cNs gate rostrocaudal propagation of excitation in the spinal cord Graphical abstract of the results 38 Chapter 1: connectivity of CSF-cNs Published article State-Dependent Modulation of Locomotion by GABAergic Spinal Sensory Neurons Current Biology, 25(23):3035-47, doi:10.1016/j.cub.2015.09.070 Kevin Fidelin,1,2,3,4 Lydia Djenoune,1,2,3,4,5 Caleb Stokes,1,2,3,4 Andrew Prendergast,1,2,3,4 Johanna Gomez,1,2,3,4 Audrey Baradel,1,2,3,4 Filippo Del Bene,4,6 and Claire Wyart1,2,3,4,* 1 Institut du Cerveau et de la Moelle épinière (ICM), 75013 Paris, France, 2INSERM UMRS 1127, 75013 Paris, France, 3CNRS UMR 7225, 75013 Paris, France, 4UPMC Univ. Paris 06, 75005 Paris, France, 5Museum National d’Histoire Naturelle, 75005 Paris, France, 6Institut Curie, CNRS UMR 3215, INSERM U934, 75005 Paris, France, *Correspondence: [email protected] Summary The cerebrospinal fluid (CSF) constitutes an interface through which chemical cues can reach and modulate the activity of neurons located at the epithelial boundary within the entire nervous system. Here, we investigate the role and functional connectivity of a class of GABAergic sensory neurons contacting the CSF in the vertebrate spinal cord and referred to as CSF-cNs. The remote activation of CSF-cNs was shown to trigger delayed slow locomotion in the zebrafish larva, suggesting that these cells modulate components of locomotor central pattern generators (CPGs). Combining anatomy, electrophysiology, and optogenetics in vivo, we show that CSF-cNs form active GABAergic synapses onto V0-v glutamatergic interneurons, an essential component of locomotor CPGs. We confirmed that activating CSF-cNs at rest induced delayed slow locomotion in the fictive preparation. In contrast, the activation of CSF-cNs promptly inhibited ongoing slow locomotion. Moreover, selective activation of rostral CSF-cNs during ongoing activity disrupted rostrocaudal propagation of descending excitation along the spinal cord, indicating that CSF-cNs primarily act at the premotor level. Altogether, our results demonstrate how a spinal GABAergic sensory neuron can tune the excitability of locomotor CPGs in a state-dependent manner by projecting onto essential components of the excitatory premotor pool. 39 Chapter 1: connectivity of CSF-cNs Introduction During active locomotion, sensory afferent neurons provide excitatory feedback to motor neurons and spinal interneurons in response to muscle contraction. Local GABAergic interneurons can modulate this pathway by inhibiting sensory afferents at the presynaptic level [1, 2]. Genetic targeting and manipulation of these GABAergic interneurons recently demonstrated the importance of presynaptic modulation of sensory afferents to control fine motor behaviors in mice [3, 4]. Although GABAergic modulation is essential for controlling excitability and spike timing of excitatory neurons throughout the nervous system [5, 6], little is known about the GABAergic modulation of descending premotor excitatory interneurons controlling rhythm and pattern generation in the spinal cord. Pharmacological manipulations showed that GABAergic neurons could modulate the burst frequency of motor neurons during fictive locomotion [7, 8], suggesting that the release of GABA can control the excitability of spinal excitatory interneurons driving fictive locomotion. Yet, the nature of GABAergic neurons mediating this effect and their targets in the spinal cord remain to be identified. Almost a century ago, Kolmer and Agduhr identified cerebrospinal fluid-contacting neurons (CSFcNs), also called KA cells in African clawed frog and zebrafish, as villiated neurons surrounding the central canal in the spinal cord of over 200 vertebrate species [9–11]. Spinal CSF-cNs are sensory neurons with unique features because they are GABAergic, intraspinal, and reside in the ventral part of the spinal cord. As CSF-cNs exhibit longitudinal axons projecting in the ventral cord, they might relay chemical information from the cerebrospinal fluid (CSF) to spinal circuits [12–14]. Despite the conservation of CSF-cNs among vertebrates, their role in sensorimotor integration is still poorly understood. Remote activation of CSF-cNs was shown to trigger delayed slow locomotor activity in headrestrained zebrafish larvae, indicating that CSF-cNs could project onto components of the slow swimming central pattern generator (CPG) [14]. However, the nature of postsynaptic targets of CSF-cNs within the slow locomotor CPG remains to be identified. 40 Chapter 1: connectivity of CSF-cNs Here, we took advantage of the zebrafish larva to optically probe the cellular and circuit mechanisms deployed by GABAergic CSF-cNs neurons to modulate locomotor activity in an intact animal. We generated a specific line to analyze the morphology of CSF-cNs, manipulate their activity, and map their functional connectivity onto locomotor CPGs. We performed channelrhodopsin-2 (ChR2)mediated activation of CSF-cNs in combination with whole-cell recordings of their targets to demonstrate that CSF-cNs form GABAergic synapses onto dbx1+/evx1+ glutamatergic commissural descending V0-v interneurons. These interneurons are essential components of the locomotor CPG in multiple vertebrate species [15–19] and are selectively active during slow locomotion in zebrafish [17–19]. We further dissected the modulatory role of CSF-cNs onto the slow CPG during specific network states. We confirmed that activating CSF-cNs induced delayed slow locomotor activity at rest. In contrast, the activation of CSF-cNs during ongoing locomotor activity inhibited slow locomotion, reducing the duration and the frequency of locomotor events. Moreover, selective activation of rostral CSF-cNs during ongoing activity led to the silencing of activity along the full length of the spinal cord, indicating that CSF-cNs primarily inhibit descending interneurons. Altogether, our results demonstrate that a single type of conserved GABAergic sensory neuron can tune the excitability of the locomotor CPG in a state-dependent manner, by modulating key excitatory premotor interneurons. Results CSF-cNs are local, ipsilateral, and ascending GABAergic sensory neurons that innervate the ventrolateral spinal cord CSF-cNs have been recently shown to selectively express the transient receptor potential channel (TRP) polycystic kidney disease 2-like 1 (Pkd2l1) [20–22]. To analyze the functional morphology underlying the connectivity of CSF-cNs, we cloned the pkd2l1 promoter and generated a specific Gal4 line (Figure 1). Fluorescent in situ hybridization against pkd2l1 revealed that the Tg(pkd2l1:gal4) line recapitulates the endogenous pkd2l1 expression profile at 3 days post fertilization (dpf) (Figure S1). In Tg(pkd2l1:gal4;UAS:ChR2-mCh) double transgenic larvae, we obtained selective expression of Channelrhodopsin-2 (ChR2) in CSF-cNs throughout the entire spinal cord (Figure 1A1). CSF-cNs are 41 Chapter 1: connectivity of CSF-cNs characterized by an apical villiated extension contacting the central canal and an elongated soma (Figures 1A2 and 1C) [23]. We found that these cells had rostral directed projections chiefly restricted to the ventrolateral spinal cord (Figures 1A2, 1A3, 1C, 1D, and 1F). We confirmed the GABAergic nature of pkd2l1+ CSF-cNs [12, 13, 21, 24] by quantifying the overlap between glutamic acid decarboxylase (Gad) expression and Pkd2l1 in Tg(pkd2l1:gal4;UAS:ChR2-mCh;gad1b:GFP) larvae (Figure 1B; 99% of mCherry+ CSF-cNs were GFP+, n = 504 cells in 5 larvae). We used sparse genetic labeling (see Supplemental Experimental Procedures) to determine the precise projection patterns of CSF-cNs axons. At 3 dpf, all CSF-cNs axons were ascending, ipsilateral, and produced local projections reaching from two to six segments away from the cell body (Figures 1C–1E; n = 88 cells in 62 larvae, mean segment projection length = 3.8 ± 0.8 segments). Regarding the extent of projections within the dorsoventral (D-V) axis, we observed that the axons of CSF-cNs mainly ran in the ventral spinal cord (Figures 1C and 1F; mean D-V axon position = 0.32 ± 0.09 where the ventral limit is 0 and the dorsal limit is 1). To map synaptic sites along the CSF-cN axons, we drove expression of Synaptophysin-GFP to visualize putative presynaptic boutons [25]. Boutons were identified as large and stable GFP+ clusters, while small Synaptophysin-GFP-containing vesicles were dim and highly mobile (Figure 1G; Movie S1). The majority of putative synaptic sites was confined in a ventral domain between 0.2 and 0.4 on the D-V axis (Figure 1I; 60% of boutons, see the black portion of the distribution), although presynaptic boutons were observed throughout the entire CSF-cNs D-V axonal projection domain (0–0.6 on the D-V axis; Figures 1H and 1I; n = 1,566 boutons from 36 cells in 27 larvae, mean bouton D-V position = 0.293 ± 0.128). This observation suggests that the primary targets of CSF-cN axons lie within the ventral spinal cord. CSF-cNs form GABAergic synapses onto premotor V0-v glutamatergic interneurons The cell bodies of a subset of glutamatergic V0-v interneurons referred to as multipolar commissural descending interneurons (MCoDs) in larval zebrafish precisely reside in the ventrolateral spinal cord, confined between 0.2 and 0.4 on the D-V axis [17, 19, 26, 27]. To test whether CSF-cNs project onto glutamatergic V0-v interneurons, we took advantage of the Tg(vglut2a:lox:DsRed:lox:GFP) transgen42 Chapter 1: connectivity of CSF-cNs ic line labeling most of the glutamatergic neurons in the spinal cord [28, 29]. In this line, we identified the large soma of DsRed+ ventrolateral excitatory interneurons at the interface of the lateral neuropil and the cluster of spinal interneurons (Figures 2A1 and 2A2). In Tg(pkd2l1:gal4;UAS:ChR2YFP;vglut2a:lox:DsRed:lox:GFP) larvae, axonal projections of CSF-cNs formed numerous boutons apposed onto the somata of vglut2a+ ventrolateral interneurons (top and bottom panels in Figures 2A1, 2A2, and 2B), suggesting that CSF-cNs synapse onto these cells. Moreover, we observed that single CSF-cN axons could project onto multiple ventrolateral vglut2a+ interneurons (Figure 2B, arrowheads). We performed targeted whole-cell recordings to measure the electrophysiological properties of ventrolateral vglut2a+ interneurons with Alexa 647 dye in the recording pipette to reconstruct their morphology (Figure 2C). The morphology and firing patterns of excitatory ventrolateral vglut2a + interneurons were similar to those described previously for glutamatergic V0-v interneurons known as MCoDs in zebrafish (Figures 2D–2G) [17, 26, 27, 30, 31]. Post hoc reconstructions of dye-filled cells demonstrated two dendrites symmetrically located on each side of the soma (Figures 2D and 2E; n = 12 cells) and multiple dendritic ramifications (Figures 2D and 2E). Analysis of presynaptic boutons made by pkd2l1-expressing cells onto dye-filled V0-v interneurons in Tg(pkd2l1:Gal4;UAS:ChR2YFP) larvae revealed dual innervation of soma and dendrites by the axons of CSF-cNs (Figure 2D, arrowheads). We made simultaneous whole-cell current clamp recordings of vglut2a+ V0-v interneurons while monitoring pooled motor output from ventral nerve root (VNR) recordings made from nearby body segments. These dual recordings showed that V0-v cells are rhythmically active during episodes of spontaneous fictive slow locomotion (15–30 Hz, Figure 2F, n = 5). In zebrafish, interneurons active during slow swimming typically exhibit input resistance greater than 400 MU [19]. We found that vglut2a + V0-v cells exhibited high input resistance (641 ± 52 MU, n = 8 cells) and that action potentials could be elicited with small depolarizing currents (Figure 2G; mean threshold current = 15.4 ± 8.3 pA, n = 43 Chapter 1: connectivity of CSF-cNs 12 cells), suggesting that these cells are highly excitable and recruited with relatively small levels of excitatory drive [17, 19]. To test whether CSF-cNs formed functional monosynaptic connections onto vglut2a+ V0-v interneurons, we elicited single spikes in ChR2-expressing CSF-cNs using brief optical activation while recording inhibitory currents in nearby vglut2a+ V0-v interneurons (Figure 3A1). Pulses of 5 ms blue (460 nm) light reliably elicited single spikes in CSF-cNs (Figure 3A2; spike delay = 4.86 ± 0.50 ms, n = 141 stimulations in 4 cells). Following single light pulses, we observed GABAergic-mediated inhibitory postsynaptic currents (IPSCs) in V0-v interneurons after a short delay (Figures 3B, 3B1, and 3C; IPSC delay = 4.94 ± 2.02 ms, time to peak = 1.58 ± 0.65 ms, n = 8 cells) and a decay time consistent with the deactivation of GABAA receptors (IPSC time decay = 25 ± 10.2 ms; [32]). Intriguingly, we found that the probability of observing an IPSC in response to each light pulse was initially low (Figure 3C; response probability = 0.18 ± 0.04, n = 8 cells) but increased during 500-ms train stimulations at 25 Hz to reach up to 0.5 (Figures 3B2 and 3E; n = 5 cells). The probability of eliciting lightevoked IPSCs increased both during a train (Figures 3D and 3E; p < 0.001, n = 5 cells) and across trains over the time course of the experiment (Figures 3D and 3F; p < 0.001, n = 5 cells), suggesting that individual CSF-cN presynaptic terminals may have a low release probability that is overcome with repeated activation. ChR2-induced IPSCs were blocked upon bath application of gabazine (Figure 3G; n = 3 cells), indicating that CSF-cNs form GABAA-mediated synapses onto V0-v interneurons. Together, these results indicate that CSF-cNs form active GABAergic synapses onto glutamatergic descending V0-v interneurons that are gradually recruited with repetitive stimulations. CSF-cNs exert a state-dependent modulation of the slow locomotor CPG Knowing that CSF-cNs project onto premotor excitatory interneurons specifically active during slow locomotion, we tested the effects of activating CSF-cNs on locomotor activity. We carefully restrained illumination to the spinal cord (see Experimental Procedures) and used a long light pulse (500 ms; Figures 4A and 4B) to elicit burst spiking in ChR2-expressing CSF-cNs (response delay = 7.9 ± 5.8 ms, 11.9 ± 4.3 spikes per light pulse, firing frequency = 23.8 ± 8.7 Hz, burst duration = 491.5 ± 10.6 44 Chapter 1: connectivity of CSF-cNs ms, n = 2 cells). In this configuration, blue light stimulation did not trigger locomotor activity in ChR2- control siblings (Figure 4C, top trace; Figure 4D; response rate = 3.6% ± 1.6%, n = 586 stimulations in 10 larvae). In contrast, there was a significantly higher response rate following the light pulse in ChR2+ larvae (Figure 4C, middle trace, and Figure 4D; response rate = 19.96%, n = 759 stimulations in 14 larvae, p < 0.05). Among the 14 ChR2+ larvae tested, we observed that blue light pulses did not induce locomotor activity in six animals (Figure 4C, bottom trace; response rate = 1.8% ± 0.8%). In 8 out of 14 ChR2+ larvae, the activation of CSF-cNs reliably triggered slow fictive swimming after a delay of 465 ± 55 ms (Figure 4C, middle trace; response rate = 33.6% ± 8.4%). Such a long delay suggested that locomotor responses followed an initial period of inhibition. We tested whether the induced locomotor response was GABA mediated on a larva with a high baseline response rate. Bath application of the GABAA receptor antagonist gabazine led to a reduction of the response rate from 0.79 to 0.16, suggesting that the rebound swimming could rely on the activation of GABAA receptors in this animal (Figure 4E). We hypothesized that the heterogeneity of the responses observed across ChR2+ larvae could be due to variations in the intrinsic excitability of the spinal locomotor circuit across fish. The excitability of spinal circuits can be modulated with the application of excitatory neurotransmitters such as NMDA [33, 34]. Bath application of low concentrations of NMDA (10–20 mM) had no effect on swimming in ChR2- control siblings (Figure 4G, left plot; response rate = 3.6% ± 1.6% at rest and 2.4% ± 1.1% with NMDA, n = 8 larvae). In contrast, the presence of NMDA led to a dramatic increase in the response rate to photostimulation in ChR2+ larvae, by converting larvae with no response into responsive larvae (Figure 4F, compare top and bottom traces; Figure 4G, right plot; the response rate went from 6.4% ± 2.4% at rest to 47% ± 11% under NMDA, n = 6 larvae, p < 0.05). We tested whether vglut2a+ V0-v interneurons contributed to the delayed motor activity following the activation of CSF-cNs at rest. We recorded V0-v in cell-attached mode and analyzed their firing in response to blue light (Figure 4H). We found that activating CSF-cNs could induce firing in V0-v (response delay = 320 ± 167 ms, n = 118 out of 311 stimulations in 3 cells). Altogether, these data 45 Chapter 1: connectivity of CSF-cNs indicate that the induction of slow locomotion at rest following CSF-cN activation involves the recruitment of V0-v interneurons. Because CSF-cNs form active GABAergic synapses onto glutamatergic V0-v premotor interneurons, we hypothesized that CSF-cN activation during ongoing locomotion might silence motor activity. We used a closed-loop ChR2 activation assay (Figures 5A and 5B) where a 500-ms light pulse was delivered at the onset of spontaneous slow fictive swimming events. In ChR2- control siblings, blue light pulses had no effect on ongoing locomotor activity (Figure 5C). In contrast, activation of ChR2+ CSFcNs led to an abrupt silencing of ongoing swimming activity (Figure 5D). The activation of CSF-cNs significantly reduced the duration of spontaneous swim bouts (Figures 5E–5G; for control fish, bout duration with light-emitting diode (LED) off [BDLED OFF] = 550 ± 53 ms, BDLED ON = 486 ± 59 ms, n = 15 larvae; whereas for ChR2-expressing fish, BDLED OFF = 840 ± 82 ms versus BDLED ON = 235 ± 33 ms, p < 0.001, n = 29 larvae). In addition, CSF-cN activation led to a significant reduction in the frequency of swim bouts (Figure 5H; for control fish, bout frequency with LED off [BFLED OFF] = 0.5, BFLED ON = 0.56, n = 7 larvae; for ChR2-expressing fish, BFLED OFF = 0.52, BFLED ON = 0.33, p < 0.05, n = 6 larvae) without altering the burst frequency (bf) within bouts (Figure S2; bfLED OFF = 22.60 ± 0.64 Hz, bfLED ON = 22.54 ± 0.80 Hz). These findings demonstrate a marked effect of CSF-cN activation on the duration and occurrence of spontaneous bouts of fictive swimming. We tested whether the silencing of locomotor activity was mediated by the activation of ionotropic GABAA receptors. Bath application of GABAA blocker gabazine (10–20 mM) led to an increase in the spontaneous bout duration (compare the left panel of Figure 5I with the left panel of Figure 5J; Figure 5K, BDLED OFF = 909 ± 117 ms, BDLED OFF/gabazine = 2.18 ± 0.64 s, p < 0.0001, n = 10 larvae), indicating that the endogenous release of GABA modulates the duration of locomotor events. Nonetheless, the presence of gabazine reduced the silencing mediated by CSF-cNs (compare Figure 5I right panel with Figure 5J right panel; Figure 5K, BDLED ON = 184 ± 38 ms, BDLED ON/gabazine = 779 ± 128 ms, p < 0.0001). Since the silencing of CSF-cNs was independent of the initial bout duration (Figure S3), we measured the silencing efficiency of CSF-cNs, i.e., their ability to reduce bout dura46 Chapter 1: connectivity of CSF-cNs tion, as the ratio of bout duration with or without the activation of ChR2+ CSF-cNs (BDLED OFF/BDLED ON). The silencing efficiency was reduced from 6.9 to 2.9 when gabazine was added into the bath (Figure 5L; p < 0.05, n = 10), indicating that the inhibition of fictive locomotion involves the activation of GABAA receptors. Together, these results demonstrate that CSF-cNs can modulate the slow locomotor CPG in a statedependent manner. CSF-cNs can trigger delayed rhythmic activity at rest, an effect enhanced by 10–20 mM NMDA in the bath. In contrast, CSF-cNs can inhibit ongoing locomotor activity, reducing both the duration and the frequency of occurrence of locomotor events. Inhibition of locomotor activity by CSF-cNs predominantly occurs at the premotor level The suppression of ongoing locomotor activity could be due to the direct inhibition of motor neurons and/or of descending premotor excitatory interneurons controlling slow locomotion. We first tested whether CSF-cN activation could silence ongoing activity in V0-v excitatory interneurons recorded in cell-attached mode (Figure 6A). We observed that spontaneous firing events in V0-v interneurons were silenced by CSF-cN activation (Figure 6B) leading to shorter episodes (Figures 6C and 6D; mean event duration with LED off [EDLED OFF] = 341 ± 50 ms, EDLED ON = 176 ± 77 ms, n = 99 and 77 episodes, respectively, recorded in n = 3 cells) containing fewer spikes (Figure 6E; mean spikes per episode with LED OFF = 15.8 ± 4.9 spikes, mean spikes per episode with LED ON = 7.7 ± 2.8 spikes). These data reveal that CSF-cNs can silence locomotor activity at the premotor level. Since glutamatergic V0-v interneurons project 15–20 segments caudally [17, 27] and are silenced by CSF-cNs, we hypothesized that silencing rostral V0-v interneurons could alter the propagation of locomotor activity to more caudal motor pools. To test this hypothesis, we restricted the optical activation of CSF-cNs to rostral (1–10) or caudal (16–25) segments while performing dual VNR recordings at rostral (5–8) and caudal (15–16) segments (Figures 7A–7C). The activation of rostral CSF-cNs strongly silenced fictive swimming activity in rostral as well as in caudal segments, occasionally abolishing caudal motor activity (Figure 7B, see stars on bottom traces; Figure 7D; n = 6 larvae). In contrast, the activation of caudal CSF-cNs had small effects on both rostral and caudal motor activity 47 Chapter 1: connectivity of CSF-cNs compared to the activation of rostral CSF-cNs (Figures 7C and 7D; n = 4 larvae; Figure 7E; silencing efficiency for rostral stimulations = 2.85 at VR 8 and 2.04 at VR 16, silencing efficiency for caudal stimulations = 1.08 at VR 8 and 1.06 at VR 16, p < 0.01). The activation of rostral CSF-cNs did not seem to modulate the rostrocaudal lag measured between segment 8 and 16 (Figure S4). Altogether, these results suggest that the ability of CSF-cNs to disrupt the excitatory drive along the spinal cord most likely relies on the modulation of premotor interneurons as these cells have long descending projections within the spinal cord. Discussion In the present study, we investigated the cellular and network mechanisms underlying the modulation of slow locomotion by CSF-cNs in larval zebrafish. To our knowledge, this work is the first investigation of the functional connectivity of a single GABAergic neuron type onto glutamatergic interneurons of the locomotor CPG. Our results highlight the complexity of this GABAergic modulatory pathway leading to antagonistic effects depending on the excitability or state of spinal motor circuits. Circuit organization of spinal CSF-cNs: projections onto excitatory elements of the slow swimming CPG Taking advantage of existing transgenic lines labeling glutamatergic interneurons [28, 29], we observed anatomical and functional connections from CSF-cNs onto vglut2a+ V0-v premotor interneurons in the spinal cord. In larval zebrafish, these cells are specifically active during slow locomotion with bursting frequencies ranging from 15 to 30 Hz and are selectively silenced at swimming frequencies above 30 Hz, possibly by glycinergic interneurons [17]. Our results demonstrate that additional GABAergic inputs to vglut2a+ V0-v originate from CSF-cNs. Given the increasing fidelity of ChR2driven IPSCs between CSF-cNs and V0-v during 25-Hz train stimulations, CSF-cNs may also contribute to the frequency-dependent suppression of V0-v activity. In mice, although glutamatergic V0-v interneurons are essential components of the locomotor CPG [15, 16], they appear critical for left-right alternation during fast locomotion [35], and their ablation selectively affects trot [36]. Further work will be necessary in zebrafish to test whether CSF-cNs can modulate left-right alternation. 48 Chapter 1: connectivity of CSF-cNs Interestingly, activation of CSF-cNs reduced the occurrence of locomotor events. This effect could be explained by the modulation of supraspinal neurons thought to control the initiation and frequency of locomotor events [37, 38]. In the rostral spinal cord, we observed that CSF-cNs projected into the lateral margins of the caudal hindbrain, reaching the somata of V0-v interneurons as well as descending excitatory fibers projecting in the spinal cord. CSF-cNs projecting onto the caudal hindbrain could delay the occurrence of locomotor events by silencing the output of hindbrain interneurons projecting in the spinal cord. However, the full connectivity map of CSF-cNs on their targets, including neurons located in the hindbrain, remains to be completed. Regarding the neurotransmitter released by CSF-cNs, the modulation of locomotion by CSF-cNs was at least partially mediated by GABAA receptors. However, there might be additional components to the effect mediated by CSF-cNs. First, gabazine in the bath failed to fully abolish the silencing of ongoing locomotion. Second, the relatively short inactivation time of GABAA receptors (<100 ms) does not match the long-lasting effects (seconds) of increasing interbout interval. CSF-cNs have been shown to express a variety of peptides [24, 39]. It is therefore plausible that other receptors for GABA and/or peptides also contribute to the modulation of locomotor activity by CSF-cNs. State-dependent modulation of locomotor activity Using electrophysiology and pharmacology, our study sheds light on the state-dependent GABAergic modulation of locomotor CPGs by CSF-cNs. On one side, we revealed the inhibitory action of CSFcNs when stimulated during ongoing locomotion. In this context, locomotor activity was silenced within 200 ms on average, suggesting that a buildup of inhibition was necessary. On the other side, activation of CSF-cNs at rest induced delayed fictive swimming that was highly dependent on the intrinsic excitability of the spinal cord. The delay of induced swimming was about 450 ms, ruling out a direct activation of locomotor CPGs. One possible explanation is that rebound activity originates from an accumulation of depolarizing inhibition as depolarizing GABA is common in immature spinal circuits [40]. Alternatively, the induction of swimming may follow a rebound from GABA A-mediated 49 Chapter 1: connectivity of CSF-cNs inhibition. Post-inhibitory rebound (PIR), a general feature of rhythmic networks including locomotor CPGs [41–43], has been proposed to regulate the timing of activation of premotor interneurons [41, 42, 44–46]. In the tadpole, PIR is an emergent property of a complex interplay of inhibition and depolarization that is modulated in a state-dependent manner [43]. Even though our data indicate that V0-v contribute to the delayed activity triggered by CSF-cNs at rest, it is unlikely that PIR originates solely from the intrinsic properties of these interneurons as we did not observe post-hyperpolarization rebound spiking in these cells. Following CSF-cN activation, PIR may rise from network interactions via other targets, leading to the rebound firing of V0-v interneurons and subsequent induction of slow swimming. Roles for a CSF-dependent GABAergic inhibition of premotor excitation Since our experiments relied on forcing the activation of CSF-cNs with light, one open question lies in identifying the physiological conditions and the timing under which these cells are normally recruited in vivo. Kolmer initially thought that CSF-cNs could form a parasagittal organ functioning as a third ear in the spinal cord [9]. Indeed, the morphology of these cells extending in the central canal is optimal to detect chemical or mechanical cues from the CSF. Previous studies in mammals indicated that CSF-cN firing was modulated by changes of extracellular pH [20, 47], but how such information is transduced and relates to locomotion is still unclear. The observation that the reliability of CSF-cNs to V0-v synaptic currents increases during 25-Hz train implies that the silencing mediated by CSF-cNs is particularly efficient with persistent spiking in the range of slow swimming frequencies. The inhibition of CSF-cNs onto V0-v interneurons could thereby build up over time during locomotor events. Future work will be necessary to complete the connectivity pattern and modulatory role of CSF-cNs in fish and mammals in order to elucidate CSF-cN modulatory function of active locomotion in vertebrates. Experimental procedures Methods and protocols used in this study, referred therein as Experimental procedures and Supplemental Experimental procedures, are described in the Material and methods section of the thesis. 50 Chapter 1: connectivity of CSF-cNs Author contributions K.F. performed electrophysiological recordings, pharmacology experiments, and imaging of spinal lines with the help of C.S. L.D. and A.P. performed single-cell morphology analysis. F.D.B. and A.B. generated transgenic animals. J.G. performed FISH experiments. K.F. and C.W. designed experiments, analyzed data, and wrote the manuscript. Acknowledgements We thank Prof. Shin-Ichi Higashijima for kindly sharing transgenic lines; Prof. Koichi Kawakami for sharing reagents; Dr. Jean Simonnet for helping to set up patch-clamp recordings; Dr. Richard Miles, Dr. Alberto Bacci, Dr. Daniel Zytnicki, Prof. Eve Marder, and members of the C.W. lab for critical reading of the manuscript; and Natalia Maties, Bodgan Buzurin, and Sophie Nunes-Figueiredo for fish care. This work received support from ICM, Ecole des Neurosciences de Paris, Fondation BettencourtSchueller, Mr. Pierre Belle, City of Paris, Atip/Avenir program, Marie Curie Actions (IRG #227200), and ERC Starting Grant Optoloco (#311673). References 1. Rudomin, P. (1990). Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 13, 499–505. 2. Rudomin, P. (2009). In search of lost presynaptic inhibition. Exp. Brain Res. 196, 139–151. 3. Betley, J.N., Wright, C.V., Kawaguchi, Y., Erde´ lyi, F., Szabo, G., Jessell, T.M., and Kaltschmidt, J.A. (2009). Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 139, 161– 174. 4. Fink, A.J., Croce, K.R., Huang, Z.J., Abbott, L.F., Jessell, T.M., and Azim, E. (2014). Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509, 43–48. 5. Cazalets, J.R., Cournil, I., Geffard, M., and Moulins, M. (1987). Suppression of oscillatory activity in crustacean pyloric neurons: implication of GABAergic inputs. J. Neurosci. 7, 2884–2893. 6. Cinelli, E., Mutolo, D., Robertson, B., Grillner, S., Contini, M., Pantaleo, T., and Bongianni, F. (2014).GABAergic and glycinergic inputsmodulate rhythmogenic mechanisms in the lamprey respiratory network. J. Physiol. 592, 1823–1838. 7. Schmitt, D.E., Hill, R.H., and Grillner, S. (2004). The spinal GABAergic system is a strong modulator of burst frequency in the lamprey locomotor network. J. Neurophysiol. 92, 2357–2367. 8. Cazalets, J.R., Bertrand, S., Sqalli-Houssaini, Y., and Clarac, F. (1998). GABAergic control of spinal locomotor networks in the neonatal rat. Ann. N Y Acad. Sci. 860, 168–180. 9. Kolmer, W. (1921). Das ‘‘Sagittalorgan’’ der Wirbeltiere. Z. Anat. Entwicklungs 60, 652–717. 10. Kolmer, W. (1931).U¨ ber das Sagittalorgan, ein Zentrales Sinnesorgan der Wirbeltiere, Insbesondere Beim Affen. Z. Zellforsch Mik. Ana. 13, 236–248. 11. Agduhr, E. (1922).U¨ ber ein Zentrales Sinnesorgan (?) bei den Vertebraten. Z. Anat. Entwicklungs 66, 223– 360. 12. Stoeckel, M.E., Uhl-Bronner, S., Hugel, S., Veinante, P., Klein, M.J., Mutterer, J., Freund-Mercier, M.J., and Schlichter, R. (2003). Cerebrospinal fluid-contacting neurons in the rat spinal cord, a gammaaminobutyric 51 Chapter 1: connectivity of CSF-cNs acidergic system expressing the P2X2 subunit of purinergic receptors, PSA-NCAM, and GAP-43 immunoreactivities: light and electron microscopic study. J. Comp. Neurol. 457, 159–174. 13. Dale, N., Roberts, A., Ottersen, O.P., and Storm-Mathisen, J. (1987). The morphology and distribution of ‘Kolmer-Agduhr cells’, a class of cerebrospinal- fluid-contacting neurons revealed in the frog embryo spinal cord by GABA immunocytochemistry. Proc. R. Soc. Lond. B Biol. Sci. 232, 193–203. 14. Wyart, C., Del Bene, F., Warp, E., Scott, E.K., Trauner, D., Baier, H., and Isacoff, E.Y. (2009). Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461, 407–410. 15. Lanuza, G.M., Gosgnach, S., Pierani, A., Jessell, T.M., and Goulding, M. (2004). Genetic identification of spinal interneurons that coordinate left left-right locomotor activity necessary for walking movements. Neuron 42, 375–386. 16. Kiehn, O., Quinlan, K.A., Restrepo, C.E., Lundfald, L., Borgius, L., Talpalar, A.E., and Endo, T. (2008). Excitatory components of the mammalian locomotor CPG. Brain Res. Brain Res. Rev. 57, 56–63. 17. McLean, D.L., Masino, M.A., Koh, I.Y., Lindquist, W.B., and Fetcho, J.R. (2008). Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat. Neurosci. 11, 1419–1429. 18. Ritter, D.A., Bhatt, D.H., and Fetcho, J.R. (2001). In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J. Neurosci. 21, 8956–8965. 19. McLean, D.L., Fan, J., Higashijima, S., Hale, M.E., and Fetcho, J.R. (2007). A topographic map of recruitment in spinal cord. Nature 446, 71–75. 20. Huang, A.L., Chen, X., Hoon, M.A., Chandrashekar, J., Guo, W., Trankner, D., Ryba, N.J., and Zuker, C.S. (2006). The cells and logic for mammalian sour taste detection. Nature 442, 934–938. 21. Djenoune, L., Khabou, H., Joubert, F., Quan, F.B., Nunes Figueiredo, S., Bodineau, L., Del Bene, F., Burckle´ , C., Tostivint, H., and Wyart, C. (2014). Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front. Neuroanat. 8, 26. 22. Orts-Del’Immagine, A., Kastner, A., Tillement, V., Tardivel, C., Trouslard, J., and Wanaverbecq, N. (2014). Morphology, distribution and phenotype of polycystin kidney disease 2-like 1-positive cerebrospinal fluid contacting neurons in the brainstem of adult mice. PLoS ONE 9, e87748. 23. Vigh, B., and Vigh-Teichmann, I. (1973). Comparative ultrastructure of the cerebrospinal fluid-contacting neurons. Int. Rev. Cytol. 35, 189–251. 24. Christenson, J., Alford, S., Grillner, S., and Ho¨ kfelt, T. (1991). Co-localized GABA and somatostatin use different ionic mechanisms to hyperpolarize target neurons in the lamprey spinal cord. Neurosci. Lett. 134, 93– 97. 25. Meyer, M.P., and Smith, S.J. (2006). Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J. Neurosci. 26, 3604–3614. 26. Hale, M.E., Ritter, D.A., and Fetcho, J.R. (2001). A confocal study of spinal interneurons in living larval zebrafish. J. Comp. Neurol. 437, 1–16. 27. Satou, C., Kimura, Y., and Higashijima, S. (2012). Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. J. Neurosci. 32, 1771–1783. 28. Kinkhabwala, A., Riley, M., Koyama, M., Monen, J., Satou, C., Kimura, Y., Higashijima, S., and Fetcho, J. (2011). A structural and functional ground plan for neurons in the hindbrain of zebrafish. Proc. Natl. Acad. Sci. USA 108, 1164–1169. 29. Koyama, M., Kinkhabwala, A., Satou, C., Higashijima, S., and Fetcho, J. (2011). Mapping a sensory-motor network onto a structural and functional ground plan in the hindbrain. Proc. Natl. Acad. Sci. USA 108, 1170– 1175. 30. Higashijima, S., Mandel, G., and Fetcho, J.R. (2004). Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J. Comp. Neurol. 480, 1–18. 31. Higashijima, S., Schaefer, M., and Fetcho, J.R. (2004). Neurotransmitter properties of spinal interneurons in embryonic and larval zebrafish. J. Comp. Neurol. 480, 19–37. 32. Roy, B., and Ali, D.W. (2014). Multiple types of GABAA responses identified from zebrafish Mauthner cells. Neuroreport 25, 1232–1236. 33. Grillner, S., McClellan, A., Sigvardt, K., Walle´ n, P., and Wile´ n, M. (1981). Activation of NMDAreceptors elicits ‘‘fictive locomotion’’ in lamprey spinal cord in vitro. Acta Physiol. Scand. 113, 549–551. 34. McDearmid, J.R., and Drapeau, P. (2006). Rhythmic motor activity evoked by NMDA in the spinal zebrafish larva. J. Neurophysiol. 95, 401–417. 35. Talpalar, A.E., Bouvier, J., Borgius, L., Fortin, G., Pierani, A., and Kiehn, O. (2013). Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500, 85–88. 36. Bellardita, C., and Kiehn, O. (2015). Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr. Biol. 25, 1426–1436, http://dx.doi.org/10.1016/j.cub.2015.04.005. 37. Shik, M.L., Severin, F.V., and Orlovsky, G.N. (1969). Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr. Clin. Neurophysiol. 26, 549. 52 Chapter 1: connectivity of CSF-cNs 38. Dubuc, R., Brocard, F., Antri, M., Fe´ nelon, K., Garie´ py, J.F., Smetana, R., Ménard, A., Le Ray, D., Viana Di Prisco, G., Pearlstein, E., et al. (2008). Initiation of locomotion in lampreys. Brain Res. Brain Res. Rev. 57, 172–182. 39. Quan, F.B., Dubessy, C., Galant, S., Kenigfest, N.B., Djenoune, L., Leprince, J., Wyart, C., Lihrmann, I., and Tostivint, H. (2015). Comparative distribution and in vitro activities of the urotensin II-related peptides URP1 and URP2 in zebrafish: evidence for their colocalization in spinal cerebrospinal fluid-contacting neurons. PLoS ONE 10, e0119290. 40. Jean-Xavier, C., Mentis, G.Z., O’Donovan, M.J., Cattaert, D., and Vinay, L. (2007). Dual personality of GABA/glycine-mediated depolarizations in immature spinal cord. Proc. Natl. Acad. Sci. USA 104, 11477– 11482. 41. Marder, E., and Bucher, D. (2001). Central pattern generators and the control of rhythmic movements. Curr. Biol. 11, R986–R996. 42. Roberts, A., and Tunstall, M.J. (1990). Mutual re-excitation with post-inhibitory rebound: a simulation study on the mechanisms for locomotor rhythm generation in the spinal cord of Xenopus embryos. Eur. J. Neurosci. 2, 11–23. 43. Soffe, S.R. (1990). Active and passive membrane properties of spinal cord neurons that are rhythmically active during swimming in Xenopus embryos. Eur. J. Neurosci. 2, 1–10. 44. Roberts, A., Li, W.C., and Soffe, S.R. (2008). Roles for inhibition: studies on networks controlling swimming in young frog tadpoles. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 194, 185–193. 45. Pirtle, T.J., and Satterlie, R.A. (2007). The role of post-inhibitory rebound in the locomotor central-pattern generator of Clione limacina. Integr. Comp. Biol. 47, 451–456. 46. Bertrand, S., and Cazalets, J.R. (1998). Postinhibitory rebound during locomotor- like activity in neonatal rat motoneurons in vitro. J. Neurophysiol. 79, 342–351. 47. Orts-Del’immagine, A., Wanaverbecq, N., Tardivel, C., Tillement, V., Dallaporta, M., and Trouslard, J. (2012). Properties of subependymal cerebrospinal fluid contacting neurones in the dorsal vagal complex of the mouse brainstem. J. Physiol. 590, 3719–3741. 48. Masino, M.A., and Fetcho, J.R. (2005). Fictive swimming motor patterns in wild type and mutant larval zebrafish. J. Neurophysiol. 93, 3177–3188. 53 Chapter 1: connectivity of CSF-cNs Figures and Supplemental Information Figure 1. The Tg(pkd2l1:gal4)icm10 stable transgenic line specifically labels CSF-cNs in the zebrafish spinal cord (A1) Complete pattern of expression of mCherry in Tg(pkd2l1:gal4;UAS:ChR2-mCh) double transgenic larvae at 4 dpf. (A2) Lateral view of the spinal cord shows that mCherry was restricted to CSF-cNs in the ventral part of the spinal cord. Seven CSF-cNs were labeled per axial segment on average. (A3) Dorsal view of the spinal cord shows that axonal projections of CSF-cNs were ipsilateral and located in the lateral margins of the spinal cord. See also Figure S1 and Table S1. (B) Overlap of mCherry (red) and GFP (gray) in the Tg(pkd2l1:gal4;UAS:ChR2-mCh;Gad1b:GFP) triple transgenic larvae confirms the GABAergic nature of CSFcNs (arrowheads). Note that the Tg(pkd2l1:gal4) line does not label all CSF-cNs. (C) Morphology of five CSFcNs located in segment 9 at 3 dpf after single-cell imaging and reconstruction. Axonal projections varied in length, in branching as well as in dorsoventral (D-V) positioning. Cells were aligned according to the D-V position of their cell body. The central canal (cc) is represented by the light red bar. (D) Mapping of CSF-cNs axonal projections across the rostrocaudal (R-C) axis. All CSF-cNs had ascending projections reaching from two to six segments away from the cell body (black circles, n = 88 cells). (E) Distribution of the number of segments covered by single axons of CSF-cNs. (F) Mean D-V positions of axons (maxima and minima) and soma of CSFcNs. (G) Single Synaptophysin-GFP CSF-cN with punctate synaptophysin clustering consistent with presynaptic boutons distributed along the entire axonal arborization (arrowheads). See also Movie S1. (H) D-V position of putative synaptic boutons for CSF-cNs sorted according to their soma position along the D-V axis (n = 36 cells). (I) Distribution of putative synaptic boutons along the D-V axis (black bars indicate that 60% of the boutons are confined in the 0.2–0.4 interval). In (A2) and (G), white solid lines delineate the ventral and dorsal limits of the spinal cord; dashed lines indicate the limits of axial segments. Scale bars are 1mm in (A1), 50 mm in (A2), (A3), and (B), 900 mm in (D), and 20 mm in (C) and (G). R, rostral; C, caudal; V, ventral; CC, central canal. (A-B) were reconstructed from Z projection stacks through the entire spinal cord. Data are represented here as mean ± SD. 54 Chapter 1: connectivity of CSF-cNs Figure 2. CSF-cNs project onto V0-v glutamatergic interneurons (A1 and A2) Z projection stack of a few optical sections imaged from the dorsal side in Tg(pkd2l1:gal4;UAS:ChR2-YFP; vglut2a:lox-DsRed-lox-GFP) triple transgenic larvae reveal the localization of glutamatergic neurons (DsRed+, magenta) relative to CSF-cN projections (YFP+, green) in rostral (A1) and caudal (A2) spinal cord. Zoom of lateral regions circled in dashed lines show that CSF-cN projections surround the DsRed+ nucleus of ventrolateral glutamatergic interneurons (arrowheads in bottom panels, A1 and A2). See also Table S1. (B) Apposition of CSF-cN axonal varicosities onto the cell bodies of two ventrolateral vglut2a + interneurons (arrowheads). (C) Ventrolateral vglut2a+ V0-v interneuron receiving projections from CSF-cNs were filled with Alexa 647 in order to image and reconstruct their morphology. (D) Z projection stack of a few optical sections imaged from the lateral side in a Tg(pkd2l1:gal4;UAS:ChR2-YFP;vglut2a:lox-DsRed-lox-GFP) triple transgenic larva labeling CSF-cNs (ChR2-YFP+, green) and glutamatergic interneurons (DsRed +, magenta) after dye filling a vglut2a+ V0-v interneuron. Arrowheads highlight axonal projections of CSF-cNs onto the soma and dendrites of the filled V0-v interneuron. (E) Typical morphology of ventrolateral vglut2a+ V0-v interneurons filled with Alexa 647. (F) Paired ventral nerve root recording (VNR) with whole-cell current clamp recording of a V0-v interneuron showing rhythmic activity during every episode of fictive slow locomotion (burst frequency ranged between 15 and 30 Hz). The region circled with dashed line is zoomed in on the right to emphasize the activity of V0-v during a fictive bout. V0-v action potentials preceded motor neurons spiking for all locomotor bursts. (G) V0-v cells are electronically compact, typically requiring only 10–20 pA of current injection to reach action potential (AP)-firing threshold. Note that the cell depicted in (G) is not the same cell as in (F). Scale bars are 50 mm in (A1) and (A2) and 10 mm in (B), (D), and (E). In each panel, Z projection stacks were reconstructed from a few optical sections (depth = 0.55 mm). 55 Chapter 1: connectivity of CSF-cNs Figure 3. CSF-cNs form active GABAergic synapses onto V0-v glutamatergic interneurons (A1) Experimental paradigm. Targeted whole-cell voltage-clamp recordings from vglut2a+ V0-v interneurons while activating ChR2+ CSF-cNs using brief (5 ms) pulses of blue light. (A2) Loose-patch recording of a ChR2+ CSF-cN shows that 5-ms blue light pulses (blue bars) reliably triggered single action potentials in CSF-cNs. The plot indicates that the spike delay relative to the onset of the light pulse was about 4–5 ms (141 stimulations from 4 cells). (B) Voltage clamp recording of a V0-v interneuron during optical stimulation of ChR2 + CSF-cNs (blue bars) using a single pulse (B1) and a train of 13 pulses at 25 Hz (B2). Holding potential V m = -96 mV and reversal potential for chloride ECl = -51 mV; therefore, IPSCs appear as inward currents. (C) Quantification of kinetic parameters of ChR2-induced IPSCs in V0-v interneurons; delay was calculated relative to the onset of the light pulse, decay time was obtained after fitting the decay with a single exponential fit, and time to peak was calculated as the difference between the time of current onset and of current peak. (D) Reliability of IPSC events increased for subsequent pulses during a trial as well as across subsequent trials recorded every 20–25 s (1st, 4th, 8th, and 15th trials shown). (E) Quantification of the response probability in V0-v interneurons for each pulse during a train. (F) Quantification of the response probability in V0-v interneurons, averaged per pulse during each train, as a function of the train number over the time course of the experiment. (G) ChR2-induced IPSCs in V0-v interneurons are blocked by application of gabazine in the bath (n = 3 cells). Blue bars represent the stimulation at 25 Hz. Red lines in (E) and (F) are linear fits. **p < 0.001 following Pearson’s linear correlation test. R is the linear correlation coefficient. Data are represented as mean ± SEM. 56 Chapter 1: connectivity of CSF-cNs Figure 4. Activation of CSF-cNs at rest can trigger delayed slow locomotion (A) Experimental paradigm showing an eye-enucleated 4 dpf ChR2+ larva mounted on its side in a glass-bottom dish after paralysis. Blue light was patterned onto the spinal cord from segment 7–8 to 27–28 through the microscope condenser. VNR signals were recorded from the axial musculature and analyzed in real time. A threshold (T) was set to trigger the LED based on the VNR signal. (B) Photoactivation protocol. LED was triggered during resting period when the animal was not fictively swimming. If the larva was not swimming, the LED was auto- 57 Chapter 1: connectivity of CSF-cNs matically activated every 4–5 s. If the larva was swimming, the LED was triggered long (500 ms to 2 s) after the onset of fictive bouts. The blue bar represents the pulse of blue light. (C and C1) Sample traces of VNR recordings showing the effects of activating ChR2 + CSF-cNs using blue light at rest (light pulses are represented by the blue bars). ChR2 - control siblings never showed swimming activity in response to blue light stimulations (top, n = 10 larvae). A responding ChR2 + responder larva showed a delayed swimming response after the onset of the light pulse (middle, n = 8 out of 14; zoom in C1). Some ChR2+ larvae did not respond to the light stimulation at rest (bottom, n = 6 out of 14). (D) Quantification of the probability to induce delayed fictive swimming after blue light stimulations in ChR2 - and ChR2+ larvae (n = 10 and 14, respectively). (E) Sample VNR traces illustrating that the induction of delayed swimming in a responsive ChR2 + larva was blocked after the addition of 20 mM gabazine in the bath. (F) Sample traces showing that 20 mM NMDA bath application transformed ChR2+ non-responder larvae into responder larvae. Note that after NMDA application, the light response of the ChR2+ non-responder larvae mimicked the response observed in responder larvae (C, middle). (G) Quantification of the probability to induce delayed fictive swimming responses in ChR2 - and ChR2+ larvae before and after NMDA application. (H and H1) Targeted cell attached recordings from a vglut2a + V0-v interneuron while activating CSF-cNs expressing ChR2 using 500-ms pulses of blue light. Delayed firing is triggered by blue light stimulations. 118 out of 311 stimulations led to delayed firing in n = 3 V0-v cells. The circled region is zoomed in on (H1) to better resolve the spiking. *p < 0.05 following Student’s t test. Data are represented as mean ± SEM. Mean values are depicted in red. 58 Chapter 1: connectivity of CSF-cNs Figure 5. Activation of CSF-cNs at the onset of ongoing fictive swimming silences locomotor activity (A) Experimental paradigm; same as in Figure 4A except that the LED was triggered at the onset of each fictive swim. (B) Photoactivation protocol. LED was triggered rapidly (10 ms) after the onset of spontaneous fictive bouts that were detected when the VNR signal had reached a manually defined threshold (T). The blue bar represents the light pulse. (C–C2) In ChR2- control siblings, profiles of fictive locomotor activity without (top) or with (bottom) blue light stimulations (blue bars) were similar (zooms in C1 and C2). (D–D2) Same as in (C) in ChR2+ larvae; a reduction of bout duration (BD) and bout frequency (BF) is associated with the optical activation of CSF-cNs (zoom on bouts in D1 and D2). (E) Distribution of spontaneous fictive bout durations in ChR2_ control siblings was similar with LED OFF (black bars) or LED ON (gray bars). The inset shows the cumulative probability of fictive bout duration in LEDOFF and LED ON conditions. (F) Same as in (E) in ChR2+ larvae. In contrast to ChR2- control siblings, the distribution and cumulative probability were different in LEDOFF (black 59 Chapter 1: connectivity of CSF-cNs bars) and LED ON (blue bars) conditions, indicating a large decrease of bout duration when the LED was ON. (G) Quantification of the mean bout duration in both conditions for ChR2 - and ChR2+ larvae. (H) Quantification of the bout frequency (BF) in ChR2- and ChR2+ larvae in recordings where the LED was alternatively ON and OFF. In control ChR2- larvae, the frequency did not change over the time course of the experiment, whereas the bout frequency reversibly decreased with LED ON in ChR2 + larvae. See also Figure S2. (I) Sample VNR traces in a ChR2+ larva illustrating the typical silencing mediated by CSF-cNs. (J) Same as in (I) after bath application of 10 mM gabazine. Note that bout duration increased upon application. The drug penetrated the spinal cord about 5 min after application. Data were collected between 5 and 15 min after application, before seizures occurred. (K) Quantification of fictive bout duration in ChR2+ larvae during either LED OFF or LED ON episodes as well as before and after gabazine was bath applied. (L) CSF-cN-mediated silencing efficiency, calculated as the relative reduction of bout duration after CSF-cN activation (BD LED OFF/BD LED ON ratio), and decreased upon gabazine treatment. See also Figure S3. In (G), (H), and (L), *p < 0.05 and **p < 0.0001 following Student’s t test. In (K), a linear mixed-effects model was applied to compare datasets, and post hoc multiple comparisons of means were performed to extract p values (**p < 0.001). Data are represented as mean ± SEM. In (G), (K), and (L), mean values are depicted in red. Figure 6. Activation of CSF-cNs silences episodes of glutamatergic V0-v interneuron activity (A) Experimental paradigm: targeted cell attached recordings from vglut2a+ V0-v interneurons while activating CSF-cNs expressing ChR2 using 500-ms pulses of blue light. (B) Episodes of V0-v activity (top: spontaneous activity; bottom: with stimulation of CSF-cNs timed to the onset of V0-v episodes). Blue bars represent blue light pulses. (C) Distribution of V0-v episode durations with LED OFF or LED ON. (D) Mean episode duration per cell with LED OFF or LED ON. (E) Mean number of spikes per episode per cell with LED OFF or LED ON. 60 Chapter 1: connectivity of CSF-cNs Figure 7. Selective activation of rostral CSF-cNs disrupts the propagation of activity to the caudal spinal cord (A) Profile of spontaneous fictive locomotor activity in ChR2 + larvae recorded at rostral (VR8) and caudal (VR16) segments. The field of photoactivation was limited by narrowing the aperture of the microscope condenser. (B) Fictive locomotor activity was silenced in the rostral VNR as well as in the caudal VNR following the activation of rostral CSF-cNs (segments 1–10). See also Figure S4. (C) In contrast, fictive locomotor activity in the same larva was not altered following the activation of caudal CSF-cNs (segments 16–25). (D) Effect of activation of caudal CSF-cNs versus rostral CSF-cNs. Mean bout duration calculated from VNR recordings at segment 8 (VR 8) and segment 16 (VR 16) in LED OFF (black) or LED ON (blue) conditions. (E) CSF-cNmediated silencing efficiency, calculated as the relative reduction of bout duration after CSF-cN activation (BD LED OFF/BD LED ON ratio), measured at segment 8 (VR 8) and 16 (VR16) during rostral or caudal activation of CSF-cNs. *p < 0.05, **p < 0.01 following Student’s t test. Data are represented as mean ± SEM. 61 Chapter 1: connectivity of CSF-cNs Figure S1 related to Figure 1. The Tg(pkd2l1:gal4)icm10 line drives expression in pkd2l1 positive cells in the zebrafish larva Fluorescent in situ hybridization (FISH) for pkd2l1 was combined with an immunohistochemistry against GFP in a Tg(pkd2l1:gal4;UAS :GCaMP5G) larva at 3 dpf. Spinal neurons expressing GCaMP5G (green) are positive for pkd2l1 (Pkd2l1+ in red), see arrowheads, n = 5 larvae. Scale bars represent 50 μm. 62 Chapter 1: connectivity of CSF-cNs Figure S2 related to Figure 5. The burst frequency is not affected by the activation of CSF-cNs during ongoing fictive slow locomotion (A) Estimation of the burst frequency (in Hz) during spontaneous fictive slow swim bouts at baseline (LEDOFF, left) and after activation of CSF-cNs (LED ON, right). The blue bar represents the light pulse. (B) Quantification of the burst frequency in LED OFF vs LED ON condition in ChR2+ larvae. The burst frequency is not altered by CSF-cN activation during ongoing slow swim events (n = 11 larvae). Figure S3 related to Figure 5. Bout duration upon CSF-cN activation did not depend on the initial bout duration The scatter plot shows the correlation between duration of fictive swimming events in LED OFF and LED ON conditions for ChR2- and ChR2+ larvae. High correlation is observed between bout duration during LEDOFF and LEDON conditions in ChR2- larvae (p < 0.001) but not in ChR2 + larvae (p > 0.05). These data demonstrate that the silencing of swimming activity does not depend of the initial duration of activity in ChR2 + fish. Red lines are linear fits. R values are correlation coefficients. Correlation significance was computed using Student’s t test. 63 Chapter 1: connectivity of CSF-cNs Figure S4 related to Figure 7. The rostrocaudal lag is not affected by the activation of rostral CSF-cNs during slow locomotion Envelope traces of VNR recordings from segment 8 and 16 show that the caudal activity follows rostral activity by several ms illustrating that the locomotor activity propagates from rostral to caudal segments. In ChR2 + larvae, the rostrocaudal lag is unaltered by the stimulation of CSF-cNs. The blue bar represents the light pulse of 500 ms. Table S1 related to Figure 1 and Figure 2. Stable transgenic lines Note: Ref S1 in Table S1 can be found in as [1] the Material and methods reference section. 64 Chapter 1: connectivity of CSF-cNs Discussion and perspectives On the specificity of CSF-cNs connectivity Our work revealed that CSF-cNs target multiple elements of the glutamatergic premotor pool in the spinal cord and that the CSF-cNs-mediated state-dependent modulation seems to predominantly occur at the premotor level. However, it remains possible that CSF-cNs target and modulate other classes of spinal neurons active during slow swimming, including motor neurons. To test this hypothesis, we analyzed the recruitment of motor neurons expressing the geneticallyencoded calcium indicator GCaMP6f during fictive slow swimming using (Chen et al., 2013; Figure 8A). The readout from ventral nerve root recordings was used to attribute each volley of calcium activity its corresponding behavior, based on the analysis of fictive swimming frequency (Masino and Fetcho, 2005; McLean et al, 2007; Figure 8A and 8C). We used the Tg(mnx1:gal4) line to specifically drive the expression of the GCaMP6f in motor neurons (Zelenchuk and Brusés, 2011; Sternberg, Severi et al., 2016, in press). The Figure 8B illustrates the pattern of expression obtained in Tg(mnx1:gal4; cry:mCherry-UAS:GCaMP6f) double transgenic larvae at 4 dpf. We imaged pools of motor neurons over one and a half segments of the spinal cord, recording an average of 25-35 motor neurons and covering the entire dorso-ventral (D-V) domain of motor neurons (from 0.1 to 0.6 on the D-V axis, Figure 8B). This approach allowed us to record from a population of motor neurons, thus capturing the contribution from both ventral secondary and early-born dorsal primary motor neurons during slow locomotor behaviors. Slow fictive locomotion is characterized by burst frequencies comprised between 20 to 30 Hz (Figure 8C, see Masino & Fetcho, 2005). We found that a rather small fraction of motor neurons was active during fictive slow swimming (25% of recorded motor neurons on average, Figure 8C, 8D, 8E). Consistent with data from single cell recordings in the zebrafish larva (Mclean et al., 2007), these neurons were found in the ventral spinal cord (0.1-0.4 on the D-V). 65 Chapter 1: connectivity of CSF-cNs Figure 8. Imaging motor neurons with GCaMP6f reveals behavior-specific patterns of motor pools recruitment (A) Experimental setup. A paralyzed larva, mounted on its side in agarose is placed under a 40X objective. Motor output is recorded at the level of ventral nerve roots (VNR). A 488 nm laser is used to record GCaMP6f signals in GCaMP expressing cells at 20 Hz. (B) Expression pattern in a Tg(mnx1:gal4;cry:mCherry; UAS:GCaMP6f) double transgenic larva at 4dpf imaged from the lateral side. The white lines delineate the ventral and dorsal limits of the spinal cord. The white dashed lines represent axial segments limits. The axis on the side illustrates the dorso-ventral segmentation of the spinal cord, with 0 being the ventral limit and 1 the dorsal limit. R is rostral, V is ventral. Scale bar is 50 µm. (C) GCaMP6f signals from individual motor neurons aligned with the VNR profile. Black trace represents the noise originating from light scattering in adjacent planes. VNR trace is enlarged (dashed lines) to better illustrate the different components. The range of slow swimming frequencies is always between 20 and 30Hz. (D) Map of motor neuron recruitment during fictive slow swimming (colored cells in magenta). (E) D-V position of active motor neurons during slow swimming. The pie plot represents the proportion of active cells. Knowing that only a small portion of ventral secondary motor neurons are active during fictive slow swimming, we looked for potential connections between CSF-cNs and these motor neurons and imaged 4dpf Tg(pkd2l1:gal4;UAS:ChR2-YFP; pargmn2ET-GFP) double transgenic larvae (Figure 9). We imaged 16 axial segments in eight larvae and found only four ventral motor neurons with putative inputs from CSF-cNs axons, suggesting that a minor portion of ventral motor neurons could be modulated (see the middle and bottom panels of Figure 9 for examples). Given the pattern of recruitment of motor neurons during fictive slow swimming and the few neurons active per segment during these episodes, it is unclear whether the motor neurons with putative inputs from CSF-cNs are participating in slow swimming. 66 Chapter 1: connectivity of CSF-cNs Figure 9. Putative connections between CSF-cNs and motor secondary neurons Z projection stacks of a few optical sections imaged from the lateral side in Tg(pkd2l1:gal4;UAS:ChR2mCherry; pargMN2ET-GFP) triple transgenic larvae reveal the localization of motor neurons (GFP +, green) relative to CSF-cN projections (mCherry+, red). Scale bars are 50 µm. A definitive confirmation of this hypothesis could be achieved by imaging the recruitment of motor neurons in a background where CSF-cNs are also labeled with a fluorescent marker. Altogether, these observations further support our idea that the modulation of slow swimming is mainly achieved through premotor circuits. However, one very notable exception should be made with regard to CSF-cNs and motor neurons interactions. When looking at CSF-cNs’ projections along the D-V axis (Figure 1), it was clear that a few axons could reach the 0.5-0.6 portion in the dorsal spinal cord, suggesting that spinal neurons in this domain could potentially receive inputs from CSF-cNs. Two of these targets have been recently identified by our lab (Hubbard et al., accepted). Early born primary motor neurons, referred to as caudal primary motor neurons (CaP) and glutamatergic commissural primary ascending interneurons (CoPA) were shown to receive strong inputs from CSF-cNs. Activating CSF-cNs using 5 ms light 67 Chapter 1: connectivity of CSF-cNs pulses, which as we showed in Figure 3 elicits single action potentials, was sufficient to silence the firing of CaP motor neurons (data not shown). Interestingly, Hubbard found that CSF-cN/CaP synapses have different properties compared to CSF-cN/V0-v synapses. There were no failures of synaptic transmission between CSF-cN and CoPA or CaP during optogenetic activation of CSF-cNs while the response probability is initially low in V0-v cells (Figure 3). However, the synaptic transmission rapidly depressed with stimulations at 1-20 Hz in CaP and CoPA whereas the response potentiated in V0v interneurons as an increase in response probability. To date, CaP motor neurons have been found to be active during embryonic motor behaviors (in particular during coiling at early developmental stages). CaP motor neurons and other early born primary motor neurons are also recruited during Mauthner-mediated escape responses and drive the first Cbend of the zebrafish tail during these events (Higashijima et al., 2003; Bhatt et al., 2007; McLean et al., 2007; Pietri et al., 2009; Knogler et al., 2014a, 2014b). CoPA neurons are recruited during tailtouch responses by Rohon-Beard neurons but do not seem to participate in other locomotor behaviors. Our imaging approach as well as many studies using single cell patch clamp recordings of motor neurons indicated that these neurons are not active during slow swimming. Altogether, these data show that CSF-cNs can tune the excitability of spinal neurons driving the initial phase of escape behaviors in addition to modulating the slow swimming circuit. Moreover, these findings illustrate the idea that CSF-cNs could potentially exert a circuit-specific (and behavior-specific) modulatory function in the spinal cord (see Chapter 2). While the specific contribution of CSF-cNs during the C-bend execution and tail-touch escapes remains to be characterized, it would be interesting to determine to what extent the force of muscle contraction scales with the activity of single CSF-cN cells. One could hypothesize that a strong C-bend contraction triggers burst spiking in CSF-cNs enabling powerful feedback on neurons mediating this response (in order to adjust the timing of CaP activation for instance) while simultaneously providing a downregulation of excitation to spinal neurons active during slower behaviors, thus optimizing the initiation of the fast response. It would also be interesting to determine whether the same CSF-cN projects onto both V0-v and CaP neurons, which would indicate that there are mechanisms regulating CSF-cN synapses in a target specific manner. 68 Chapter 1: connectivity of CSF-cNs CSF-cNs target V3 interneurons, a second class of ventral glutamatergic interneurons Among the classes of glutamatergic interneurons identified in the ventral the spinal cord of larval zebrafish, unipolar commissural descending (UCoD) and ventromedial (VeMe) interneurons could represent potential targets of CSF-cNs (Hale et al., 2001; Higashijima et al., 2004a, 2004b). UCoDs are difficult to target because their precise location in the spinal cord is unclear and because there is no specific marker to genetically target them. In fact, it is unclear whether UCoDs and MCoDs are distinct subtypes. VeMe interneurons are easier to study because they are the ventral-most interneurons in the spinal cord and can be visualized using the Vglut2a reporter line. Figures 10A and 10B illustrate the putative connection between CSF-cNs and VeMe interneurons. Cell bodies of the two types appear very close in space. Comparatively, the number of putative CSF-cNs presynaptic boutons on VeMe somata seems higher than what we found for V0-v interneurons, suggesting that the synaptic properties might be different between these two targets. Given their ventro-medial position in the spinal cord, we wondered whether VeMe interneurons were homologs of mouse spinal V3 interneurons, which derive from the nkx2.2a progenitor domain and express the transcription factor sim1 (Zhang et al., 2008). We imaged Tg(vglut2a:DsRed; nkx2.2aGFP) double transgenic larvae and found that ventro-medial vglut2a+ cells were also nkx2.2a+ (Figure 10C and 10D). It is unclear whether these nkx2.2a+/ vglut2a+ also express sim1 but we believe that the anatomy, genetic identity, and position in the cord are, when taken together, are good arguments to name zebrafish VeMe interneurons V3 homologs. In mouse, genetic ablation of sim1+ spinal neurons suggest that V3 interneurons regulate the rhythmicity of locomotion, notably by projecting onto both motor neurons and premotor glutamatergic interneurons (Zhang et al., 2008). Current clamp recordings of V3 interneurons revealed that they receive rhythmic excitation during fictive locomotion, suggesting that they are part of locomotor central pattern generators (Zhang et al., 2008). In zebrafish, the function and connectivity of V3 interneurons is unknown. To determine whether V3 are active during locomotion in zebrafish, we recorded their activity in the fictive swimming configuration using GCaMP imaging (Figure 10E). 69 Chapter 1: connectivity of CSF-cNs Figure 10. Connections between CSF-cNs and V3 glutamatergic interneurons (A-B) Z projection stack of a few optical sections imaged from the lateral side in Tg(pkd2l1:gal4;UAS:ChR2YFP; vglut2a:lox:DsRed:lox:GFP) triple transgenic larvae reveal the location of glutamatergic neurons (DsRed+, magenta) relative to CSF-cN projections (YFP+, green). (C-D) Z projection stack of a few optical sections imaged from the lateral side in Tg(nkx2.2a-GFP; vglut2a:lox:DsRed:lox:GFP) double transgenic larvae reveal the localization of V3interneurons (DsRed+, magenta) relative to nkx2.2a+ cells (GFP+, white). (E) Imaging setup, same as in Figure 9, except that here zebrafish larvae are mounted dorsal side up, thus enabling us to isolate the layer of V3 interneurons. White dashed lines delineate the lateral borders of the spinal cord. (F) V3 interneurons appear active during spontaneous fictive slow swimming events. Paired calcium imaging and fictive locomotion recordings revealed that V3 are indeed rhythmically active during spontaneous slow fictive swimming (Figure 10F). These observations suggest that silencing of V3 activity by CSF-cNs during slow swimming could, in addition to the silencing of V0-v interneurons, contribute to the modulation of slow locomotion. In mouse, V3 interneurons fire action potentials after injections of hyperpolarizing currents (Zhang et al., 2008; Borowska et al., 2013). This feature suggests that V3 interneurons could rebound from inhibition and it is proposed to be an important mechanism for the modulation of spinal neurons rhythmicity. As a matter of fact, post-inhibitory rebound is a crucial feature of rhythmic neurons in many rhythmic neuronal circuits in several species (Marder and Bucher, 2001; Grillner, 2003). The stimulation of CSF-cNs at rest is associated with a delayed slow swimming response and we hypothesized 70 Chapter 1: connectivity of CSF-cNs that this “rebound” effect could be mediated by a rebound activity in V3 interneurons, which would place V3 interneurons at the core of the state-dependent modulation we have described in this chapter (Figure 4). Further work will aim to determine the intrinsic properties of V3 interneurons, the properties of their synapse with CSF-cNs as well as their diagram of connectivity in the spinal cord. On the modulation of bout generation Our stimulation assay revealed that CSF-cNs can modulate the bout frequency when larvae present high level of spontaneous locomotion (Figure 5). While we hypothesized that the co-release of GABA and peptides could explain this effect, we would like to discuss the possible contribution of hindbrain neurons. Using optogenetics, chx10+ hindbrain neurons were shown to drive the initiation of locomotion while their silencing was sufficient to stop ongoing locomotion (Kimura et al., 2013). This suggests that glutamatergic hindbrain neurons provide the necessary and sufficient excitation to drive locomotion (Hägglund et al., 2010). Our characterization of CSF-cNs’ projections together with our imaging assay revealed that CSF-cNs from the segment 1-5 can send projections deep into the lateral hindbrain, thus forming putative synapses with hindbrain glutamatergic neurons (Figure 1, 2, 11). However the nature of these hindbrain neurons is unknown (Kinkhabwala et al., 2011). Using backfill injections from segment 1-10, we found that hindbrain neurons potentially receiving inputs from CSFcNs shared anatomical features with spinal V0-v interneurons with two dendrites on each side of the soma. Further work will be necessary to determine whether these neurons are part of the V0 group and whether they are important for initiating locomotion. Chapter 2 will describe in details the nature of a second class of putative hindbrain targets. The problem of chloride homeostasis in developing neuronal networks While our data point to a contribution of V3 interneurons in the state-dependent modulation of locomotion, it also remains possible that CSF-cNs directly activate V0-v and V3 interneurons because of GABA being depolarizing at 4 dpf. The polarity switch of GABA occurs when the potassium-chloride co-transporter KCC2 is expressed during development (Ben Ari, 2002). This allows the extrusion of chloride from neurons, counter-balances the influx of chloride through the sodium-potassium-chloride 71 Chapter 1: connectivity of CSF-cNs co-transporter NKCC1, and reverses the concentration gradient of chloride, with chloride being more concentrated outside the cell. In zebrafish, it has been shown that KCC2 is expressed as soon as 2 dpf, a stage where the shift from excitation to inhibition (E/I) mediated by GABAergic inputs occurs in retinal ganglion cells (Reynolds et al., 2008; Zhang et al., 2010). These data suggest that the channel is functional as soon as it is expressed even though the state of KCC2 phosphorylation, which is an important factor for its regulation (Kahle et al., 2013), is not known during these experiments. In addition, it is unclear if such E/I shift occurs at the same stage in the spinal cord and GABA could be inhibitory via depolarizing blocks. To remove the ambiguity, we will perform perforated patch clamp recordings on identified CSF-cNs targets in order to probe the net effect of GABAergic inputs on spinal neurons active during locomotion. Figure 10. Backfill injections in the spinal cord reveal that CSF-cNs projections (green) target V0-v like neurons in the hindbrain (red). Dorsal view of the hindbrain in a 4 dpf larva. The dashed square is enlarged on the bottom left panel. Rostral is up. 72 Chapter 1: connectivity of CSF-cNs On the role of CSF-contacting neurons during slow swimming Following the idea that sensory feedback pathways from the periphery are integrated in spinal central pattern generating circuits (see introduction), studies from our lab have demonstrated that CSF-cNs constitute a special sensory interface located in the spinal cord itself and capable of detecting both pH variations and tail bending during locomotion. In addition we showed that these cells can modulate multiple excitatory elements within motor circuits. However, the precise contribution of CSF-cNs during slow locomotion remains to be fully characterized. To tackle this problem, one would need to silence the output of CSF-cNs during swimming or to eliminate CSF-cNs from the circuit. Such approaches are challenging because they rely on potent silencing tools and on our ability to track and analyze locomotion in moving animals. Among the genetically encoded tools available in zebrafish, nitroreductase (NTR, Curado et al., 2007) is particularly powerful for eliminating cells of interest provided that specific promoters are available to drive its expression. Here, the use of the Tg(pkd2l1:gal4) line to drive NTR expression in CSF-cNs is a problem because the line labels a subset of heart cells. Any behavioral defects could be attributed to heart dysfunction and would therefore be difficult to interpret. To circumvent this problem, we generated a pkd2l1 “direct” line (no UAS-Gal4), which led to a cleaner expression without noticeable heart expression. However, the expression of NTR was very dim and it was not possible to eliminate CSF-cNs (Dr. Prendergast, personal communication). Alternatively, we tried to silence CSF-cNs using Halorhodopsin (NpHR, Zhang et al., 2007) in head-embedded preparations where the fish can move its tail. We failed to see any modification of swimming activity in these experiments but this could be due to the high illumination power required by NpHR, which was not possible to reach when illuminating the whole tail in our paradigm. Looking for potent silencing tools, our lab started to collaborate with the teams of Dr. Suster and Dr. Kawakami, who had generated a zebrafish optimized version of the botulinum toxin light chain (BtxLC, Annex 1) as a mean to silence neuronal circuits. Our lab provided its expertise in electrophysiology and behavior analysis to validate and calibrate the use of Btx-LC in zebrafish (see the manuscript 73 Chapter 1: connectivity of CSF-cNs by Sternberg, Severi et al., in Annex 1 and data in Chapter 2). In particular, this work showed that Btx-LC is nontoxic, have no side effects, and is very efficient at silencing neuronal activity -we indeed verified that CSF-cNs synapses were silenced when CSF-cNs express Btx-LC. In the meantime, Böhm and Prendergast used Btx-LC to investigate the nature of CSF-cNs’ inputs and investigated the putative role of CSF-cNs during acoustic escapes responses in freely swimming larvae (Böhm, Prendergast et al., 2016, Figure 11). At the time, the tracking software used for isolating features such as the tail angle and the tail-beat frequency could not efficiently track spontaneous slow swimming because the signal to noise ratio of these events was too low. Therefore, it was not possible to use this assay for investigating behavioral defects during slow swimming in a high throughput manner. With the help of Dr. Mirat who developed the first version of our tracking software (Mirat et al., 2013), Prendergast and Tseng in the lab refined the analysis and it is now possible to resolve slow swimming. The analysis of the slow swimming dataset in larvae expressing Btx-LC in CSF-cNs will be available soon and should provide useful insights on the role of CSF-cNs during this particular locomotor behavior. 74 Chapter 1: connectivity of CSF-cNs Can CSF-cNs modulate distinct locomotor behaviors using circuit-specific mechanisms? Analysis of the contribution of CSF-cNs to evoked fast locomotion Böhm and Prendergast used Btx-LC to investigate the contribution of CSF-cNs during acoustic escape responses in freely swimming larvae and found that the mean swimming frequency was significantly decreased when about 70% of CSF-cNs are silenced (Figure 11). To further identify if fast frequencies are specifically affected in these escapes, they analyzed the frequency band above (30Hz) to isolate the fast component and also found a decrease of the swimming frequency (Figure 12). These data suggest that CSF-cNs can silence/inhibit premotor circuits during slow locomotion while their inputs appear critical for the execution of escape responses at an optimal speed. If this idea is true, it means that CSF-cNs can target spinal neurons involved in slow and fast locomotion, which are thought to be part of distinct microcircuits (McLean et al., 2008; Ampatzis et al., 2014). The data from Hubbard in the lab already points to such hypothesis as CSF-cNs can modulate neurons involved in the initial part of touched-evoked escape responses. However, these neurons (CaP motor neurons and CoPA interneurons) are not involved in controlling the frequency of locomotion, suggesting that the effect reported by Böhm and Prendergast is driven by the contribution of a new, yet unidentified, target. In order to form a coherent picture of the function of CSF-cNs during locomotion, it becomes critical to better understand the mechanisms of modulation that these cells perform on each of their targets. We pointed to experiments that will determine the polarity of CSF-cNs inputs on targets involved in slow swimming but there seems to be an important, and likely different, modulation of the circuit driving fast locomotion. The work presented in Chapter 2 aims to identity the targets of CSF-cNs that control the frequency of locomotion and unravel the circuit mechanisms deployed by CSF-cNs to differentially modulate slow and fast locomotion. 75 Chapter 1: connectivity of CSF-cNs Figure 11. Silencing the output of CSF-cN leads to a reduction of swimming frequency during the escape (A) Experimental setup monitoring at high speed the escape response of freely swimming zebrafish larvae isolated in separated swim arenas triggered by 10 ms, 500 Hz acoustic stimuli. Acoustic stimuli were repeated five times per larva with 2 min inter trial interval. (B) Superimposed images showing a typical acoustic escape, (C) tracking corresponding to the same escape, swim distance (1) is derived from swim bladder position. (C) kinematic analysis relied on the measure of the tail angle over time α(t) enabling to measure latency (2), escape duration (3), C-bend amplitude (4) and number of oscillations and tail beat frequency (TBF) based on detection of subsequent peaks (5). Speed is derived from swim distance divided by duration. Scale bar: 2 mm. (D) Silencing of vesicular release in CSF-cNs by Botulinum toxin (Btx-LC) causes behavioral deficits, in particular, a reduction of TBF. TBF is significantly reduced in Btx-LC+ larvae (p = 0.0042), siblings represented in black (368 escapes from 128 larvae) and Btx-LC+ larvae in green (177 escapes from 74 larvae). Adapted from Boehm, Prendergast et al., 2016. Figure 12. Kinematic variables separately analyzed for fast components of acoustic escape responses in Tg(pkd2l1:gal4;UAS:BoTxBLC-GFP) larvae. The left group of each subplot represents control siblings, whereas the right group represents mechanosensory deprived fish. Courtesy of Brian Tseng and Dr. Andrew Prendergast. 76 Chapter 2: speed modulation by CSF-cNs Chapter 2- Modulation of spinal circuits controlling the frequency of locomotion by cerebrospinal fluid-contacting neurons In larval zebrafish, motor neurons are recruited in a topographic manner, from ventral to dorsal according to the swimming frequency (McLean et al., 2007). Ventral motor neurons are active during slow swimming (20-30 Hz on average) while more dorsal motor neurons are incrementally recruited as the speed of locomotion increases (30-80 Hz). At the premotor level, glutamatergic V0-v interneurons are only active during slow swimming while V2a interneurons are active across the entire frequency range and follow the same recruitment topography as for motor neurons (McLean et al., 2007; McLean et al., 2008; Ampatzis et al., 2014). These observations led to the idea that V2a interneurons control the frequency of locomotion, a hypothesis supported by their microcircuit organization where ventral V2a interneurons preferentially drive the recruitment of ventral motor neurons and dorsal V2a preferentially activate dorsal primary motor neurons (Ampatzis et al., 2014). The source of excitation driving the activation of spinal V2a interneurons has not yet been identified but hindbrain V2a neurons seem to play a critical role in initiating swimming (Eklöf-Ljunggren et al., 2012; Kimura et al., 2013; Eklöf-Ljunggren et al., 2014). The organization of hindbrain circuits with regard to speed modulation is not well understood but it is plausible that hindbrain and spinal V2a neurons may be connected in order to control the speed dependent recruitment of spinal microcircuits. The role of V2a interneurons in controlling the frequency of locomotion in zebrafish prompted us to ask whether these neurons could be at the core of the circuit-specific modulation mediated by CSF-cNs. Calcium imaging and two-photon laser ablation of V2a interneurons during experiments where locomotion is induced by electrical stimulation have highlighted the importance of V2a neurons for the production of fast locomotor frequencies (Eklöf-Ljunggren et al., 2012). There are several ways of inducing fast locomotor behaviors in larval zebrafish notably through electrical stimulation of hindbrain circuits or by applying water or air puff to activate the vestibular system and each of these stimu- 77 Chapter 2: speed modulation by CSF-cNs lations can potentially engage distinct descending pathways. We first determined the contribution of V2a neurons during acoustic escape responses, which we know are modulated by CSF-cNs (Figure 11, 12, 13, and 14, Böhm, Prendergast et al., 2016). BoTxBLC-mediated silencing of V2a interneurons confirms their critical role in fast locomotion We tested how V2a silencing impacts fast and slow locomotor regimes during active locomotion. Expressing BoTxBLC-GFP under the control of the chx10 promoter targeted most V2a interneurons (Figure 13A1-13A2). Consequently, acoustic escape responses in these larvae show a large reduction in the distance traveled in responsive trials compared to control siblings (Figure 13A3-13A4). In Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae assayed in a head-embedded and tail-free configuration (Figure 13B-13C), the maximum bend angle, average tail-beat frequency, and response duration were all significantly reduced compared to control siblings during the acoustic escape response (Figure 13D). Although the Tg(chx10:gal4) transgenic line recapitulated chx10 expression in the hindbrain and rostral spinal cord, a few neurons with axons exiting the spinal cord were labeled caudally (Figure 13A2), which could contribute to the response by affecting muscle contraction. To circumvent this confound, we performed fictive ventral nerve root (VNR) recordings in 4 dpf paralyzed larvae, which allowed monitoring of the motor neuron output upstream of the neuromuscular junction. In control siblings, fast escape responses induced by otic vesicle stimulation consisted of a few large amplitude bursts (Phase 1), followed by fast frequency swimming (~35-60 Hz, Phase 2), which transitioned to slow frequency swimming (<35 Hz, Phase 3) (Figure 13E). Silencing V2a output selectively disrupted fast swimming (Figure 13F); the response duration was not significantly reduced (Figure 13G). Fast locomotor frequencies above 40 Hz were abolished, while lower frequencies were unaffected (Figure 13H-I). 78 Chapter 2: speed modulation by CSF-cNs Figure 13. V2a interneurons are critical for induced fast-frequency swimming (A) Comparison of control siblings and Tg(chx10:gal4; UAS:BoTxBLC-GFP) 5 dpf larvae in a freely swimming acoustic escape assay. (A1) Whole larva lateral view. (A2) Lateral view of the spinal cord with borders denoted by dotted line. (A3) Trajectory of swimming larvae in the assay. Control siblings are Tg(UAS:BoTxBLC-GFP) larvae. (A4) The average distance traveled during a responsive trial (mm) (n = 11 control larvae, 81 responses out of 120 trials and 12 BoTxBLC-GFP+ larvae, 107 responses out of 120 trials). (B-D) Comparison of control siblings and Tg(chx10:gal4; UAS:BoTxBLC-GFP) 5 dpf larvae in a head-embedded, tail-free acoustic escape 79 Chapter 2: speed modulation by CSF-cNs assay. (B) Left: control larva (Tg(UAS:BoTxBLC-GFP)), Right: Tg(chx10:gal4;UAS:BoTxBLC-GFP) larva with its head embedded in agarose and the tail free to move; dotted line indicates agar border. (C) Example tail-bend angle (degrees) extracted for head-restrained control larvae (Tg(UAS:BoTxBLC-GFP) alone, black, top) and BoTxBLC-GFP (Tg(chx10:gal4; UAS:BoTxBLC-GFP), teal, bottom); dotted line indicates the time of stimulus delivery. (D) Kinematic analysis of head-embedded acoustic-induced escape responses. Maximum bend angle in the trial (left, degrees), average tail-beat frequency within a bout (center, Hz) and average bout duration (right, ms) (n = 12 control larvae, 104 trials. n = 12 Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae, 118 trials). (E-I) Analysis of fictive VNR recordings during escape responses in 4 dpf larvae. (E) Schematic of the otic vesicle stimulation used to evoke fast escape responses in combination with fictive VNR recordings (left). Three phases of the response can be distinguished in the escape response (right). (F) Representative traces of fictive escape responses from 3 larvae for control (F1) and Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae (F2). Red asterisks indicate the stimulus onset. (G-H) Response duration (G, s) and mean burst frequency (H, Hz) for n = 57 stimulations for 5 control larvae and n = 54 stimulations for 5 BoTxBLC-GFP+ larvae. (I) Distribution of burst frequencies (Hz) in control larvae (grey) and BoTxBLC-GFP+ larvae (teal). Inset: cumulative distribution function. (J-N) Calcium transients, imaged in motor neurons as a function of dorsoventral position, when V2as are silenced (BoTxBLC-GFP+) or not (control) with simultaneous fictive VNR recording. (J) Schematic of the calcium imaging configuration performed in combination with otic vesicle stimulation and VNR recordings as described in (E). (K) GCaMP5G expression in Tg(mnx1:GCaMP5G) with ROIs overlaid (top), calcium transients (center) and fictive VNR recording during a fictive escape in ventral motor neurons (bottom). Control larva: purple ROIs, black ΔF/F trace (left). BoTxBLC-GFP+ larva: teal ROIs, teal ΔF/F trace (right). (L1-L3) Peak ΔF/F response for an individual ROI, color-coded by larva, with the average across larvae indicated by a black line. Comparison of control vs. BoTxBLC-GFP+ larvae for ventral motor neurons at dorso-ventral positions: 0-0.2 (L1), 0.2-0.3 (L2), or 0.3-0.4 (L3). (M) Same as (K) but for dorsal motor neurons. (N1-N3) Same as (L1-L3) but for dorso-ventral positions: 0.4-0.5 (N1) or 0.5-1 (N2). For (L) and (N), n = 199 ROIs from 55 stimulations with swimming episodes for 5 control larvae and n = 102 ROIs from n = 30 stimulations with swimming episodes for 4 BoTxBLC-GFP+ larvae. Scale bars are 100 µm in (A1), 25 µm in (A2), 5 mm in (A3), 1 mm in (B), 20 µm in (K and M). For (A4), (D), (G), and (H), each data point is the average across all trials for a single larva. Control sibling Tg(UAS:BoTxBLC-GFP) larvae are in grey and Tg(chx10:gal4; UAS:BoTxBLCGFP) are in teal. Means are shown ± S.E.M. in black. p-values ** <0.01, *** <0.001, **** <0.0001. Motor neurons are incrementally recruited along the dorsoventral axis with swimming frequency. To investigate how motor neuron recruitment was affected when V2as were silenced, we generated a novel transgenic line, Tg(mnx1:GCaMP5G), in which motor neurons express the genetically-encoded calcium sensor GCaMP5G, and coupled calcium imaging of motor neurons with otic-vesicle stimulation and VNR recordings (Experimental Procedures, Figure 13J-13N). The amplitude of calcium transients varied as a function of the dorsoventral position within the motor pool in a different manner for control Tg(mnx1:GCaMP5G) larvae and V2a silenced Tg(mnx1:GCaMP5G; chx10:gal4; UAS:BoTxBLC-GFP) larvae (Figure 13K, 13M, p = 0.000005). Overall, the average peak ΔF/F amplitude was highest in the most dorsal motor neurons in control larvae. Ventral motor neurons exhibited similar ΔF/F amplitude whether V2as were silenced or not (Figure 13L). In contrast, the activity of dorsal motor neurons was largely reduced in V2a-silenced larvae compared to controls (Figure 13N). A reduction in the recruitment of dorsal motor neuron is consistent with our observations that 80 Chapter 2: speed modulation by CSF-cNs fast locomotor frequencies (> 30 Hz) in both active and fictive locomotion are abolished when V2as are silenced. Silencing of V2a interneurons decreases the locomotor frequency during spontaneous slow swimming Despite the role of hindbrain V2a neurons in initiating locomotion in zebrafish larva, the V2a contribution to spontaneous slow swimming has not been directly addressed. In freely swimming larvae, we noted that the occurrence of spontaneous slow swimming was largely reduced in Tg(chx10:gal4;UAS:BoTxBLC-GFP) (Figure 14A-14D), indicating that these neurons normally drive spontaneous locomotion. We observed that the rarely-occurring swim bouts were on average longer in duration (Figure 14E). In V2a silenced larvae, fictive slow swim bouts occurred less frequently and tended to last longer than in control siblings (Figure 14F-14H). In contrast to fast escapes, in which a reduction of frequency occurred via a suppression of the highest frequencies (Figure 14I), in the slow regime, swim frequency was reduced by a downward shift of locomotor frequencies (Figure 14I-14J). Taken together, the active and fictive data demonstrate that V2a interneurons contribute to generation of spontaneous swim events and setting the range of locomotor frequencies during slow locomotion. Given the contribution of V2a interneurons in acoustic escape responses and the ability of CSF-cNs to modulate this behavior, we tested if V2a interneurons are targeted by CSF-cNs and if yes, to what extent the profile of innervation resembles what we have identified for other targets such as V0-v and V3 interneurons. 81 Chapter 2: speed modulation by CSF-cNs Figure 14. V2a interneurons drive most of spontaneous slow swimming and adjust the locomotor frequency in the slow regime (A-E) Spontaneous slow swimming assay comparing control and Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae at 5 dpf. (A) The trajectory of a 5 dpf larvae spontaneously swimming for the first minute of a four minute recording. Left: control sibling (Tg(UAS:BoTxBLC-GFP) alone, Right: Tg(chx10:gal4; UAS:BoTxBLC-GFP) larva recorded simultaneously. (B) Average distance traveled (mm, measured during the first minute of the trial). (C) Rastergram of the timing of swim bout initiation over the four minute spontaneous swimming trial recorded. The y-axis represents a different individual larva (Top: control siblings, Bottom: Tg(chx10:gal4;UAS:BoTxBLCGFP)). (D) Mean frequency of bout occurrence (bouts per second, Hz) measured over a four minute trial. (E) Average bout duration (ms) across all bouts recorded spontaneously in a four minute trial. (B-E) n = 8 control and 8 BoTxBLC-GFP+ larvae. (F) Schematic and example trace of fictive slow swimming in a control (top) and a BoTxBLC-GFP+ (bottom) larva. Boxes indicate expanded regions at right. Note the reduced burst frequency in BoTxBLC-GFP+ larvae when V2as are silenced. (G) Reduction in frequency of bout occurrence (bouts per second) in BoTxBLC-GFP+ larvae compared to control siblings (n = 1265 bouts for 8 control larvae, 938 bouts for 8 BoTxBLC-GFP+ larvae). (H) Fictive bout duration (s). (I) Mean burst frequency during fictive slow swim bouts (Hz). (H-I) n = 741 bouts from 8 control larvae and 497 bouts from 8 BoTxBLC-GFP+ larvae. (J) Distribution of burst frequencies (Hz) in control larvae (grey) and BoTxBLC-GFP+ larvae (teal). Inset: cumulative distribution function. For (B), (D), (E), (G), (H), and (I): Control siblings (grey, Tg(UAS:BoTxBLCGFP)) were compared to Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae (teal) and each point represents a single larva. Means are shown ± S.E.M. in black. p-value * <0.05, ** <0.01, *** <0.001, **** <0.0001. Student’s t-test for (D) and (G). 82 Chapter 2: speed modulation by CSF-cNs Topographic organization of CSF-cNs inputs onto V2a interneurons We imaged Tg(pkd2l1:gal4;UAS:ChR2-YFP;chx10:lox-DsRed-lox-GFP) triple transgenic larvae and found two types of anatomical contacts between CSF-cNs and V2a neurons (Figure 15). We noted that putative presynaptic structures along CSF-cN axons were positioned onto axons of V2a neurons (Figure 15A) or closer to the soma possibly in the vicinity of the axon initial segment (Figure 15B). We also found putative synaptic boutons surrounding cell bodies on ventral V2a neurons both in the hindbrain and in the spinal cord (Figure 15D, 15E). It was unclear whether axo-axonic contacts originated from ventral or more dorsal V2a neurons. To disentangle the two possibilities, we performed backfilling injections from caudal regions of the spinal cord (from segments 15 to 20). This technique allowed us to better resolve axonal processes that can be sometimes difficult to image and track using our transgenic reporter lines, while recording the position of the cell body of the labeled neuron (Figure 15C, 15F, 15G). Using this technique, we confirmed the presence of axo-axonic contact between descending interneurons and CSF-cNs (Figure 15F). In addition, we mapped the position of cell bodies of V2a receiving either axo-somatic or axo-axonic inputs from CSF-cNs or the cell bodies of descending interneurons labelled in our backfill approach. This mapping strategy revealed that V2a and descending interneurons that receive axo-somatic inputs preferentially lie in the ventral spinal cord (between 0 and 0.45 on the D-V axis, Figure 15G), which is reminiscent of what we found for ventral V0-v and V3 interneurons. In contrast, V2a and descending neurons receiving axo-axonic inputs were preferentially restricted to the dorsal spinal cord (between 0.45 and 0.7 on the D-V axis, Figure 15G). While we cannot exclude that ventral neurons including V2a, V0-v and V3 neurons receive axo-axonic inputs, we did not observe such contacts. First, axons of V0-v cross the midline and can hardly be seen in the vglut2a transgenic reporter line. Second, it is possible that backfill injections are biased towards axons projecting to more dorsal portions of the spinal cord, which is why we could not label ventrally projecting cells as efficiently. Finally, we may have only targeted long-projecting propriospinal neurons at the expense of cells with more local axonal projections such as V3 interneurons. Apart from this, it remains clear that ventral glutamatergic neurons seem to be mainly targeted at the cell body 83 Chapter 2: speed modulation by CSF-cNs level. In contrast, we rarely observed dorsal neurons with inputs on their soma, the dorsal-most being around 0.5 on the D-V axis (Figure 15, except CaP and CoPA neurons, see Chapter 1). Figure 15. Anatomy of connections between CSF-cNs and V2a neurons (A) Z projection stack of a few optical sections imaged from the dorsal side in Tg(pkd2l1:gal4;UAS:ChR2-YFP; chx10:lox:DsRed:lox:GFP) triple transgenic larvae reveal the localization of V2a neurons (DsRed +, red) relative to CSF-cN projections (YFP+, green). Arrowheads point to putative axo-axonic contact between axons of the two cell types. (B) Apposition of CSF-cN axonal varicosities onto a region close from the soma of a chx10 + interneurons (arrowhead). (C) Schematic for the backfilling injections. Alexa 647 is injected in the spinal cord where it is up taken by damaged axons and transported back up to the soma. (D, arrowheads) Examples of perisomatic innervation of V2a cell bodies by axonal processes from CSF-cNs. (E, arrowheads) Same as in (D) but in the caudal portion of the hindbrain. (F) Examples of backfilled descending neurons. Close up panels highlight putative axo-axonic contacts. (G) Map of cell body positions of V2a and descending interneurons imaged in the chx10 transgenic reporter line or after backfilling injections. 84 Chapter 2: speed modulation by CSF-cNs Discussion and perspectives Experimental strategy to probe the modulation of V2a neurons by CSF-cNs First, we will analyze the properties of V2a-v/CSF-cN synapses in order to determine if this projection shares properties with the one identified onto V0-v neurons. Since V0-v, V2a-v, and V3 share a common pattern of somatic innervation, it is likely that their firing and outputs are similarly modulated by CSF-cNs. The identified projections suggest that CSF-cNs have the ability to modulate most of the glutamatergic premotor drive involved in slow swimming generation identified so far. Undoubtedly, confirmation of the functional connectivity using electrophysiology combined with a more careful analysis of the slow swimming after silencing the vesicular release of CSF-cNs using Btx-LC will allow us to formulate definitive conclusions on the modulatory role of CSF-cNs during slow locomotion. Next, we will take advantage of the connectivity map of V2a interneurons identified recently to probe the putative modulation of synaptic transmission between dorsal V2a interneurons and their target motor neurons by performing paired recording between the two types while activating CSF-cNs projecting their axons onto V2a axons. This experiment is critical to understand if the net effect of the axonic modulation potentially mediated by CSF-cNs differs from the somatic modulation found for V0-v neurons and other ventral targets. Work in the pyramidal cortex in mouse tackled this question by looking at the effect of pyramidal neurons output modulation by GABAergic Chandelier cells that specifically target pyramidal neurons at the axonic level, more precisely at the site of their axon initial segments (Szabadics et al., 2006). Here, the net effect of GABAergic currents is reversed and depolarizes pyramidal neurons probably because the concentration of chloride is higher in the small axonic structure compared to the extracellular medium, thus shifting the ECl potential to more positive values. Even though these results were obtained in a completely different structure, in a different model organism, and were subject to controversy in the field of cortical circuits, they nonetheless set the ground for similarities in the zebrafish spinal cord given the ability of CSF-cNs to target axonal processes. It is currently difficult to predict the outcome of the paired recording experiments but one pos- 85 Chapter 2: speed modulation by CSF-cNs sibility is that inputs from CSF-cNs at the axon level increase motor neuron excitability, thus yielding to the generation of more action potentials or to an increase of the firing frequency. Alternatively, and because CSF-cNs are activated during the muscle contraction, thus several milliseconds after motor neurons receive the initial volley of excitatory drive from V2a neurons, inputs from CSF-cNs could contribute to the maintenance of the optimal firing frequency of motor neurons by controlling the timing of glutamatergic inputs to motor neuron while the movement is executed. Some assembly required: building a global picture of CSF-cNs-mediated modulation of spinal central pattern generators The work presented in this manuscript allowed us to define the connectivity diagram of CSF-cNs onto excitatory premotor interneurons in the spinal cord (Figure 16). The challenge now lies in getting a unified picture of their modulatory role in the spinal cord. Altogether, the proposed set of experiments combining electrophysiology and behavior analysis will enable us to determine if there is a circuitspecific and behavior-dependent modulation of locomotion mediated by this peculiar sensory feedback pathway in zebrafish. These results will contribute to the identification of a previously uncharacterized mechanism of circuit modulation in the spinal cord by revealing axonic modulation of premotor circuits by sensory feedback. We believe this observation is new and important because it redefines how we approach sensory processing in the spinal cord. In particular, it brings a new model of circuit modulation contrasting with the classical view where central circuits can modulate sensory feedback pathway at the presynaptic level. Future projects should determine how the connections present at the level of the brainstem versus those found in the spinal cord contribute to the modulation of motor circuits. Are V2a interneurons at the core of a global mechanosensory feedback loop? Work by Dr. Knafo in the lab recently addressed the contribution of glutamatergic sensory pathways during locomotion by targeting the expression of the optical sensor Aequorin in motor neurons, comparing the pattern of recruitment of motor neurons in active vs. fictive swimming, and by 86 Chapter 2: speed modulation by CSF-cNs Figure 16. Map of connectivity of CSF-cNs onto the locomotor CPG in zebrafish manipulating the activity of isl2b+ mechanosensory neurons using BoTxBLC (Knafo et al., in preparation, see Annex 2). Knafo and colleagues observed a decrease of swimming frequency when most of the glutamatergic mechanosensory neurons (including trigeminal neurons, Rohon-Beard cells and DRG neurons) were genetically silenced. I joined the project and analyzed the putative connectivity between these sensory neurons and V2a interneurons in the zebrafish spinal cord. Interestingly, I found that the dorsal-most V2a interneurons, i.e. the V2a neurons controlling fast swimming, were embedded in the bundle of axons from both RB and DRG neurons (Figure 17, Annex 2). This observation is interesting because it suggests that different sources of sensory feedback collectively target the V2a population in order to dynamically tune the frequency of ongoing locomotion in zebrafish. I will soon test the functional connectivity between V2a interneurons and RB/DRG cells using electrophysiology and optogenetics in order to determine the nature of the sensory modulation that could explain the effect we observed on swimming frequency. 87 Chapter 2: speed modulation by CSF-cNs Figure 17. Isl2b+ mechanosensory neurons (RB and DRG neurons) project onto dorsal V2a interneurons. (A) Z-projection stack of the spinal cord imaged from the lateral side in a 4dpf Tg(isl2b:gal4;UAS:aequorinGFP;chx10:DsRed) triple transgenic larva. The white dashed lines delineate the ventral and dorsal limits of the spinal cord; these limits define the ventro-dorsal (D/V) axis from 0 to 1. The axon bundle from isl2b+ Dorsal Root Ganglia (DRG) and Rohon Beard (RB) neurons contact the soma of dorsal chx10+ V2a interneurons. The white square is enlarged in the right panel to stress the anatomical connections (arrow). (B) Dendrites of dorsal V2a interneurons are targeted by axonal processes from isl2b+ sensory neurons (arrows). (C) Positions of V2a neurons receiving putative inputs from isl2b+ cells (mean D/V position = 0.72 ± 0.04. N = 12 cells in n = 4 fish. For each panel rostral side is on the left. Scale bar in (A) is 50 µm and 10 µm in (B). 88 Conclusions and future directions The work presented in this thesis led to the first description of the functional connectivity of cerebrospinal fluid-contacting neurons. It is now clear that CSF-cNs have the ability to modulate distinct circuits in the spinal cord and as a consequence, distinct locomotor behaviors. It is important to pursue the connectivity mapping effort to have a precise picture of the circuit formed by CSF-cNs. New optical techniques will help us refine our optogenetic paradigm notably by photostimulating only one or a subset of CSF-cNs at a time while recording or imaging postsynaptic targets in 3D (see Annex 3). This approach will help us determine if there are different functional subsets of CSF-cNs in the spinal cord. However, there are several challenges to tackle in order to fully understand the role of these sensory neurons. First, future work should focus on the identifying and classify the types of sensory cues that mediate the activation of CSF-cNs in vivo. We know that CSF-cNs respond to pH changes, a feature that is likely shared across species, and axial bending, a feature that is likely specific to swimming vertebrates. What we do not know yet is whether CSF-cNs can detect compounds flowing in the CSF, and if yes, which ones. The morphology of CSF-cNs suggest that they are sensing but also releasing molecules in the CSF. What for? A thorough analysis of the genetic profile of CSF-cNs will help us identifying genes and/or receptors expressed by these neurons and guide our search for novel sensory cues as well as potential distal targets that are not necessarily present in the spinal cord. Once we have a better picture of the genetic and sensory identity of CSF-cNs, it will be important to reconcile the different chemical responses to the modulation of locomotion. Are chemical and mechanosensory responses linked or independent in fish? Finally, once we have a good grasp on the function of these neurons in zebrafish, it will become critical to assess the role of these neurons in mammals and in limbed vertebrates in particular. It is currently unknown whether CSF-cNs target and modulate locomotor circuits in limbed vertebrates. In addition, it remains unclear if CSF-cNs are capable of sensing molecules from the CSF in mammals. Altogether our approaches combining genetics, optophysiology, and behavior analysis should enable us to test most of these questions in the future. 89 Experimental procedures Materials and methods Animal care Animal handling and procedures were validated by ICM and the National Ethics Committee (Comité National de Réflexion Ethique sur l’Expérimentation Animale, Ce5/2011/056) in agreement with EU legislation. Adults were reared at a maximal density of eight animals per liter in a 14/10 hr light/dark cycle environment. Fish were fed live artemia twice a day, and feeding regime was supplemented with solid extracts matching the fish developmental stage (ZM Systems). Larvae were raised at 28.5_C with a 14/10 day/night light cycle. Experiments were performed at room temperature (22-C–25-C) on 3–5 dpf larvae Generation of transgenic animals To generate the Tg(pkd2l1:gal4)icm10 transgenic line expressing the Gal4 reporter gene in pkd2l1 expressing cells, we amplified 3.8 kbp of genomic sequence immediately upstream of the predicted ATG site for the zebrafish pkd2l1 gene (ENSDARG00000022503). Analyzing the genomic locus in various Teleostian species, the second intron of the predicted transcript was remarkably conserved in length. We amplified this sequence from zebrafish cDNA as it was likely to contain regulatory elements recapitulating pkd2l1 expression. Both PCR fragments were cloned in the pCRII vector using the TOPO TA cloning kit according to manufacturer’s instructions (Invitrogen). The correct DNA inserts were verified by sequencing. Both DNA fragments were then subcloned in the pT2KhspGFF plasmid [2] using standard molecular biology techniques. The 3.8 kb promoter region was cloned upstream of the Gal4 (GFF) sequence in place of the hsp promoter present in the pT2KhspGFF plasmid, while the intronic sequence was placed after the Gal4 stop codon and before the SV40 poly-A site. To generate a stable transgenic line, a solution containing 20 ng/μl plasmid DNA, 50 ng/μl tol2 transposase mRNA was injected into Tg(UAS:Kaede)s1999t embryos [3] to reveal Gal4 expression. Injected embryos were screened at 2 dpf under a dissecting fluorescent microscope. Embryos showing Gal4 expression in the spinal cord were raised and outcrossed to obtain a stable line. We generated the Tg(UAS:ChR2-YFP)icm11 line by injecting the original published construct UAS:ChR2(H134R)90 Experimental procedures eYFP [4]. The Tg(UAS:GCaMP5G)icm08 line was generated by regular cloning in the PT2 14xUAS vector from Prof. Kawakami's lab. 50 ng/μl of this vector were injected with Tol2 RNA into eggs at the one-cell stage from the outcross of Et(e1b:Gal4-VP16)s1020t [3] with wild type TL. Potential founders were screened later on by outcrossing to wild type animals. Generation of animals with mosaic labeling of CSF-cNs To trace individual CSF-cNs, we took advantage of multiple approaches relying on the specific Tg(pkd2l1:Gal4)icm10 line. For 36 cells, we injected the UAS:synaptophysin-GFP [5] DNA construct at 60 ng/μl into single cell-stage embryos. We reconstructed 29 cells after imaging highly variegated carriers of the Tg(pkd2l1:gal4;UAS:ChR2-mCherry) constructs. For 22 cells, the Tg(pkd2l1:tagRFP) construct generated with a three-fragment Gateway recombineering reaction (Invitrogen, Carlsbad, CA, USA) was injected into wild-type embryos at 25 ng/μl. For one cell, we used the Tg(Brn3c:Gal4:UAS:GFP) transgenic line (BGUG) to label a subset of Gal4 expressing cells [3, 6]. 3 dpf larvae selected for single CSF-cN expression were fixed with 4% PFA for 3 hours and immunostained using one of following antibodies: chicken anti-GFP (1:500, Abcam, Cambridge, UK), mouse antimCherry (1:500, Clontech, Mountain View, CA, USA) and rabbit anti-tagRFP (1:500, Evrogen, Moscow, Russia). Morphological analysis of isolated CSF-cNs Images of single CSF-cNs were acquired using an FV1000 confocal microscope (Olympus, Tokyo, Japan) equipped with a 40x water immersion objective using the 473 nm and 543 nm laser lines. For each cell, multiple z-stacks (step size 1 μm) were acquired to capture the entire arborization of the axon. Multiple stacks from a single cell were combined using the Grid/Collection stitching plugin in Fiji [7] or the XuvTools stitching software [S8]. Cell morphology was reconstructed into a threedimension image using the Simple Neurite Tracer (SNT) plugin in Fiji [9]. The total axon length and branching hierarchy were obtained from SNT. Other morphological parameters were extracted using a custom-made MATLAB script. The dorsoventral (D-V) soma positions were measured from the center of the cell body and normalized to the limits of the spinal cord. Counting of presynaptic boutons was 91 Experimental procedures performed in a semi-automated manner using a custom-made MATLAB script. To distinguish boutons from vesicles, we imaged live segments of CSF-cNs axons (n = 23, Movie S1). We noticed that vesicles were recognizable as they were small and rapidly migrating along the axon while boutons were bright and immotile (Movie S1). Fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC) The procedure used here relied on published protocols [10]. Briefly, whole-mount ISH were performed on 3 dpf larvae fixed in 4% PFA in PBS overnight at 4◦C. To reveal pkd2l1 expression, probes [10] were detected with anti-DIG antibodies (Roche Diagnostics, France). pkd2l1 FISH was performed before IHC against GFP: embryos were washed and immunostained with the chicken anti-GFP antibody antibody overnight at 4◦C, and then incubated with the corresponding Alexa conjugated secondary antibodies IgG (1:500, Life Technologies) combined with DAPI (2.5 μg/ml, Life Technologies). Live imaging of spinal neurons Zebrafish larvae were imaged using an upright microscope (Examiner Z1, Zeiss) equipped with a spinning disk head (CSU-X1, Yokogawa) and a modular laser light source (LasterStack, 3i Intelligent Imaging Innovations). Z projection stacks were acquired using Slidebook software (3i) and reconstructed online using Fiji (http://fiji.sc/Fiji). Calcium imaging of spinal neurons Zebrafish larvae were imaged using a custom spinning disk microscope (Intelligent Imaging Innovation, Denver, USA) equipped with a set of water-immersion objectives (Zeiss 20X, 40X, NA=1). Recordings were acquired using Slidebook® software at 10-20 Hz at 488nm. Gain and binning were optimized to maximize signal to noise ratio. Z projection stacks showed full pattern of expression using Fiji (43). Positions of cells along the D-V axis were computed using Fiji and Matlab. Calcium signals were extracted online using custom MATLAB scripts. Regions of interest (ROIs) were manually designed and calcium signals time series were extracted as the mean fluorescence from individual ROIs at each time point of the recording. We observed that out-of- focus signals varied between ani92 Experimental procedures mals, from dorsal to ventral spinal cord regions in a behavior-dependent manner. To estimate the contribution of out-of- focus signals we systematically picked two background ROIs, one placed below the ventral limit of the spinal cord to capture out-of- focus signals at the level of ventral motor neurons during slow swimming, the second in the dorsal-most part of the spinal cord to capture out-of- focus signals in the dorsal spinal cord during the escape. We estimated the maximum out-of- focus signals observed during each behavior and used this value as a threshold for discriminating active from silent motor neurons. Sample preparation for fictive locomotion recordings and optogenetic stimulations 3 dpf Tg(pkd2l1:gal4;UAS:ChR2-YFP) larvae were screened for dense labeling and bright expression of ChR2-YFP in CSF-cNs under a dissecting fluoroscope (Leica). Larvae were anaesthetized in 0.02% Tricaine-Methiodide (MS-222, Sigma-Aldrich) diluted in fish facility water and then mounted upsidedown (ventral side facing up) in glass-bottom dishes (MatTek) filled with 1.5% low-melting-point agarose. We surgically removed the eyes using a thin tungsten pin in order to avoid light-evoked locomotion with blue light during ChR2 stimulation. Following the surgery, larvae were transferred in cold ACSF (concentrations in mM: 134 NaCl, 2.9 KCl, 1.2 MgCl2, 10 HEPES, 10 glucose, and 2.1 CaCl2; 290 mOsm, adjusted to pH 7.7–7.8 with NaOH) for 3–5 min. Larvae were then transferred in fish facility water to recover for 24 hr at 28_C. The following day, larvae were mounted on their side and immobilized by injecting 0.5 nl of 0.5 mM a-Bungarotoxin in the ventral axial musculature (Tocris). A portion of agarose was removed using a sharp razorblade in order to expose 2–3 segments. Fictive locomotion recordings and optogenetic stimulation Our recording protocol is based on published procedures [17, 19, 48]. VNR recordings were acquired using a MultiClamp 700A amplifier, a Digidata series 1322A Digitizer, and pClamp 8.2 software (Axon Instruments, Molecular Devices). A blue LED (UHP-Mic-LED-460, Prizmatix) was used to activate ChR2. The light was delivered on the fish spinal cord through the microscope condenser, typically 20 segments from segment 7–8 to 27–28 with 14 mW/mm2. To time the optical activation of ChR2 after the onset of fictive swimming bouts, we designed a closed-loop program in which the LED was 93 Experimental procedures turned on via transistor-transistor logic (TTL) pulses 10 ms or 500 ms after the fictive motor output reached an arbitrary threshold. Parameters describing the fictive locomotion were extracted using custom-made MATLAB scripts. Bout frequency was analyzed in larvae with basal level of swimming activity above 0.3 Hz. Pharmacology 4 dpf larvae were pinned down in Sylgard-coated, glass-bottom dishes filled with ACSF with thin tungsten pins through the notochord. Skin was removed from segments 5–6 to the end of the tail using sharp forceps. GABAA receptor antagonist gabazine (SR95531, Tocris) or NMDA (Tocris) was bath applied at either 10 or 20 mM final concentrations. Fluorescence-guided whole-cell recordings Whole-cell recording were performed in head-off larvae in the same configuration as pharmacology experiments. After removing the skin, one to two segments were dissected using glass suction pipettes. Patch pipettes (1B150F-4, WPI) were designed to reach a tip resistance of 11–15 MU and were filled with potassium-containing internal solution (concentrations in mM: K-gluconate 115, KCl 15, MgCl2 2, Mg-ATP 4, HEPES free acid 10, EGTA 0.5, 290 mOsm, adjusted to pH 7.2 with KOH and supplemented with Alexa 647 at 4 mM final concentration). To resolve evoked inhibitory postsynaptic currents in voltage-clamp mode, cells were held at around -80 mV, away from the calculated chloride reversal potential (-51 mV). We calculated the liquid junction potential in our experiments (-19 mV) but did not correct for it since it did not affect the outcome of our experiments. Kinetic parameters of light-evoked currents and IPSCs were extracted and analyzed using custom made MATLAB scripts. Statistics Linear correlation in datasets was calculated using a Pearson’s linear correlation test. Comparisons between two groups of data were performed using a Student’s t test. A linear mixed-effects model was used to test the interaction between the LED and gabazine. The level of significance was p < 0.05 for all datasets. 94 Experimental procedures Accession numbers The three new zebrafish transgenic lines Tg(pkd2l1:gal4)icm10, Tg(UAS:ChR2-YFP)icm11, and Tg(UAS:GCaMP5G)icm08 have been deposited in the Zebrafish Model Organism database under ID codes ZFIN: ZDB-FISH-150901-9831, ZDB-FISH-150901-19823, and ZDB-FISH-150901-6255. Supplemental References 1. Satou, C., Kimura, Y., Hirata, H., Suster, M. L., Kawakami, K., and Higashijima, S. (2013). Transgenic tools to characterize neuronal properties of discrete populations of zebrafish neurons. Development 140, 3927–31. 2. Asakawa, K., Suster, M. L., Mizusawa, K., Nagayoshi, S., Kotani, T., Urasaki, A., Kishimoto, Y., Hibi, M., and Kawakami, K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci U S A 105, 1255–60. 3. Scott, E. K., Mason, L., Arrenberg, A. B., Ziv, L., Gosse, N. J., Xiao, T., Chi, N. C., Asakawa, K., Kawakami, K., and Baier, H. (2007). Targeting neural circuitry in zebrafishusing GAL4 enhancer trapping. Nat Methods 4, 323–6. 4. Schoonheim, P. J., Arrenberg, A. B., Del Bene, F., and Baier, H. (2010). Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J Neurosci 30, 7111–20. 5. Meyer, M. P., and Smith, S. J. (2006). Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci 26, 3604–14. 6. Xiao, T., Roeser, T., Staub, W., and Baier, H. (2005). A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development 132, 2955–67. 7. Preibisch, S., Saalfeld, S., and Tomancak, P. (2009). Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–5. 8. Emmenlauer, M., Ronneberger, O., Ponti, A., Schwarb, P., Griffa, A., Filippi, A., Nitschke, R., Driever, W., and Burkhardt, H. (2009). XuvTools: free, fast and reliable stitching of large 3D datasets. J Microsc 233, 42–60. 9. Longair, M. H., Baker, D. A., and Armstrong, J. D. (2011). Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 27, 2453–4. 10. Djenoune, L., Khabou, H., Joubert, F., Quan, F. B., Nunes Figueiredo, S., Bodineau, L., Del Bene, F., Burcklé, C., Tostivint, H., and Wyart, C. (2014). Investigation of spinal cerebrospinal fluidcontacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front Neuroanat 8, 26. 95 References Acerbo, M. J., Hellmann, B., & Güntürkün, O. (2003). Catecholaminergic and dopamine-containing neurons in the spinal cord of pigeons: an immunohistochemical study. Journal of Chemical Neuroanatomy, 25(1), 19–27. Agduhr, E. (1922).U¨ ber ein Zentrales Sinnesorgan (?) bei den Vertebraten. Z. Anat. Entwicklungs 66, 223– 360. Akay, T., Tourtellotte, W. G., Arber, S., & Jessell, T. M. (2014). Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proceedings of the National Academy of Sciences, 201419045. http://doi.org/10.1073/pnas.1419045111 Alaynick, W. A., Jessell, T. M., & Pfaff, S. L. (2011). SnapShot: spinal cord development. Cell, 146(1), 178– 178.e1. http://doi.org/10.1016/j.cell.2011.06.038 Alvarez, F. J., & Fyffe, R. E. W. (2007). The continuing case for the Renshaw cell. The Journal of Physiology, 584(Pt 1), 31–45. http://doi.org/10.1113/jphysiol.2007.136200 Alvarez, F. J., Jonas, P. C., Sapir, T., Hartley, R., Berrocal, M. C., Geiman, E. J., et al. (2005). Postnatal phenotype and localization of spinal cord V1 derived interneurons. The Journal of Comparative Neurology, 493(2), 177–192. http://doi.org/10.1002/cne.20711 Ampatzis, K., Song, J., Ausborn, J., & Manira, El, A. (2014). Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron, 83(4), 934–943. http://doi.org/10.1016/j.neuron.2014.07.018 Anden, N. E., Lundberg, A., Rosengren, E., & Vyklicky, L. (1963). The effect of DOPA on spinal reflexes from the FRA (flexor reflex afferents). Experientia, 19, 654–655. Anden, N. E., Jukes, M. G., & Lundberg, A. (1964). Spinal reflexes and monoamine liberation. Nature, 202, 1222–1223. Arber, S. (2012). Motor circuits in action: specification, connectivity, and function. Neuron, 74(6), 975–989. http://doi.org/10.1016/j.neuron.2012.05.011 Armstrong, D. M. (1986). Supraspinal contributions to the initiation and control of locomotion in the cat. Progress in Neurobiology, 26(4), 273–361. Azim, E., Jiang, J., Alstermark, B., & Jessell, T. M. (2014a). Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature, 508(7496), 357–363. http://doi.org/10.1038/nature13021 Azim, E., Fink, A. J. P., & Jessell, T. M. (2014b). Internal and External Feedback Circuits for Skilled Forelimb Movement. Cold Spring Harbor Symposia on Quantitative Biology, 79, 81–92. http://doi.org/10.1101/sqb.2014.79.024786 Baldiserra, F., Hultborn, H., Illert, M. (1981). Integration in spinal neuronal systems. Handbook of Physiology. The Nervous System. Motor Control. American Physiological Society Baldissera, F., Cavallari, P., Fournier, E., Pierrot-Deseilligny, E., & Shindo, M. (1987). Evidence for mutual inhibition of opposite Ia interneurones in the human upper limb. Experimental Brain Research, 66(1), 106– 114. Bagnall, M. W., & McLean, D. L. (2014). Modular organization of axial microcircuits in zebrafish. Science, 343(6167), 197–200. http://doi.org/10.1126/science.1245629 96 References Barreiro-Iglesias, A., Villar-Cerviño, V., Anadón, R., & Rodicio, M. C. (2008). Descending brain-spinal cord projections in a primitive vertebrate, the lamprey: cerebrospinal fluid-contacting and dopaminergic neurons. The Journal of Comparative Neurology, 511(6), 711–723. http://doi.org/10.1002/cne.21863 Bellardita, C., & Kiehn, O. (2015). Phenotypic Characterization of Speed-Associated Gait Changes in Mice Reveals Modular Organization of Locomotor Networks. Current Biology http://doi.org/10.1016/j.cub.2015.04.005 Ben-Ari, Y. (2002). Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience, 3(9), 728–739. http://doi.org/10.1038/nrn920 Bennis, M., Calas, A., Geffard, M., & Gamrani, H. (1990). Distribution of dopamine immunoreactive systems in brain stem and spinal cord of the chameleon. Biological Structures and Morphogenesis, 3(1), 13– 19. Berkowitz, A., Roberts, A., & Soffe, S. R. (2010). Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Frontiers in Behavioral Neuroscience, 4, 36. http://doi.org/10.3389/fnbeh.2010.00036 Bernhardt, R. R., Chitnis, A. B., Lindamer, L., & Kuwada, J. Y. (1990). Identification of spinal neurons in the embryonic and larval zebrafish. The Journal of Comparative Neurology, 302(3), 603–616. http://doi.org/10.1002/cne.903020315 Betley, J. N., Wright, C. V. E., Kawaguchi, Y., ErdElyi, F., SzabO, G., Jessell, T. M., & Kaltschmidt, J. A. (2009). Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell, 139(1), 161–174. http://doi.org/10.1016/j.cell.2009.08.027 Bhatt, D. H., McLean, D. L., Hale, M. E., & Fetcho, J. R. (2007). Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron, 53(1), 91–102. http://doi.org/10.1016/j.neuron.2006.11.011 Bikoff, J. B., Gabitto, M. I., Rivard, A. F., Drobac, E., Machado, T. A., Miri, A., et al. (2016). Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell, 165(1), 207–219. http://doi.org/10.1016/j.cell.2016.01.027 Biró, Z., Hill, R. H., & Grillner, S. (2008). The activity of spinal commissural interneurons during fictive locomotion in the lamprey. Journal of Neurophysiology, 100(2), 716–722. http://doi.org/10.1152/jn.90206.2008 Boothby, K. M., & Roberts, A. (1992). The stopping response of Xenopus laevis embryos: pharmacology and intracellular physiology of rhythmic spinal neurones and hindbrain neurones. The Journal of Experimental Biology, 169, 65–86. Borowska, J., Jones, C. T., Zhang, H., Blacklaws, J., Goulding, M., & Zhang, Y. (2013). Functional subpopulations of V3 interneurons in the mature mouse spinal cord. Journal of Neuroscience, 33(47), 18553–18565. http://doi.org/10.1523/JNEUROSCI.2005-13.2013 Böhm, U. L., Prendergast, A., Djenoune, L., Nunes Figueiredo, S., Gomez, J., Stokes, C., et al. (2016). CSFcontacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nature Communications, 7, 10866. http://doi.org/10.1038/ncomms10866 Brand, A. H., & Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118(2), 401–415. Brette, R., and Destexhe, A. (2012). Handbook of Neural Activity Mesurement. Cambridge University Press 97 References Brodin, L., Dale, N., Christenson, J., Storm-Mathisen, J., Hökfelt, T., & Grillner, S. (1990). Three types of GABA-immunoreactive cells in the lamprey spinal cord. Brain Research, 508(1), 172–175. Brown, T.G. (1911). The intrinsic factors in the act of progression in the mammal. Proc. R. Soc. Lond. B, 84, 309-319 Brown, T.G., Sherrington, C.S. (1912). The rule of reflex response in the limb reflexes of the mammal and its exceptions. The Journal of Physiology, 44(3):125-30. Brown, T. G. (1914). On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. The Journal of Physiology, 48(1), 18–46. http://doi.org/10.1111/(ISSN)1469-7793 Buchanan, J. T., & Grillner, S. (1987). Newly identified “glutamate interneurons” and their role in locomotion in the lamprey spinal cord. Science, 236(4799), 312–314. Buchanan, J. T., & Grillner, S. (1988). A new class of small inhibitory interneurones in the lamprey spinal cord. Brain Research, 438(1-2), 404–407. Buchanan, J. T., Grillner, S., Cullheim, S., & Risling, M. (1989). Identification of excitatory interneurons contributing to generation of locomotion in lamprey: structure, pharmacology, and function. Journal of Neurophysiology, 62(1), 59–69. Buhl, E., Soffe, S. R., & Roberts, A. (2015). Sensory initiation of a co-ordinated motor response: synaptic excitation underlying simple decision-making. The Journal of Physiology, 593(19), 4423–4437. http://doi.org/10.1113/JP270792 Burke, R. E. (1999). The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Experimental Brain Research, 128(3), 263–277. Buschges, A., & Manira, A. E. (1998). Sensory pathways and their modulation in the control of locomotion. Current Opinion in Neurobiology, 8(6), 733–739. Carlsson, A., Falck, B., Fuxe, K., & Hillarp, N. A. (1964). Cellular localization of monoamines in the spinal cord. Acta Physiologica Scandinavica, 60(1-2), 112–119. http://doi.org/10.1111/j.17481716.1964.tb02874.x Caspary, T., & Anderson, K. V. (2003). Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nature Reviews Neuroscience, 4(4), 289–297. http://doi.org/10.1038/nrn1073 Chen, T.-W., Wardill, T. J., Sun, Y., Pulver, S. R., Renninger, S. L., Baohan, A., et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature, 499(7458), 295–300. http://doi.org/10.1038/nature12354 Chiba, A., & Oka, S. (1999). Serotonin-immunoreactive structures in the central nervous system of the garfish Lepisosteus productus (Semionotiformes, Osteichthyes). Neuroscience Letters, 261(1-2), 73–76. Christenson, J., Bongianni, F., Grillner, S., & Hökfelt, T. (1991). Putative GABAergic input to axons of spinal interneurons and primary sensory neurons in the lamprey spinal cord as shown by intracellular Lucifer yellow and GABA immunohistochemistry. Brain Research, 538(2), 313–318. Clarke, J. D., & Roberts, A. (1984). Interneurones in the Xenopus embryo spinal cord: sensory excitation and activity during swimming. The Journal of Physiology, 354, 345–362. Clarke, J. D., Hayes, B. P., Hunt, S. P., & Roberts, A. (1984). Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. The Journal of Physiology, 348, 511–525. http://doi.org/10.1111/(ISSN)1469-7793 98 References Conte, A., Khan, N., Defazio, G., Rothwell, J. C., & Berardelli, A. (2013). Pathophysiology of somatosensory abnormalities in Parkinson disease. Nature Reviews. Neurology, 9(12), 687–697. http://doi.org/10.1038/nrneurol.2013.224 Conway, B. A., Hultborn, H., & Kiehn, O. (1987). Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research, 68(3), 643–656. Crone, S. A., Quinlan, K. A., Zagoraiou, L., Droho, S., Restrepo, C. E., Lundfald, L., et al. (2008). Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron, 60(1), 70–83. http://doi.org/10.1016/j.neuron.2008.08.009 Curado, S., Anderson, R. M., Jungblut, B., Mumm, J., Schroeter, E., & Stainier, D. Y. R. (2007). Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Developmental Dynamics, 236(4), 1025–1035. http://doi.org/10.1002/dvdy.21100 Dale, N., Roberts, A., Ottersen, O. P., & Storm-Mathisen, J. (1987). The morphology and distribution of “Kolmer-Agduhr cells,” a class of cerebrospinal-fluid-contacting neurons revealed in the frog embryo spinal cord by GABA immunocytochemistry. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. Royal Society (Great Britain), 232(1267), 193–203. http://doi.org/10.1098/rspb.1987.0068 Deisseroth, K. (2015). Optogenetics: 10 years of microbial opsins in neuroscience. Nature Neuroscience, 18(9), 1213–1225. http://doi.org/10.1038/nn.4091 Del Bene, F., & Wyart, C. (2012). Optogenetics: a new enlightenment age for zebrafish neurobiology. Developmental Neurobiology, 72(3), 404–414. http://doi.org/10.1002/dneu.20914 Delcomyn, F. (1980). Neural basis of rhythmic behavior in animals. Science, 210(4469), 492–498. Delmas, P. (2005). Polycystins: polymodal receptor/ion-channel cellular sensors. Pflugers Archiv European Journal of Physiology, 451(1), 264–276. http://doi.org/10.1007/s00424-005-1431-5 Dietz, V. (2002). Proprioception and locomotor disorders. Nature Reviews Neuroscience, 3(10), 781–790. http://doi.org/10.1038/nrn939 Djenoune, L., Khabou, H., Joubert, F., Quan, F. B., Nunes Figueiredo, S., Bodineau, L., et al. (2014). Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Frontiers in Neuroanatomy, 8, 26. http://doi.org/10.3389/fnana.2014.00026 Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L., & Tank, D. W. (2007). Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron, 56(1), 43–57. http://doi.org/10.1016/j.neuron.2007.08.003 Douglass, A. D., Kraves, S., Deisseroth, K., Schier, A. F., & Engert, F. (2008). Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Current Biology, 18(15), 1133–1137. http://doi.org/10.1016/j.cub.2008.06.077 Duysens, J., & Pearson, K. G. (1980). Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Research, 187(2), 321–332. Eccles, J. C., Fatt, P., & Koketsu, K. (1954). Cholinergic and inhibitory synapses in a pathway from motoraxon collaterals to motoneurones. The Journal of Physiology, 126(3), 524–562. http://doi.org/10.1111/(ISSN)1469-7793 Eccles, J. C., Kostyuk, P. G., & Schmidt, R. F. (1962a). Central pathways responsible for depolarization of primary afferent fibres. The Journal of Physiology, 161(2), 237–257. 99 References Eccles, J. C., Kostyuk, P. G., & Schmidt, R. F. (1962b). Presynaptic inhibition of the central actions of flexor reflex afferents. The Journal of Physiology, 161(2), 258–281. http://doi.org/10.1111/(ISSN)1469-7793 Eccles, J. C., Schmidt, R. F., & Willis, W. D. (1962c). Presynaptic inhibition of the spinal monosynaptic reflex pathway. The Journal of Physiology, 161(2), 282–297. http://doi.org/10.1111/(ISSN)1469-7793 Edin, B. (2001). Cutaneous afferents provide information about knee joint movements in humans. The Journal of Physiology, 531(Pt 1), 289–297. Eklöf-Ljunggren, E., Haupt, S., Ausborn, J., Dehnisch, I., Uhlén, P., Higashijima, S.-I., & Manira, El, A. (2012). Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proceedings of the National Academy of Sciences, 109(14), 5511–5516. http://doi.org/10.1073/pnas.1115377109 Eklöf-Ljunggren, E., Haupt, S., Ausborn, J., Ampatzis, K., & Manira, El, A. (2014). Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. Journal of Neuroscience, 34(1), 134–139. http://doi.org/10.1523/JNEUROSCI.4087-13.2014 Faucherre, A., Nargeot, J., Mangoni, M. E., & Jopling, C. (2013). piezo2b regulates vertebrate light touch response. Journal of Neuroscience, 33(43), 17089–17094. http://doi.org/10.1523/JNEUROSCI.052213.2013 Fernández-López, B., Villar-Cerviño, V., Valle-Maroto, S. M., Barreiro-Iglesias, A., Anadón, R., & Rodicio, M. C. (2012). The glutamatergic neurons in the spinal cord of the sea lamprey: an in situ hybridization and immunohistochemical study. PLoS ONE, 7(10), e47898. http://doi.org/10.1371/journal.pone.0047898 Fidelin, K., & Wyart, C. (2014). Inhibition and motor control in the developing zebrafish spinal cord. Current Opinion in Neurobiology, 26, 103–109. http://doi.org/10.1016/j.conb.2013.12.016 Fidelin, K., Djenoune, L., Stokes, C., Prendergast, A., Gomez, J., Baradel, A., et al. (2015). State-Dependent Modulation of Locomotion by GABAergic Spinal Sensory Neurons. Current Biology, 25(23), 3035–3047. http://doi.org/10.1016/j.cub.2015.09.070 Fink, A. J. P., Croce, K. R., Huang, Z. J., Abbott, L. F., Jessell, T. M., & Azim, E. (2014). Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature, 509(7498), 43–48. http://doi.org/10.1038/nature13276 Flourens, M.-J.-P. (1824). Experimental studies on the properties and functions of the nervous system in vertebrate animals. Chez Crevot, Paris. Freusberg, A. (1874). Reflex movements in dogs. Pflügers Arch. Ges. Physiol, 9, 358–391. Geertsen, S. S., Stecina, K., Meehan, C. F., Nielsen, J. B., & Hultborn, H. (2011). Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. The Journal of Physiology, 589(1), 119–134. http://doi.org/10.1113/jphysiol.2010.199125 Gosgnach, S., Lanuza, G. M., Butt, S. J. B., Saueressig, H., Zhang, Y., Velasquez, T., et al. (2006). V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature, 440(7081), 215–219. http://doi.org/10.1038/nature04545 Gossard, J. P., Brownstone, R. M., Barajon, I., & Hultborn, H. (1994). Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Experimental Brain Research, 98(2), 213– 228. Goulding, M., & Pfaff, S. L. (2005). Development of circuits that generate simple rhythmic behaviors in vertebrates. Current Opinion in Neurobiology, 15(1), 14–20. http://doi.org/10.1016/j.conb.2005.01.017 Goulding, M. (2009). Circuits controlling vertebrate locomotion: moving in a new direction. Nature Reviews Neuroscience, 10(7), 507–518. http://doi.org/10.1038/nrn2608 100 References Gouti, M., Metzis, V., & Briscoe, J. (2015). The route to spinal cord cell types: a tale of signals and switches. Trends in Genetics : TIG, 31(6), 282–289. http://doi.org/10.1016/j.tig.2015.03.001 Green, C. S., & Soffe, S. R. (1998). Roles of ascending inhibition during two rhythmic motor patterns in Xenopus tadpoles. Journal of Neurophysiology, 79(5), 2316–2328. Grillner, S. (1969). The influence of DOPA on the static and the dynamic fusimotor activity to the triceps surae of the spinal cat. Acta Physiologica Scandinavica, 77(4), 490–509. http://doi.org/10.1111/j.17481716.1969.tb04592.x Grillner, S. (2003). The motor infrastructure: from ion channels to neuronal networks. Nature Reviews Neuroscience, 4(7), 573–586. http://doi.org/10.1038/nrn1137 Grillner, S., & Jessell, T. M. (2009). Measured motion: searching for simplicity in spinal locomotor networks. Current Opinion in Neurobiology, 19(6), 572–586. http://doi.org/10.1016/j.conb.2009.10.011 Grillner, S., & Manira, A. E. (2015). The intrinsic operation of the networks that make us locomote. Current Opinion in Neurobiology, 31C, 244–249. http://doi.org/10.1016/j.conb.2015.01.003 Grillner, S., & Rossignol, S. (1978). On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Research, 146(2), 269–277. Grillner, S., Williams, T., & Lagerbäck, P. A. (1984). The edge cell, a possible intraspinal mechanoreceptor. Science, 223(4635), 500–503. Guertin, P., Angel, M. J., Perreault, M. C., & McCrea, D. A. (1995). Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. The Journal of Physiology, 487(1), 197–209. http://doi.org/10.1111/(ISSN)1469-7793 Guglielmone, R., & Panzica, G. C. (1985). Early appearance of catecholaminergic neurons in the central nervous system of precocial and altricial avian species. A fluorescence-histochemical study. Cell and Tissue Research, 240(2), 381–384. Hale, M. E., Ritter, D. A., & Fetcho, J. R. (2001). A confocal study of spinal interneurons in living larval zebrafish. The Journal of Comparative Neurology, 437(1), 1–16. Halpern, M. E., Rhee, J., Goll, M. G., Akitake, C. M., Parsons, M., & Leach, S. D. (2008). Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish, 5(2), 97–110. http://doi.org/10.1089/zeb.2008.0530 Harrison, P. J., Hultborn, H., Jankowska, E., Katz, R., Storai, B., & Zytnicki, D. (1984). Labelling of interneurones by retrograde transsynaptic transport of horseradish peroxidase from motoneurones in rats and cats. Neuroscience Letters, 45(1), 15–19. Hayes, B. P., & Roberts, A. (1983). The anatomy of two functional types of mechanoreceptive “free” nerveending in the head skin of Xenopus embryos. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. Royal Society (Great Britain), 218(1210), 61–76. Hägglund, M., Borgius, L., Dougherty, K. J., & Kiehn, O. (2010). Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nature Neuroscience, 13(2), 246–252. http://doi.org/10.1038/nn.2482 Heathcote, R. D., & Chen, A. (1993). A nonrandom interneuronal pattern in the developing frog spinal cord. The Journal of Comparative Neurology, 328(3), 437–448. http://doi.org/10.1002/cne.903280309 Hiebert, G. W., Whelan, P. J., Prochazka, A., & Pearson, K. G. (1996). Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. Journal of Neurophysiology, 75(3), 1126–1137. 101 References Higashijima, S.-I., Masino, M. A., Mandel, G., & Fetcho, J. R. (2003). Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. Journal of Neurophysiology, 90(6), 3986– 3997. http://doi.org/10.1152/jn.00576.2003 Higashijima, S.-I., Mandel, G., & Fetcho, J. R. (2004a). Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. The Journal of Comparative Neurology, 480(1), 1–18. http://doi.org/10.1002/cne.20278 Higashijima, S.-I., Schaefer, M., & Fetcho, J. R. (2004b). Neurotransmitter properties of spinal interneurons in embryonic and larval zebrafish. The Journal of Comparative Neurology, 480(1), 19–37. http://doi.org/10.1002/cne.20279 Higashijima, S.-I., Masino, M. A., Mandel, G., & Fetcho, J. R. (2004c). Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. Journal of Neuroscience, 24(25), 5827–5839. http://doi.org/10.1523/JNEUROSCI.5342-03.2004 Holtz, A., Levi, R. (2010). Spinal cord injury. Oxford University Press Hsu, L.-J., Zelenin, P. V., Grillner, S., Orlovsky, G. N., & Deliagina, T. G. (2013). Intraspinal stretch receptor neurons mediate different motor responses along the body in lamprey. The Journal of Comparative Neurology, 521(16), 3847–3862. http://doi.org/10.1002/cne.23382 Huang, A. L., Chen, X., Hoon, M. A., Chandrashekar, J., Guo, W., Tränkner, D., et al. (2006). The cells and logic for mammalian sour taste detection. Nature, 442(7105), 934–938. http://doi.org/10.1038/nature05084 Hultborn, H., & Udo, M. (1972a). Convergence in the reciprocal Ia inhibitory pathway of excitation from descending pathways and inhibition from motor axon collaterals. Acta Physiologica Scandinavica, 84(1), 95–108. http://doi.org/10.1111/j.1748-1716.1972.tb05159.x Hultborn, H., & Udo, M. (1972b). Convergence of large muscle spindle (Ia) afferents at interneuronal level in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiologica Scandinavica, 84(4), 493–499. http://doi.org/10.1111/j.1748-1716.1972.tb05199 Hultborn, H. (2001). State-dependent modulation of sensory feedback. The Journal of Physiology, 533(Pt 1), 5–13. Hultborn, H. (2006). Spinal reflexes, mechanisms and concepts: From Eccles to Lundberg and beyond. Progress in Neurobiology, 78(3-5), 215–232. http://doi.org/10.1016/j.pneurobio.2006.04.001 Hultborn, H., Jankowska, E., & Lindström, S. (1971). Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. The Journal of Physiology, 215(3), 613–636. Illert, M., & Tanaka, R. (1978). Integration in descending motor pathways controlling the forelimb in the cat. 4. Corticospinal inhibition of forelimb motoneurones mediated by short propriospinal neurones. Experimental Brain Research, 31(1), 131–141. Jalalvand, E., Robertson, B., Wallén, P., & Grillner, S. (2016a). Ciliated neurons lining the central canal sense both fluid movement and pH through ASIC3. Nature Communications, 7, 10002. http://doi.org/10.1038/ncomms10002 Jalalvand, E., Robertson, B., Tostivint, H., Wallén, P., & Grillner, S. (2016b). The Spinal Cord Has an Intrinsic System for the Control of pH. Current Biology. http://doi.org/10.1016/j.cub.2016.03.048 Jalalvand, E., Robertson, B., Wallén, P., Hill, R. H., & Grillner, S. (2014). Laterally projecting cerebrospinal fluid-contacting cells in the lamprey spinal cord are of two distinct types. The Journal of Comparative Neurology, 522(8), http://doi.org/10.1002/cne.23584 102 References Jankowska, E., Johannisson, T., & Lipski, J. (1981). Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. The Journal of Physiology, 310, 381–402. Jankowska, E. (1992). Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology, 38(4), 335–378. Jankowska, E. (2001). Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. The Journal of Physiology, 533(Pt 1), 31–40. http://doi.org/10.1111/j.14697793.2001.0031b.x Jankowska, E. (2008). Spinal interneuronal networks in the cat: Elementary components. Brain Research Reviews, 57(1), 46–55. http://doi.org/10.1016/j.brainresrev.2007.06.022 Jankowska, E., & Hammar, I. (2002). Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Research. Brain Research Reviews, 40(1-3), 19–28. Jankowska, E., & Roberts, W. J. (1972). Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. The Journal of Physiology, 222(3), 623–642. http://doi.org/10.1111/(ISSN)1469-7793 Jankowska, E., Jukes, M. G., Lund, S., & Lundberg, A. (1965). Reciprocal innervation through interneuronal inhibition. Nature, 206(980), 198–199. Jankowska, E., Jukes, M. G., Lund, S., & Lundberg, A. (1967a). The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiologica Scandinavica, 70(3), 369–388. http://doi.org/10.1111/j.17481716.1967.tb03636.x Jankowska, E., Jukes, M. G., Lund, S., & Lundberg, A. (1967b). The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiologica Scandinavica, 70(3), 389–402. http://doi.org/10.1111/j.1748-1716.1967.tb03637.x Jessell, T. M. (2000). Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Reviews. Genetics, 1(1), 20–29. http://doi.org/10.1038/35049541 Jordan, L. M. (1998). Initiation of locomotion in mammals. Annals of the New York Academy of Sciences, 860, 83–93. Kahle, K. T., Deeb, T. Z., Puskarjov, M., Silayeva, L., Liang, B., Kaila, K., & Moss, S. J. (2013). Modulation of neuronal activity by phosphorylation of the K–Cl cotransporter KCC2. Trends in Neurosciences, 36(12), 726–737. http://doi.org/10.1016/j.tins.2013.08.006 Kiehn, O. (2016). Decoding the organization of spinal circuits that control locomotion. Nature Reviews Neuroscience, 17(4), 224–238. http://doi.org/10.1038/nrn.2016.9 Kimura, Y., Okamura, Y., & Higashijima, S.-I. (2006). alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. Journal of Neuroscience, 26(21), 5684–5697. http://doi.org/10.1523/JNEUROSCI.4993-05.2006 Kimura, Y., Satou, C., Fujioka, S., Shoji, W., Umeda, K., Ishizuka, T., et al. (2013). Hindbrain V2a neurons in the excitation of spinal locomotor circuits during zebrafish swimming. Current Biology : CB, 23(10), 843– 849. http://doi.org/10.1016/j.cub.2013.03.066 Kinkhabwala, A., Riley, M., Koyama, M., Monen, J., Satou, C., Kimura, Y., et al. (2011). A structural and functional ground plan for neurons in the hindbrain of zebrafish. Proceedings of the National Academy of Sciences, 108(3), 1164–1169. http://doi.org/10.1073/pnas.1012185108 103 References Knogler, L. D., & Drapeau, P. (2014). Sensory gating of an embryonic zebrafish interneuron during spontaneous motor behaviors. Frontiers in Neural Circuits, 8, 121. http://doi.org/10.3389/fncir.2014.00121 Knogler, L. D., Ryan, J., Saint-Amant, L., & Drapeau, P. (2014). A hybrid electrical/chemical circuit in the spinal cord generates a transient embryonic motor behavior. Journal of Neuroscience, 34(29), 9644–9655. http://doi.org/10.1523/JNEUROSCI.1225-14.2014 Kohashi, T., & Oda, Y. (2008). Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. Journal of Neuroscience, 28(42), 10641–10653. http://doi.org/10.1523/JNEUROSCI.1435-08.2008 Kolmer, W. (1921). Das ‘‘Sagittalorgan’’ der Wirbeltiere. Z. Anat. Entwicklungs 60, 652–717. Kolmer, W. (1931).U¨ ber das Sagittalorgan, ein Zentrales Sinnesorgan der Wirbeltiere, Insbesondere Beim Affen. Z. Zellforsch Mik. Ana. 13, 236–248. Krillaars, D. J., Brownstone, R. M., Noga, B. R., & Jordan, L. M. (1994). Mechanical entrainment of fictive locomotion in the decerebrate cat. Journal of Neurophysiology, 71(6), 2074–2086. Lam, T., & Pearson, K. G. (2001). Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. Journal of Neurophysiology, 86(3), 1321–1332. LaMotte, C. C. (1987). Vasoactive intestinal polypeptide cerebrospinal fluid-contacting neurons of the monkey and cat spinal central canal. The Journal of Comparative Neurology, 258(4), 527–541. http://doi.org/10.1002/cne.902580405 Lanuza, G. M., Gosgnach, S., Pierani, A., Jessell, T. M., & Goulding, M. (2004). Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron, 42(3), 375–386. Lee, S. K., & Pfaff, S. L. (2001). Transcriptional networks regulating neuronal identity in the developing spinal cord. Nature Neuroscience, 4, 1183–1191. http://doi.org/10.1038/nn750 Li, W. C., Higashijima, S.-I., Parry, D. M., Roberts, A., & Soffe, S. R. (2004). Primitive roles for inhibitory interneurons in developing frog spinal cord. Journal of Neuroscience, 24(25), 5840–5848. http://doi.org/10.1523/JNEUROSCI.1633-04.2004 Li, W. C., Perrins, R., Soffe, S. R., Yoshida, M., Walford, A., & Roberts, A. (2001). Defining classes of spinal interneuron and their axonal projections in hatchling Xenopus laevis tadpoles. The Journal of Comparative Neurology, 441(3), 248–265. http://doi.org/10.1002/cne.1410 Li, W.-C., Soffe, S. R., & Roberts, A. (2003). The spinal interneurons and properties of glutamatergic synapses in a primitive vertebrate cutaneous flexion reflex. Journal of Neuroscience, 23(27), 9068–9077. Liao, J. C., & Fetcho, J. R. (2008). Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. Journal of Neuroscience, 28(48), 12982–12992. http://doi.org/10.1523/JNEUROSCI.3330-08.2008 Lundberg, A., Malmgren, K., & Schomburg, E. D. (1975). Convergence from Lb, cutaneous and joint afferents in reflex pathways to motoneurones. Brain Research, 87(1), 81–84. Marder, E., & Bucher, D. (2001). Central pattern generators and the control of rhythmic movements. Current Biology, 11(23), R986–96. Marlinskiĭ, V. V. (1983). Depolarization of primary afferents induced by evoked activation of intersegmental connections of the substantia gelatinosa of the spinal cord in the cat. Neurophysiology, 15(3), 288–294. 104 References Masino, M. A., & Fetcho, J. R. (2005). Fictive swimming motor patterns in wild type and mutant larval zebrafish. Journal of Neurophysiology, 93(6), 3177–3188. http://doi.org/10.1152/jn.01248.2004 McClellan, A. D., & Grillner, S. (1983). Initiation and sensory gating of “fictive” swimming and withdrawal responses in an in vitro preparation of the lamprey spinal cord. Brain Research, 269(2), 237–250. McCrea, D. A. (2001). Spinal circuitry of sensorimotor control of locomotion. The Journal of Physiology, 533(Pt 1), 41–50. McCrea, D. A., & Rybak, I. A. (2008). Organization of mammalian locomotor rhythm and pattern generation. Brain Research Reviews, 57(1), 134–146. http://doi.org/10.1016/j.brainresrev.2007.08.006 McCrea, D. A., Pratt, C. A., & Jordan, L. M. (1980). Renshaw cell activity and recurrent effects on motoneurons during fictive locomotion. Journal of Neurophysiology, 44(3), 475–488. McLean, D. L. (2013). Optogenetics: illuminating sources of locomotor drive. Current Biology : CB, 23(10), R441–3. http://doi.org/10.1016/j.cub.2013.04.015 McLean, D. L., Fan, J., Higashijima, S.-I., Hale, M. E., & Fetcho, J. R. (2007). A topographic map of recruitment in spinal cord. Nature, 446(7131), 71–75. http://doi.org/10.1038/nature05588 McLean, D. L., Masino, M. A., Koh, I. Y. Y., Lindquist, W. B., & Fetcho, J. R. (2008). Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nature Neuroscience, 11(12), 1419– 1429. http://doi.org/10.1038/nn.2225 McNeill, A.R. (2002). Principles of animal locomotion. Princeton University Press Menelaou, E., VanDunk, C., & McLean, D. L. (2014). Differences in the morphology of spinal V2a neurons reflect their recruitment order during swimming in larval zebrafish. The Journal of Comparative Neurology, 522(6), 1232–1248. http://doi.org/10.1002/cne.23465 Mentis, G. Z., Siembab, V. C., Zerda, R., O'Donovan, M. J., & Alvarez, F. J. (2006). Primary afferent synapses on developing and adult Renshaw cells. Journal of Neuroscience, 26(51), 13297–13310. http://doi.org/10.1523/JNEUROSCI.2945-06.2006 Mirat, O., Sternberg, J. R., Severi, K. E., & Wyart, C. (2013). ZebraZoom: an automated program for highthroughput behavioral analysis and categorization. Frontiers in Neural Circuits, 7, 107. http://doi.org/10.3389/fncir.2013.00107 Montgomery, K. L., Iyer, S. M., Christensen, A. J., Deisseroth, K., & Delp, S. L. (2016). Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Science Translational Medicine, 8(337), 337rv5–337rv5. http://doi.org/10.1126/scitranslmed.aad7577 Moran-Rivard, L., Kagawa, T., Saueressig, H., Gross, M. K., Burrill, J., & Goulding, M. (2001). Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron, 29(2), 385–399. Mullins, O. J., Hackett, J. T., Buchanan, J. T., & Friesen, W. O. (2011). Neuronal control of swimming behavior: Comparison of vertebrate and invertebrate model systems. Progress in Neurobiology, 93(2), 244–269. http://doi.org/10.1016/j.pneurobio.2010.11.001 Naumann, E. A., Kampff, A. R., Prober, D. A., Schier, A. F., & Engert, F. (2010). Monitoring neural activity with bioluminescence during natural behavior. Nature Neuroscience, 13(4), 513–520. http://doi.org/10.1038/nn.2518 Nishimaru, H., Restrepo, C. E., & Kiehn, O. (2006). Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. Journal of Neuroscience, 26(20), 5320–5328. http://doi.org/10.1523/JNEUROSCI.5127-05.2006 105 References Ochi, J., Yamamoto, T., & Hosoya, Y. (1979). Comparative study of the monoamine neuron system in the spinal cord of the lamprey and hagfish. Archivum Histologicum Japonicum = Nihon Soshikigaku Kiroku, 42(3), 327–336. Ohta, Y., Dubuc, R., & Grillner, S. (1991). A new population of neurons with crossed axons in the lamprey spinal cord. Brain Research, 564(1), 143–148. Orlovsky, G.N., Deliagina, T.G., Grillner, S. (1999). Neural control of locomotion: from mollusk to man. Oxford University Press Orts-Del'Immagine, A., Seddik, R., Tell, F., Airault, C., Er-Raoui, G., Najimi, M., et al. (2016). A single polycystic kidney disease 2-like 1 channel opening acts as a spike generator in cerebrospinal fluid-contacting neurons of adult mouse brainstem. Neuropharmacology, 101, 549–565. http://doi.org/10.1016/j.neuropharm.2015.07.030 Orts-Del'Immagine, A., Wanaverbecq, N., Tardivel, C., Tillement, V., Dallaporta, M., & Trouslard, J. (2012). Properties of subependymal cerebrospinal fluid contacting neurones in the dorsal vagal complex of the mouse brainstem. The Journal of Physiology, 590(Pt 16), 3719–3741. http://doi.org/10.1113/jphysiol.2012.227959 Palanca, A. M. S., Lee, S.-L., Yee, L. E., Joe-Wong, C., Trinh, L. A., Hiroyasu, E., et al. (2013). New transgenic reporters identify somatosensory neuron subtypes in larval zebrafish. Developmental Neurobiology, 73(2), 152–167. http://doi.org/10.1002/dneu.22049 Panek, I., Bui, T., Wright, A. T. B., & Brownstone, R. M. (2014). Cutaneous afferent regulation of motor function. Acta Neurobiologiae Experimentalis, 74(2), 158–171. Parent, A., & Northcutt, R. G. (1982). The monoamine-containing neurons in the brain of the garfish, Lepisosteus osseus. Brain Research Bulletin, 9(1-6), 189–204. Pearson, K. G. (1995). Proprioceptive regulation of locomotion. Current Opinion in Neurobiology, 5(6), 786–791. Pearson, K. G., & Collins, D. F. (1993). Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. Journal of Neurophysiology, 70(3), 1009–1017. Perrins, R., Walford, A., & Roberts, A. (2002). Sensory activation and role of inhibitory reticulospinal neurons that stop swimming in hatchling frog tadpoles. Journal of Neuroscience, 22(10), 4229–4240. Petracca, Y. L., Sartoretti, M. M., Di Bella, D. J., Marin-Burgin, A., Carcagno, A. L., Schinder, A. F., & Lanuza, G. M. (2016). The late and dual origin of cerebrospinal fluid-contacting neurons in the mouse spinal cord. Development, 143(5), 880–891. http://doi.org/10.1242/dev.129254 Pierani, A., Moran-Rivard, L., Sunshine, M. J., Littman, D. R., Goulding, M., & Jessell, T. M. (2001). Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron, 29(2), 367–384. Pietri, T., Manalo, E., Ryan, J., Saint-Amant, L., & Washbourne, P. (2009). Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Developmental Neurobiology, 69(12), 780–795. http://doi.org/10.1002/dneu.20741 Portugues, R., Severi, K. E., Wyart, C., & Ahrens, M. B. (2013). Optogenetics in a transparent animal: circuit function in the larval zebrafish. Current Opinion in Neurobiology, 23(1), 119–126. http://doi.org/10.1016/j.conb.2012.11.001 Pratt, C. A., & Jordan, L. M. (1987). Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. Journal of Neurophysiology, 57(1), 56–71. 106 References Ramsey, I. S., Delling, M., & Clapham, D. E. (2006). An introduction to TRP channels. Annual Review of Physiology, 68(1), 619–647. http://doi.org/10.1146/annurev.physiol.68.040204.100431 Reali, C., Fernández, A., Radmilovich, M., Trujillo-Cenóz, O., & Russo, R. E. (2011). GABAergic signalling in a neurogenic niche of the turtle spinal cord. The Journal of Physiology, no–no. http://doi.org/10.1113/jphysiol.2011.214312 Renshaw, B. (1941). Influence of discharge of motoneurons upon excitation of neighboring motoneurons. The Journal of Neurophysiology, 4:167 Renshaw, B. (1946). Observations on interaction of nerve impulses in the gray matter and on the nature of central inhibition. The American Journal of Physiology, 146, 443–448. Reyes, R., Haendel, M., Grant, D., Melancon, E., & Eisen, J. S. (2004). Slow degeneration of zebrafish Rohon-Beard neurons during programmed cell death. Developmental Dynamics, 229(1), 30–41. http://doi.org/10.1002/dvdy.10488 Reynolds, A., Brustein, E., Liao, M., Mercado, A., Babilonia, E., Mount, D. B., & Drapeau, P. (2008). Neurogenic role of the depolarizing chloride gradient revealed by global overexpression of KCC2 from the onset of development. Journal of Neuroscience, 28(7), 1588–1597. http://doi.org/10.1523/JNEUROSCI.379107.2008 Ritter, D. A., Bhatt, D. H., & Fetcho, J. R. (2001). In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. Journal of Neuroscience, 21(22), 8956–8965. Roberts, A. (1980). The function and role of two types of mechanoreceptive ‘free’ nerve endings in the head skin of amphibian embryos. The Journal of Comparative Physiology A,135:341–348 Roberts, A. (2000). Early functional organization of spinal neurons in developing lower vertebrates. Brain Research Bulletin, 53(5), 585–593. Roberts, A., & Sillar, K. T. (1990). Characterization and Function of Spinal Excitatory Interneurons with Commissural Projections in Xenopus laevis embryos. The European Journal of Neuroscience, 2(12), 1051– 1062. Roberts, A., Li, W. C., Soffe, S. R., & Wolf, E. (2008). Origin of excitatory drive to a spinal locomotor network. Brain Research Reviews, 57(1), 22–28. http://doi.org/10.1016/j.brainresrev.2007.06.015 Roberts, A., Soffe, S. R., Wolf, E. S., Yoshida, M., & Zhao, F. Y. (1998). Central circuits controlling locomotion in young frog tadpoles. Annals of the New York Academy of Sciences, 860, 19–34. Roberts, B. L., & Meredith, G. E. (1987). Immunohistochemical study of a dopaminergic system in the spinal cord of the ray, Raja radiata. Brain Research, 437(1), 171–175. Rossignol, S., Dubuc, R., & Gossard, J.-P. (2006). Dynamic sensorimotor interactions in locomotion. Physiological Reviews, 86(1), 89–154. http://doi.org/10.1152/physrev.00028.2005 Rudomin, P. (2009). In search of lost presynaptic inhibition. Experimental Brain Research, 196(1), 139–151. http://doi.org/10.1007/s00221-009-1758-9 Rudomin, P., & Schmidt, R. F. (1999). Presynaptic inhibition in the vertebrate spinal cord revisited. Experimental Brain Research, 129(1), 1–37. Sanes, J. N., Mauritz, K. H., Dalakas, M. C., & Evarts, E. V. (1985). Motor control in humans with large-fiber sensory neuropathy. Human Neurobiology, 4(2), 101–114. Sapir, T., Geiman, E. J., Wang, Z., Velasquez, T., Mitsui, S., Yoshihara, Y., et al. (2004). Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. Journal of Neuroscience, 24(5), 1255–1264. http://doi.org/10.1523/JNEUROSCI.3187-03.2004 107 References Satou, C., Kimura, Y., & Higashijima, S.-I. (2012). Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. Journal of Neuroscience, 32(5), 1771–1783. http://doi.org/10.1523/JNEUROSCI.5500-11.2012 Saueressig, H., Burrill, J., & Goulding, M. (1999). Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development, 126(19), 4201–4212. Scott, E. K., Mason, L., Arrenberg, A. B., Ziv, L., Gosse, N. J., Xiao, T., et al. (2007). Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nature Methods. http://doi.org/10.1038/nmeth1033 Selverston, A.I., Paul S.G. Stein, P.S.G., Douglas G. Stuart, D.S., Grillner, S. (1999). Neurons, Networks and Motor behaviors. The MIT Press Sherrington, C. S. (1906a). Integrative action of the nervous system. Constable, London Sherrington, C. S. (1906b). Observations on the scratch-reflex in the spinal dog. The Journal of Physiology, 34(1-2), 1–50. http://doi.org/10.1111/(ISSN)1469-7793 Sherrington, C. S. (1910). Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. The Journal of Physiology, 40(1-2), 28–121. http://doi.org/10.1111/(ISSN)1469-7793 Shik, M. L., Severin, F. V., & Orlovskiĭ, G. N. (1966). Control of walking and running by means of electric stimulation of the midbrain. Biofizika, 11(4), 659–666. Sillar, K. T., & Roberts, A. (1988). A neuronal mechanism for sensory gating during locomotion in a vertebrate. Nature, 331(6153), 262–265. http://doi.org/10.1038/331262a Sillar, K. T., & Roberts, A. (1992). The role of premotor interneurons in phase-dependent modulation of a cutaneous reflex during swimming in Xenopus laevis embryos. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 12(5), 1647–1657. Sims, T. J. (1977). The development of monamine-containing neurons in the brain and spinal cord of the salamander, Ambystoma mexicanum. The Journal of Comparative Neurology, 173(2), 319–336. http://doi.org/10.1002/cne.901730208 Soffe, S. R. (1991). Triggering and gating of motor responses by sensory stimulation: behavioural selection in Xenopus embryos. Proceedings. Biological Sciences / the Royal Society, 246(1317), 197–203. http://doi.org/10.1098/rspb.1991.0145 Soffe, S. R. (1997). The pattern of sensory discharge can determine the motor response in young Xenopus tadpoles. Journal of Comparative Physiology. a, Sensory, Neural, and Behavioral Physiology, 180(6), 711– 715. Spitzer, N. C. (1984). On the basis of delayed depolarization and its role in repetitive firing of Rohon-Beard neurones in Xenopus tadpoles. The Journal of Physiology, 357, 51–65. http://doi.org/10.1111/(ISSN)14697793 Stoeckel, M.-E., Uhl-Bronner, S., Hugel, S., Veinante, P., Klein, M.-J., Mutterer, J., et al. (2003). Cerebrospinal fluid-contacting neurons in the rat spinal cord, a gamma-aminobutyric acidergic system expressing the P2X2 subunit of purinergic receptors, PSA-NCAM, and GAP-43 immunoreactivities: light and electron microscopic study. The Journal of Comparative Neurology, 457(2), 159–174. http://doi.org/10.1002/cne.10565 Stuart, D. G. (1999). The segmental motor system--advances, issues, and possibilities. Progress in Brain Research, 123, 3–28. Stuart, D. G., & Hultborn, H. (2008). Thomas Graham Brown (1882. Brain Research Reviews, 59(1), 74–95. http://doi.org/10.1016/j.brainresrev.2008.06.001 108 References Szabadics, J., Varga, C., Molnár, G., Oláh, S., Barzó, P., & Tamás, G. (2006). Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science, 311(5758), 233–235. http://doi.org/10.1126/science.1121325 Szobota, S., Gorostiza, P., Del Bene, F., Wyart, C., Fortin, D. L., Kolstad, K. D., et al. (2007). Remote control of neuronal activity with a light-gated glutamate receptor. Neuron, 54(4), 535–545. http://doi.org/10.1016/j.neuron.2007.05.010 Takeoka, A., Vollenweider, I., Courtine, G., & Arber, S. (2014). Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell, 159(7), 1626–1639. http://doi.org/10.1016/j.cell.2014.11.019 Talpalar, A. E., Bouvier, J., Borgius, L., Fortin, G., Pierani, A., & Kiehn, O. (2013). Dual-mode operation of neuronal networks involved in left-right alternation. Nature, 500(7460), 85–88. http://doi.org/10.1038/nature12286 Tourtellotte, W. G., & Milbrandt, J. (1998). Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nature Genetics, 20(1), 87–91. http://doi.org/10.1038/1757 Unzer, J. (1771). The Principles of Physiology. Weidmanns, Erben and Reich, Leipzig. Vigh, B., & Vigh-Teichmann, I. (1973). Comparative ultrastructure of the cerebrospinal fluid-contacting neurons. International Review of Cytology, 35, 189–251. Vigh, B., & Vigh-Teichmann, I. (1998). Actual problems of the cerebrospinal fluid-contacting neurons. Microscopy Research and Technique, 41(1), 57–83. http://doi.org/10.1002/(SICI)10970029(19980401)41:1<57::AID-JEMT6>3.0.CO;2-R Vigh, B., Vigh-Teichmann, I., & Aros, B. (1977). Special dendritic and axonal endings formed by the cerebrospinal fluid contacting neurons of the spinal cord. Cell and Tissue Research, 183(4), 541–552. Vigh, B., Vigh-Teichmann, I., Manzano e Silva, M. J., & van den Pol, A. N. (1983). Cerebrospinal fluidcontacting neurons of the central canal and terminal ventricle in various vertebrates. Cell and Tissue Research, 231(3), 615–621. Wallace, J. A., Mondragon, R. M., Allgood, P. C., Hoffman, T. J., & Maez, R. R. (1987). Two populations of tyrosine hydroxylase-positive cells occur in the spinal cord of the chick embryo and hatchling. Neuroscience Letters, 83(3), 253–258. Wallen, P., & Williams, T. L. (1984). Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. The Journal of Physiology, 347, 225–239. http://doi.org/10.1111/(ISSN)1469-7793 Wenner, P., O'Donovan, M. J., & Matise, M. P. (2000). Topographical and physiological characterization of interneurons that express engrailed-1 in the embryonic chick spinal cord. Journal of Neurophysiology, 84(5), 2651–2657. Whelan, P. J., Hiebert, G. W., & Pearson, K. G. (1995). Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Experimental Brain Research, 103(1), 20–30. Wickersham, I. R., & Sullivan, H. A. (2015a). Rabies viral vectors for monosynaptic tracing and targeted transgene expression in neurons. Cold Spring Harbor Protocols, 2015(4), 375–385. http://doi.org/10.1101/pdb.prot072389 Wickersham, I. R., Sullivan, H. A., Pao, G. M., Hamanaka, H., Goosens, K. A., Verma, I. M., & Seung, H. S. (2015b). Lentiviral vectors for retrograde delivery of recombinases and transactivators. Cold Spring Harbor Protocols, 2015(4), 368–374. http://doi.org/10.1101/pdb.prot075879 109 References Wilson, D. M., & Wyman, R. J. (1965). Motor output patterns during random and rhythmic stimulation of locust thoracic ganglia. Biophysical Journal, 5(2), 121–143. Windhorst, U. (2007). Muscle proprioceptive feedback and spinal networks. Brain Research Bulletin, 73(46), 155–202. http://doi.org/10.1016/j.brainresbull.2007.03.010 Woo, S.-H., Lukacs, V., de Nooij, J. C., Zaytseva, D., Criddle, C. R., Francisco, A., et al. (2015). Piezo2 is the principal mechanotransduction channel for proprioception. Nature Neuroscience, 18(12), 1756–1762. http://doi.org/10.1038/nn.4162 Wyart, C., Del Bene, F., Warp, E., Scott, E. K., Trauner, D., Baier, H., & Isacoff, E. Y. (2009). Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature, 461(7262), 407–410. http://doi.org/10.1038/nature08323 Yulis, C. R., & Lederis, K. (1988). Occurrence of an anterior spinal, cerebrospinal fluid-contacting, urotensin II neuronal system in various fish species. General and Comparative Endocrinology, 70(2), 301–311. Zagoraiou, L., Akay, T., Martin, J. F., Brownstone, R. M., Jessell, T. M., & Miles, G. B. (2009). A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron, 64(5), 645–662. http://doi.org/10.1016/j.neuron.2009.10.017 Zelenchuk, T. A., & Brusés, J. L. (2011). In Vivo labeling of zebrafish motor neurons using an mnx1 enhancer and Gal4/UAS. Genesis, 49(7), 546–554. http://doi.org/10.1002/dvg.20766 Zhang, F., Wang, L.-P., Brauner, M., Liewald, J. F., Kay, K., Watzke, N., et al. (2007). Multimodal fast optical interrogation of neural circuitry. Nature, 446(7136), 633–639. http://doi.org/10.1038/nature05744 Zhang, R.-W., Wei, H.-P., Xia, Y.-M., & Du, J.-L. (2010). Development of light response and GABAergic excitation-to-inhibition switch in zebrafish retinal ganglion cells. The Journal of Physiology, 588(Pt 14), 2557– 2569. http://doi.org/10.1113/jphysiol.2010.187088 Zhang, Y., Narayan, S., Geiman, E., Lanuza, G. M., Velasquez, T., Shanks, B., et al. (2008). V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron, 60(1), 84–96. http://doi.org/10.1016/j.neuron.2008.09.027 Zhong, G., Sharma, K., & Harris-Warrick, R. M. (2011). Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord. Nature Communications, 2, 274. http://doi.org/10.1038/ncomms1276 110 Annex 1 Annex 1 Article (in press) Optimization of a neurotoxin to investigate the contribution of excitatory interneurons to speed modulation in vivo 111 Annex1 Optimization of a neurotoxin to investigate the contribution of excitatory interneurons to speed modulation in vivo Jenna R. Sternberg1-4,8, Kristen E. Severi1-4,8, Kevin Fidelin1-4, Johanna Gomez1-4, Hideshi Ihara5, Yara Alcheikh1-4, Jeffrey M. Hubbard1-4, Koichi Kawakami6#, Maximiliano Suster6-7, Claire Wyart1-4# Affiliations 1. Institut du Cerveau et de la Moelle épinière (ICM), 75013 Paris, France 2. INSERM UMRS 1127, 75013 Paris, France 3. CNRS UMR 7225, 75013 Paris, France 4. UPMC Univ Paris 06, 75005 Paris, France 5. Department of Biological Science, Graduate School of Science, Osaka Prefecture University, Sakai, Osaka 599-8531, Japan 6. Division of Molecular and Developmental Biology, National Institute of Genetics, and Department of Genetics, and SOKENDAI (The Graduate University for Advanced Studies), Mishima, Shizuoka 444-8540, Japan 7. Neural Circuits and Behaviour Group, Uni Research AS, Thormølensgate 55, Bergen 5008, Norway 8. Co-first author # To whom correspondence should be addressed: [email protected], [email protected] Keywords botulinum toxin, locomotion, V2a interneurons, chx10, zebrafish Summary Precise control of speed during locomotion is essential to adapt behavior in different environmental contexts [1-4]. A central question in locomotion lies in understanding which neural populations set locomotor frequency during slow and fast regimes. Tackling this question in vivo requires additional non-invasive tools to silence large populations of neurons during active locomotion. Here we generated a stable transgenic line encoding a zebrafish-optimized botulinum neurotoxin light chain fused to GFP to silence synaptic output over large populations of motor neurons or interneurons while monitoring active locomotion. By combining calcium imaging, electrophysiology, optogenetics, and behavior, we show that expression of BoTxBLC-GFP abolished synaptic release while maintaining characterized activity patterns and without triggering off-target effects. As chx10+ V2a interneurons (V2as) are well-characterized as the main population driving the frequency-dependent recruitment of motor neurons during fictive locomotion [5-14], we validated our silencing method by testing the effect of silencing chx10+ V2as during active and fictive locomotion. Silencing V2as selectively abolished fast locomotor frequencies during escape responses. In addition, spontaneous slow locomotion occurred less often and at frequencies lower than controls. Overall, this silencing approach confirms that V2a excitation is critical for the production of fast stimulus-evoked swimming and also reveals a role for V2a excitation in the production of slower spontaneous locomotor behavior. Altogether these results establish BoTxBLC-GFP as an ideal tool for in vivo silencing to probe the development and function of neural circuits from the synaptic to the behavioral level. Results Generation of a zebrafish-optimized transgenic line for botulinum neurotoxin light chain In order to understand the neural circuits underlying behavior, efficient tools to silence distinct classes of neurons in behaving animals are necessary. Botulinum toxins are potent poisons that block vesicu112 Annex 1 lar release at the synaptic cleft by cleaving SNARE proteins and thus eliminate synaptic transmission [15-18]. The efficacy and specificity of action of botulinum toxin light chains (BoTxLCs) make them ideal tools to silence neurons. However, stable transgenic lines optimized for expression have not yet been generated and characterized in zebrafish. We utilized the two-part Gal4/UAS combinatorial gene expression system [19, 20] to generate a stable and efficient zebrafish-optimized transgenic line encoding BoTxLC fused to GFP to abolish synaptic release in vivo. To test the efficiency of neuronal silencing of different botulinum neurotoxin serotypes (Figure 1A), we expressed distinct serotypes fused to GFP under the control of a 5X repeat of UAS (Figure 1B). We quantified the touch escape response of embryos transiently expressing either the bacterial or zebrafish codon-optimized variants of each serotype under a ubiquitous driver (Figure 1C) [19]. The bacterial forms were unable to induce paralysis, whereas the zebrafish codon-optimized form of the B serotype paralyzed 72% of embryos (Figure 1C). We established a stable transgenic line for the B serotype (Tg(UAS:BoTxBLC-GFP)). Offspring of Tg(UAS:BoTxBLC-GFP) fish mated to Tg(SAGFF73A:gal4) fish [19] (Table S1), which expresses ubiquitously, were unable to generate spontaneous and touch-evoked movement (>99%, data not shown). At least the 10th generation of Tg(UAS:BoTxBLC-GFP) fish were used for the remainder of this article. Expression of BoTxBLC-GFP in spinal motor neurons selectively abolishes coiling at the embryonic stage To test the efficiency and evaluate off-target effects of BoTxBLC-GFP, we performed a behavioral assay using Gal4 lines driving expression in either motor neurons or in spinal interneurons [5, 20, 21]. Embryos 17-25 hours post-fertilization (hpf) perform a gap junction driven stereotyped coiling behavior [22, 23]. To determine if BoTxBLC-GFP expressed in motor neurons could block muscle contraction, we used Tg(s1020t:gal4), which drives expression in a large subset of motor neurons [24, 25] (Figure S1). Embryos lost the ability to coil when BoTxBLC-GFP was expressed in this line (Figure 1D, Movie S1). We then went on to test Gal4 lines expressing in interneurons that do not drive embryonic coiling [23]. Coiling persisted when embryos expressed BoTxBLC-GFP in chx10+ interneurons (V2as) or in GABAergic pkd2l1+ cerebrospinal fluid-contacting neurons (CSF-cNs) (Figure 1E-1F) [14, 26]. Together these results indicate that BoTxBLC-GFP efficiently blocks synaptic output without off-target effects in zebrafish embryos. BoTxBLC-GFP expressing motor neurons maintain their characteristic activity To test whether the loss of coiling in Tg(s1020t:gal4; UAS:BoTxBLC-GFP) embryos (Figure 1D) was not due to off-target effects of BoTxBLC-GFP expression, we performed calcium imaging in motor neurons expressing both BoTxBLC-GFP and GCaMP5G [21, 27, 28] (Figure 1G-1H). Motor neurons 113 Annex1 in Tg(s1020t:gal4; UAS:BoTxBLC-GFP; UAS:GCaMP5G) embryos showed calcium transients occurring at comparable frequencies to non-BoTxBLC-GFP expressing Tg(s1020t:gal4; UAS:GCaMP5G) embryos at 24 hpf (Figure 1H-1I, Movie S2). This result indicates that expression of BoTxBLC-GFP silenced motor output without noticeably affecting motor neuron properties, suggesting paralysis resulted from a loss of presynaptic release rather than cell death. BoTxBLC-GFP expression in essential neuronal populations effectively disrupts the escape response in freely swimming larvae To test the utility of BoTxBLC-GFP in an active behavioral assay, we measured the escape response to an acoustic stimulus, which at 5 days post-fertilization (dpf) consists of a high amplitude turn followed by fast swimming [29, 30]. The expression of BoTxBLC-GFP in Tg(s1020t:gal4) (Figure 1J, Figure S1), targeting a large subset of motor neurons, or in Tg(vglut2a:gal4), targeting glutamatergic neurons (Figure 1K)[31], severely disrupted the escape response (Movie S3). When the larva responded, the distance traveled was significantly reduced (Figure 1J4, 1K4), confirming that expression of BoTxBLC-GFP in essential motor circuit populations leads to a predictable massive deficit in locomotion at larval stages. BoTxBLC-GFP completely blocks synaptic release in vivo To directly test the capacity of BoTxBLC-GFP to abolish vesicular release, we took advantage of a reliable monosynaptic connection between GABAergic cerebrospinal fluid-contacting neurons (CSFcNs) and caudal primary motor neurons (CaP) (Hubbard et al., in revision). Using transgenic Tg(pkd2l1:gal4; UAS:ChR2-mCherry) larvae, we elicited single spikes with 5ms light pulse in CSFcNs while performing whole-cell recordings in CaPs [21, 26] (Figure 2A-2B). Light-mediated spiking of CSF-cNs induced large short-latency inhibitory postsynaptic currents (IPSCs) in CaPs (Hubbard et al., in revision) that were strongly reduced by bath application of GABAAR antagonist gabazine (Figure 2B2). In Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP; UAS:ChR2-mCherry) larvae, light-mediated spiking in CSF-cNs did not generate IPSCs in CaPs (Figure 2C). The identity of the CaP was confirmed by filling the cells with Alexa 488 dye (Figure 2D-2E). In 59 out of 60 trials, expression of BoTxBLCGFP abolished the evoked current in CaPs (Figure 2F). Despite the block of synaptic release, Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP) transgenic larvae did not exhibit any defects in spinal cord formation (Figure S2). Furthermore, CSF-cNs retained their standard morphology, and the animals lived to adulthood (data not shown). These results demonstrate the complete loss of vesicular release from BoTxBLC-GFP expressing neurons in vivo. 114 Annex 1 BoTxBLC-mediated silencing of V2a interneurons confirms their critical role in fast locomotion V2a interneurons are known to drive motor neuron activation, and their recruitment is frequencydependent during fictive locomotion [8, 9, 14]. Therefore, we tested how V2a silencing impacts fast and slow locomotor regimes during active locomotion. Expressing BoTxBLC-GFP under the control of the chx10 promoter [5] targeted most V2a interneurons (Figure 3A1-3A2). Consequently, acoustic escape responses in these larvae show a large reduction in the distance traveled in responsive trials compared to control siblings (Figure 3A3-3A4, Movie S3). In Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae assayed in a head-embedded and tail-free configuration (Figure 3B-3C, Movie S4), the maximum bend angle, average tail-beat frequency, and response duration were all significantly reduced compared to control siblings during the acoustic escape response (Figure 3D). Although the Tg(chx10:gal4) transgenic line recapitulated chx10 expression in the hindbrain and rostral spinal cord [5], a few neurons with axons exiting the spinal cord were labeled caudally (Figure 3A2), which could contribute to the response by affecting muscle contraction. To circumvent this confound, we performed fictive ventral nerve root (VNR) recordings in 4 dpf paralyzed larvae, which allowed monitoring of the motor neuron output upstream of the neuromuscular junction. In control siblings, fast escape responses induced by otic vesicle stimulation [32] consisted of a few large amplitude bursts (Phase 1), followed by fast frequency swimming (~35-60 Hz, Phase 2), which transitioned to slow frequency swimming (<35 Hz, Phase 3) (Figure 3E). Silencing V2a output selectively disrupted fast swimming (Figure 3F); the response duration was not significantly reduced (Figure 3G). Fast locomotor frequencies above 40 Hz were abolished, while lower frequencies were unaffected (Figure 3H-I). Motor neurons are incrementally recruited along the dorsoventral axis with swimming frequency [7]. To investigate how motor neuron recruitment was affected when V2as were silenced, we generated a novel transgenic line, Tg(mnx1:GCaMP5G), in which motor neurons express the genetically-encoded calcium sensor GCaMP5G, and coupled calcium imaging of motor neurons with otic-vesicle stimulation and VNR recordings [28, 33] (Table S1, Experimental Procedures, Figure 3J-3N). The amplitude of calcium transients varied as a function of the dorsoventral position within the motor pool in a different manner for control Tg(mnx1:GCaMP5G) larvae and V2a silenced Tg(mnx1:GCaMP5G; chx10:gal4; UAS:BoTxBLC-GFP) larvae (Figure 3K, 3M, p = 0.000005). Overall, the average peak ΔF/F amplitude was highest in the most dorsal motor neurons in control larvae. Ventral motor neurons exhibited similar ΔF/F amplitude whether V2as were silenced or not (Figure 3L). In contrast, the activity of dorsal motor neurons was largely reduced in V2a-silenced larvae compared to controls (Figure 3N). A reduction in the recruitment of dorsal motor neuron is consistent with our observations that fast locomotor frequencies (> 40 Hz) in both active and fictive locomotion are abolished when V2as are silenced. 115 Annex1 Silencing of V2a interneurons decreases the locomotor frequency during spontaneous slow swimming Despite the role of hindbrain V2a neurons in initiating locomotion in zebrafish larva [5], the V2a contribution to spontaneous slow swimming has not been directly addressed. In freely swimming larvae, we noted that the occurrence of spontaneous slow swimming was largely reduced in Tg(chx10:gal4;UAS:BoTxBLC-GFP) (Figure 4A-4D, Movie S5), indicating that these neurons normally drive spontaneous locomotion. We observed that the rarely-occurring swim bouts were on average longer in duration (Figure 4E). In V2a silenced larvae, fictive slow swim bouts occurred less frequently and tended to last longer than in control siblings (Figure 4F-4H). In contrast to fast escapes, in which a reduction of frequency occurred via a suppression of the highest frequencies (Figure 3I), in the slow regime, swim frequency was reduced by a downward shift of locomotor frequencies (Figure 4I-4J). Taken together, the active and fictive data demonstrate that V2a interneurons contribute to generation of spontaneous swim events and setting the range of locomotor frequencies during slow locomotion. Discussion Silencing with the optimized botulinum toxin light chain Silencing large populations of neurons in vivo is critical to understanding their role in circuits that control behavior. To be readily implemented, silencing tools should be genetically-encoded, efficient, and non-toxic. BoTxBLC-GFP is efficient at blocking presynaptic vesicular release, as demonstrated here by probing monosynaptically connected pairs of neurons in vivo. Neurotoxins have been used extensively for eliminating neuronal activity. Persistent problems for diphtheria use include off-target damage and establishing stable transgenic lines, while in the case of tetanus, detection of fluorescence is difficult in vivo[19, 34-36]. The stable trangenic BoTxBLC-GFP has been effective for many generations and fluorescence could be detected in vivo. Codon-optimization of BoTxBLC-GFP was critical for expression and silencing in zebrafish, demonstrating the need to design model-specific tools. Other strategies such as Nitroreductase, KillerRed, or transient receptor potential channels developed for targeted cell ablation or silencing in zebrafish work in vivo but require a chemical cofactor or light delivery [34-37]. Using chemically-mediated methods, ablations can require long incubation times, depending on the expression level, and if driver lines express in non-neuronal tissues [36], these tissues will be ablated. Although opsins can silence with precise spatial and temporal resolution, light delivery can interfere with light sensitive behaviors, and opsins are currently unfeasible for silencing 116 Annex 1 across large spatial regions during active behavior because they require high power density [38-40]. BoTxBLC-GFP offers a straightforward alternative to these existing methods, potently and chronically silencing in neuronal tissue without additional cofactors or off-target effects. Implementing inducible expression in the future would allow dissection of the relative contributions of acute versus chronic silencing. The contribution of V2a interneurons to locomotion chx10+ V2a glutamatergic neurons are involved in initiation [5], termination [6], left-right alternation [41], and setting locomotor frequency [7, 11, 42] in vertebrate species. V2a interneurons recorded during fictive locomotion in zebrafish are recruited in a frequency-dependent manner along the dorsoventral axis and are necessary for generation of high-frequency swimming [8, 12]. Here we investigated the effects of silencing a majority of V2as during two motor behaviors: slow spontaneous swimming and fast escape responses. The escape response in zebrafish larva includes a wide range of locomotor frequencies from 15-100 Hz [2, 43]. Fast components of the escape were suppressed when V2a interneurons were silenced, confirming the critical role of V2as in fast locomotion [8, 44]. By performing calcium imaging on the motor pool, we showed that this suppression effect corresponds to the diminished recruitment of dorsal motor neurons essential for fast swimming. This suggests the excitation of motor neurons by V2as is necessary to generate fast locomotor frequencies. Spontaneous slow locomotion occurs in a narrow range of locomotor frequencies (20-30 Hz) [2, 43], during which a subset of ventral V2as is active [8]. Spontaneous active slow swimming rarely occurred when V2as were silenced, and those swim bouts lasted longer. These effects could be related to hindbrain V2a populations involved in initiating locomotion [5] or controlling the duration of locomotor events [6]. In addition, we observed that V2as adjust the locomotor frequency in the slow regime. Further investigation should clarify how excitation by V2as from the hindbrain and spinal cord contribute to this effect. The remaining source of excitation driving slow swimming when V2as are silenced could be glutamatergic V0-v neurons, referred to as MCoDs in zebrafish, which are selectively recruited at these frequencies [8], suggesting that MCoDs and V2as may work in concert during slow locomotion. Our results confirm important contributions for V2as in fast and slow regimes of locomotion. Previous work showed that spinal V2as are a heterogeneous population of neurons based on their dorsoventral positioning, morphology, intrinsic properties, and connectivity [8, 9, 12]. Our results, obtained by silencing of the V2a population in the hindbrain and spinal cord, indicate speed-dependent roles for V2as. While V2as are critical for sustaining fast locomotion, they appear to adjust locomotor frequency and modulate bout duration in the slow regime. New genetic tools will be required to distinguish how hindbrain and spinal cord V2as accomplish these unique functions. 117 Annex1 Experimental procedures Generation of stable transgenic zebrafish lines 25 ng μl-1 of Tol2 transposase mRNA and either pT2S-UAS:zBoTxBLC-GFP or pT2S-UAS:zBoTxBLC were co-injected into one-cell stage embryos of the TAB (AB/TUB) strain. 188 injected embryos from seven unique clutches were raised. 47 embryos injected with pT2S-UAS:zBoTxBLC-GFP were screened after mating with selected Gal4FF lines for behavioral phenotypes and GFP fluorescence. 13 embryos injected with pT2S-UAS:zBoTxBLC were screened by PCR. We used Gal4FF drivers (Tg(SAGFF73A) and others) for screening the UAS founder fish. We selected six founders, identified single integrations by Southern blot, and mapped the integration sites from F1 progeny (834 F1 fish examined). A single homozygous F2 line Tg(UAS:zBoTxBLC-GFP)34b has been stably maintained for over 10 generations used for the experiments shown for all figure panels except Figure 1C. The sequences are available on the zTrap database (http://kawakami.lab.nig.ac.jp/ztrap/). The Tg(mnx1:GCaMP5G)icm25 stable transgenic line was generated based on the mnx1 promoter [33] integrated in a 5’ entry gateway clone [45] and combined to the pME-GCaMP5G and the 3’ polyA entry clone into the destination vector carrying tol2 sites and cryaa:venus [46]. Table S1 lists all transgenic lines used in this study, long form names, and expression patterns if applicable. Supplemental information The supplemental information contains Supplemental Experimental Procedures, 1 table (Table S1), 2 figures (Figure S1, S2), and 5 movies (S1-5). Author contributions MS generated the BoTx constructs and lines in KK’s lab, which provided the fish facility and technical assistance to map the integration. MS conceived and initiated the BoTx project and generated the plasmid constructs and stable transgenic line Tg(UAS:BoTxBLC-GFP). MS actively worked to validate and maintain active carriers over generations from the line used in this manuscript. JRS, KES, and CW conceived this study with input from KF. JRS performed and analyzed coiling experiments, in vivo patch recordings, calcium imaging, VNR recordings, and cell counting. KES performed and analyzed larval behavioral experiments with help from YA. KF performed and analyzed VNR recordings. JG generated the Tg(mnx1:GCaMP5G) transgenic line. HI provided the original clones for 118 Annex 1 BoTxLC serotypes. JH provided unpublished essential electrophysiological data. CW and KK supervised research. JRS, KES, and CW wrote and revised the manuscript with feedback from KF, KK, and MS. Acknowledgements We thank Prof. Shin-Ichi Higashijima for the Tg(chx10:gal4) line, Prof. Shunji Kozaki provided bacterial BoTx plasmids, Tom Auer and Filippo Del Bene for the mnx1 promoter, David McLean for useful feedback, Urs Böhm, Andrew Prendergast, and Steven Knafo for support with behavior and tracking, Vincent Guillemot for assistance with statistics, Sophie Nunes Figueiredo, Natalia Maties, and Bogdan Buzurin for fish care, Ruben Portugues, Ana Faustino, and David Lyons for helpful comments on the manuscript, the Plateforme d'Imagerie Cellulaire Pitié-Salpêtrière, the cloning facility, and the Biostatistics facility at ICM. This work received support from the JSPS postdoc fellowship (MS), and the chair d’excellence of the Ecole des Neurosciences de Paris (ENP), European Research Council (ERC) starting grant Optoloco #311673, and the National Institute of Health (NIH) Brain Initiative #5U01NS090501 (CW). References 1. 2. 3. 4. 5. 6. 7. 8. Severi, K.E., Portugues, R., Marques, J.C., O'Malley, D.M., Orger, M.B., and Engert, F. (2014). Neural control and modulation of swimming speed in the larval zebrafish. Neuron 83, 692-707. Budick, S.A., and O'Malley, D.M. (2000). Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J. Exp. Biol. 203, 2565–2579. Talpalar, A.E., Bouvier, J., Borgius, L., Fortin, G., Pierani, A., and Kiehn, O. (2013). Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500, 85-88. Gosgnach, S., Lanuza, G.M., Butt, S.J., Saueressig, H., Zhang, Y., Velasquez, T., Riethmacher, D., Callaway, E.M., Kiehn, O., and Goulding, M. (2006). V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440, 215-219. Kimura, Y., Satou, C., Fujioka, S., Shoji, W., Umeda, K., Ishizuka, T., Yawo, H., and Higashijima, S. (2013). Hindbrain V2a neurons in the excitation of spinal locomotor circuits during zebrafish swimming. Curr. Biol. 23, 843-849. Bouvier, J., Caggiano, V., Leiras, R., Caldeira, V., Bellardita, C., Balueva, K., Fuchs, A., and Kiehn, O. (2015). Descending Command Neurons in the Brainstem that Halt Locomotion. Cell 163, 1191-1203. McLean, D.L., Fan, J., Higashijima, S., Hale, M.E., and Fetcho, J.R. (2007). A topographic map of recruitment in spinal cord. Nature 446, 71-75. McLean, D.L., Masino, M.A., Koh, I.Y., Lindquist, W.B., and Fetcho, J.R. (2008). Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat. Neurosci. 11, 1419-1429. 119 Annex1 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 120 Menelaou, E., Vandunk, C., and McLean, D.L. (2014). Differences in the morphology of spinal V2a neurons reflect their recruitment order during swimming in larval zebrafish. J. Comp. Neurol. 522, 1232-1248. Dougherty, K.J., and Kiehn, O. (2010). Functional organization of V2a-related locomotor circuits in the rodent spinal cord. Ann. N. Y. Acad. Sci. 1198, 85-93. Ljunggren, E.E., Haupt, S., Ausborn, J., Ampatzis, K., and El Manira, A. (2014). Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. J. Neurosci. 34, 134-139. Ampatzis, K., Song, J., Ausborn, J., and El Manira, A. (2014). Separate microcircuit modules of distinct V2a interneurons and motoneurons control the speed of locomotion. Neuron 83, 934-943. Ritter, D.A., Bhatt, D.H., and Fetcho, J.R. (2001). In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J. Neurosci. 21, 8956–8965. Kimura, Y., Okamura, Y., and Higashijima, S. (2006). alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J. Neurosci. 26, 5684-5697. Kao, I., Drachman, D.B., and Price, D.L. (1976). Botulinum toxin: mechanism of presynaptic blockade. Science 193, 1256-1258. Schiavo, G., Benfenati, F., Poulain, B., Rossetto, O., Polverino de Laureto, P., DasGupta, B.R., and Montecucco, C. (1992). Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359, 832-835. Sutton, R.B., Fasshauer, D., Jahn, R., and Brunger, A.T. (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347-353. Brunger, A.T., Jin, R., and Breidenbach, M.A. (2008). Highly specific interactions between botulinum neurotoxins and synaptic vesicle proteins. Cell. Mol. Life Sci. 65, 2296-2306. Asakawa, K., Suster, M.L., Mizusawa, K., Nagayoshi, S., Kotani, T., Urasaki, A., Kishimoto, Y., Hibi, M., and Kawakami, K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 105, 1255-1260. Scott, E.K., Mason, L., Arrenberg, A.B., Ziv, L., Gosse, N.J., Xiao, T., Chi, N.C., Asakawa, K., Kawakami, K., and Baier, H. (2007). Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4, 323-326. Fidelin, K., Djenoune, L., Stokes, C., Prendergast, A., Gomez, J., Baradel, A., Del Bene, F., and Wyart, C. (2015). State-dependent modulation of locomotion by GABAergic spinal sensory neurons. Curr. Biol. 25, 3035-3047. Saint-Amant, L., and Drapeau, P. (1998). Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 37, 622-632. Saint-Amant, L., and Drapeau, P. (2000). Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J. Neurosci. 20, 3964-3972. Warp, E., Agarwal, G., Wyart, C., Friedmann, D., Oldfield, C.S., Conner, A., Del Bene, F., Arrenberg, A.B., Baier, H., and Isacoff, E.Y. (2012). Emergence of patterned activity in the developing zebrafish spinal cord. Curr. Biol. 22, 93-102. Wyart, C., Del Bene, F., Warp, E., Scott, E.K., Trauner, D., Baier, H., and Isacoff, E.Y. (2009). Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461, 407-410. Annex 1 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. Djenoune, L., Khabou, H., Joubert, F., Quan, F.B., Nunes Figueiredo, S., Bodineau, L., Del Bene, F., Burckle, C., Tostivint, H., and Wyart, C. (2014). Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Frontiers in neuroanatomy 8, 26. Lauterbach, M.A., Ronzitti, E., Sternberg, J.R., Wyart, C., and Emiliani, V. (2015). Fast calcium imaging with optical sectioning via HiLo microscopy. PloS one 10, e0143681. Akerboom, J., Chen, T.W., Wardill, T.J., Tian, L., Marvin, J.S., Mutlu, S., Calderon, N.C., Esposti, F., Borghuis, B.G., Sun, X.R., et al. (2012). Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819-13840. Kohashi, T., Nakata, N., and Oda, Y. (2012). Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish. J. Neurosci. 32, 5810-5820. Bohm, U.L., Prendergast, A., Djenoune, L., Nunes Figueiredo, S., Gomez, J., Stokes, C., Kaiser, S., Suster, M., Kawakami, K., Charpentier, M., et al. (2016). CSFcontacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nat. Commun. 7, 10866. Satou, C., Kimura, Y., Hirata, H., Suster, M.L., Kawakami, K., and Higashijima, S.-I. (2013). Transgenic tools to characterize neuronal properties of discrete populations of zebrafish neurons. Development 140, 3927-3931. Kohashi, T., and Oda, Y. (2008). Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J. Neurosci. 28, 10641-10653. Zelenchuk, T.A., and Bruses, J.L. (2011). In vivo labeling of zebrafish motor neurons using an mnx1 enhancer and Gal4/UAS. Genesis 49, 546-554. Lee, A., Mathuru, A.S., Teh, C., Kibat, C., Korzh, V., Penney, T.B., and Jesuthasan, S. (2010). The habenula prevents helpless behavior in larval zebrafish. Curr. Biol. 20, 2211-2216. Curado, S., Anderson, R.M., Jungblut, B., Mumm, J., Schroeter, E., and Stainier, D.Y. (2007). Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev. Dyn. 236, 1025-1035. Curado, S., Stainier, D.Y., and Anderson, R.M. (2008). Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nature protocols 3, 948954. Chen, S., Chiu, C.N., McArthur, K.L., Fetcho, J.R., and Prober, D.A. (2015). TRP channel mediated neuronal activation and ablation in freely behaving zebrafish. Nat. Methods 13, 147-150. Mattis, J., Tye, K.M., Ferenczi, E.A., Ramakrishnan, C., O'Shea, D.J., Prakash, R., Gunaydin, L.A., Hyun, M., Fenno, L.E., Gradinaru, V., et al. (2011). Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159-172. Friedmann, D., Hoagland, A., Berlin, S., and Isacoff, E.Y. (2015). A spinal opsin controls early neural activity and drives a behavioral light response. Curr. Biol. 25, 6974. Rihel, J., and Schier, A.F. (2012). Behavioral screening for neuroactive drugs in zebrafish. Dev. Neurobiol. 72, 373-385. Crone, S.A., Quinlan, K.A., Zagoraiou, L., Droho, S., Restrepo, C.E., Lundfald, L., Endo, T., Setlak, J., Jessell, T.M., Kiehn, O., et al. (2008). Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron 60, 70-83. 121 Annex1 42. 43. 44. 45. 46. 122 Dougherty, K.J., Zagoraiou, L., Satoh, D., Rozani, I., Doobar, S., Arber, S., Jessell, T.M., and Kiehn, O. (2013). Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron 80, 920-933. Mirat, O., Sternberg, J.R., Severi, K.E., and Wyart, C. (2013). ZebraZoom: an automated program for high-throughput behavioral analysis and categorization. Front. Neural Circuits 7, 107. Eklof-Ljunggren, E., Haupt, S., Ausborn, J., Dehnisch, I., Uhlen, P., Higashijima, S., and El Manira, A. (2012). Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc. Natl. Acad. Sci. USA 109, 5511-5516. Kwan, K.M., Fujimoto, E., Grabher, C., Mangum, B.D., Hardy, M.E., Campbell, D.S., Parant, J.M., Yost, H.J., Kanki, J.P., and Chien, C.B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099. Ni, T.T., Lu, J., Zhu, M., Maddison, L.A., Boyd, K.L., Huskey, L., Ju, B., Hesselson, D., Zhong, T.P., Page-McCaw, P.S., et al. (2012). Conditional control of gene function by an invertible gene trap in zebrafish. Proc. Natl. Acad. Sci. USA 109, 15389-15394. Annex 1 Figures and Supplemental information 123 Annex1 Figure 1. Generation and validation of the transgenic line Tg(UAS:BoTxBLC-GFP) (A) Botulinum serotype B targets synaptobrevin, a SNARE complex protein essential for neurotransmitter release (after Brunger et al., 2008; Sutton et al., 1998, A-G denote cleavage sites of distinct Botulinum serotypes). (B) Design of the zebrafish codon-optimized UAS:BoTxLC-GFP constructs for the A, B, C, and E serotypes tested in transient. (C) Percentage of Tg(SAGFF73:gal4) (embryos paralyzed upon transient expression of BoTx serotypes, with and without codon optimization for zebrafish (embryos tested from left to right n = 11, 16, 18, 20, 20, 25, 16, 30) . (D) Expression pattern of Tg(1020t:gal4; UAS:BoTxBLC-GFP) double transgenic embryos (left) and reduction in coiling frequency (right) n = 14 BoTxBLC-GFP+ embryos and 14 control siblings). (E) Same as (D) for Tg(chx10:gal4; UAS:BoTxBLC-GFP), n = 11 BoTxBLC-GFP+ embryos and 12 control siblings. (F) Same as (D) for Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP), n = 16 BoTxBLC-GFP+ embryos and 15 control siblings. (G, H) Representative examples of calcium imaging in Tg(s1020t:gal4; UAS:GCaMP5G) control (G) and Tg(s1020t:gal4; UAS:GCaMP5G; UAS:BoTxBLCGFP) (H) embryos. (G1, H1) imaging plane, (G2, H2) selected ROIs, left and right hemicords indicated in magenta and green, respectively, and corresponding ΔF/F traces (right). Differences in ∆F/F amplitude result from higher levels of baseline GFP expression in BoTxBLC-GFP+ embryos. (I) Frequency of calcium transients in control and BoTxBLC-GFP+ embryos. Each point represents the average frequency of all ROIs within an embryo (n = 8 BoTxBLC-GFP+ and 8 non-sibling control embryos). (J) Comparison of control siblings and Tg(s1020t:gal4; UAS:BoTxBLC-GFP) 5 dpf larvae in a freely swimming acoustic escape assay. (J1) Whole larva lateral view. (J2) Lateral view of the spinal cord. (J3) Trajectories of swimming larvae in the assay. Control siblings are Tg(UAS:BoTxBLC-GFP) only. (J4) The average distance traveled during a responsive trial (mm) (n = 12 control larvae, 109 responses out of 118 trials and 11 BoTxBLC-GFP+ larvae, 72 responses out of 118 trials). (K) Same as (J) for Tg(vglut2a:gal4; UAS:BoTxBLC-GFP) (n = 12 control larvae, 98 responses out of 128 trials and 10 BoTxBLC-GFP+ larvae, 67 responses out of 128 trials). Controls sibling embryos for (D-F) had either the Gal4 or the UAS transgene, or neither transgene. Insets for (D-F) are Z-projection stacks of a few optical sections imaged on the lateral side between segments 7 and 10. Scale bars are 500 µm in (D-F), 20 µm for insets in (D-F), 20 µm in (G-H), 100 µm for (J1) and (K1), 25 µm for (J2) and (K2), and 5 mm for (J3) and (K3). (D), (E), (F), (J1), (J2), (K1), and (K2) are lateral views with dorsal up. (G-H) are dorsal views. Rostral is to the left in all panels. In (DF) individual points correspond to coiling frequency for one embryo. For (J4) and (K4), the average responses of each larva are represented by a single grey point for control or teal point for BoTxBLCGFP+ larvae respectively. Means are shown ± S.E.M. in black. p-values *** < 0.001, **** <0.0001, Student's t-test for (D-F), and (I). L, left. R, right. See also Figure S1, Table S1, Movie S1-S3. 124 Annex 1 Figure 2. ChR2-mediated activation of presynaptic neurons expressing BoTxBLC-GFP demonstrates a complete block of synaptic release in vivo (A) Expression of BoTxBLC-GFP and ChR2-mCherry in Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP; UAS:ChR2-mCherry). GFP and mCherry co-labeling in a CSF-cN innervating a CaP primary motor neuron (innervation of a CaP by a CSF-cN is indicated by an arrowhead). (B) Full field light-mediated activation of CSF-cNs in Tg(pkd2l1:gal4; UAS:ChR2-mCherry) induces a monosynaptic IPSC in a CaP that is reduced by gabazine. (B1) Schematic representation of light-mediated activation of ChR2+ CSF-cNs coupled with a whole-cell recording of a CaP. (B2) Top: individual traces from one CaP (grey), average of ten trials (black). Bottom: Gabazine strongly reduces the induced IPSC (individual traces from one CaP (grey), average of ten trials (purple). (C) No IPSC was observed after lightmediated activation of CSF-cNs in Tg(pkd2l1:gal4; UAS:ChR2-mCherry;UAS:BoTxBLC-GFP) larvae: (C1) schematic representation of experimental setup, (C2) individual traces from one CaP (grey), average of ten trials (teal). (D, E) Confirmation of CaP identity after filling with Alexa 488 dye. (D) Cell body with Alexa 488 (top), ChR2-mCherry (middle), merge (bottom), and axonal projections onto ventral musculature in (E) (black double arrowhead, CaP soma, double white arrowhead, axon exiting the spinal cord). (F) Quantification of peak IPSC amplitude in control and BoTxBLC-GFP+ larvae (control: n = 3 cells from 3 larvae, 60 trials; gabazine: n= 2 cells from 2 larvae, 40 trials; BoTxBLC-GFP+: n = 3 cells from 3 larvae, 60 trials). (A) and (E) are Z-projection stacks from a few optical sections imaged on the lateral side. (D) is a single plane image. All images are oriented with rostral to the left and dorsal up. Scale bars are 10 µm (approximated in (D)).* p < 0.05, one-way ANOVA with repeated measures. R, rostral, C, caudal, D, dorsal, V, ventral. See also Figure S2, Table S1. 125 Annex1 Figure 3. V2a interneurons are critical for induced fast-frequency swimming (A) Comparison of control siblings and Tg(chx10:gal4; UAS:BoTxBLC-GFP) 5 dpf larvae in a freely swimming acoustic escape assay. (A1) Whole larva lateral view. (A2) Lateral view of the spinal cord with borders denoted by dotted line. (A3) Trajectory of swimming larvae in the assay. Control siblings are Tg(UAS:BoTxBLC-GFP) larvae. (A4) The average distance traveled during a responsive trial (mm) (n = 11 control larvae, 81 responses out of 120 trials and 12 BoTxBLC-GFP+ larvae, 107 responses out of 120 trials). (B-D) Comparison of control siblings and Tg(chx10:gal4; UAS:BoTxBLC-GFP) 5 dpf larvae in a head-embedded, tail-free acoustic escape assay. (B) Left: control larva (Tg(UAS:BoTxBLCGFP)), Right: Tg(chx10:gal4;UAS:BoTxBLC-GFP) larva with its head embedded in agarose and the 126 Annex 1 tail free to move; dotted line indicates agar border. (C) Example tail-bend angle (degrees) extracted for head-restrained control larvae (Tg(UAS:BoTxBLC-GFP) alone, black, top) and BoTxBLC-GFP (Tg(chx10:gal4; UAS:BoTxBLC-GFP), teal, bottom); dotted line indicates the time of stimulus delivery. (D) Kinematic analysis of head-embedded acoustic-induced escape responses. Maximum bend angle in the trial (left, degrees), average tail-beat frequency within a bout (center, Hz) and average bout duration (right, ms) (n = 12 control larvae, 104 trials. n = 12 Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae, 118 trials). (E-I) Analysis of fictive VNR recordings during escape responses in 4 dpf larvae. (E) Schematic of the otic vesicle stimulation used to evoke fast escape responses in combination with fictive VNR recordings (left). Three phases of the response can be distinguished in the escape response (right). (F) Representative traces of fictive escape responses from 3 larvae for control (F1) and Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae (F2). Red asterisks indicate the stimulus onset. (G-H) Response duration (G, s) and mean burst frequency (H, Hz) for n = 57 stimulations for 5 control larvae and n = 54 stimulations for 5 BoTxBLC-GFP+ larvae. (I) Distribution of burst frequencies (Hz) in control larvae (grey) and BoTxBLC-GFP+ larvae (teal). Inset: cumulative distribution function. (J-N) Calcium transients, imaged in motor neurons as a function of dorsoventral position, when V2as are silenced (BoTxBLC-GFP+) or not (control) with simultaneous fictive VNR recording. (J) Schematic of the calcium imaging configuration performed in combination with otic vesicle stimulation and VNR recordings as described in (E). (K) GCaMP5G expression in Tg(mnx1:GCaMP5G) with ROIs overlaid (top), calcium transients (center) and fictive VNR recording during a fictive escape in ventral motor neurons (bottom). Control larva: purple ROIs, black ΔF/F trace (left). BoTxBLC-GFP+ larva: teal ROIs, teal ΔF/F trace (right). (L1-L3) Peak ΔF/F response for an individual ROI, color-coded by larva, with the average across larvae indicated by a black line. Comparison of control vs. BoTxBLC-GFP+ larvae for ventral motor neurons at dorso-ventral positions: 0-0.2 (L1), 0.2-0.3 (L2), or 0.3-0.4 (L3). (M) Same as (K) but for dorsal motor neurons. (N1-N3) Same as (L1-L3) but for dorso-ventral positions: 0.4-0.5 (N1) or 0.5-1 (N2). For (L) and (N), n = 199 ROIs from 55 stimulations with swimming episodes for 5 control larvae and n = 102 ROIs from n = 30 stimulations with swimming episodes for 4 BoTxBLC-GFP+ larvae. Scale bars are 100 µm in (A1), 25 µm in (A2), 5 mm in (A3), 1 mm in (B), 20 µm in (K and M). For (A4), (D), (G), and (H), each data point is the average across all trials for a single larva. Control sibling Tg(UAS:BoTxBLC-GFP) larvae are in grey and Tg(chx10:gal4; UAS:BoTxBLC-GFP) are in teal. Means are shown ± S.E.M. in black. p-values ** <0.01, *** <0.001, **** <0.0001. See also Table S1, Movie S3-4. 127 Annex1 Figure 4. V2a interneurons drive most of spontaneous slow swimming and adjust the locomotor frequency in the slow regime (A-E) Spontaneous slow swimming assay comparing control and Tg(chx10:gal4; UAS:BoTxBLCGFP) larvae at 5 dpf. (A) The trajectory of a 5 dpf larvae spontaneously swimming for the first minute of a four minute recording. Left: control sibling (Tg(UAS:BoTxBLC-GFP) alone, Right: Tg(chx10:gal4; UAS:BoTxBLC-GFP) larva recorded simultaneously. (B) Average distance traveled (mm, measured during the first minute of the trial). (C) Rastergram of the timing of swim bout initiation over the four minute spontaneous swimming trial recorded. The y-axis represents a different individual larva (Top: control siblings, Bottom: Tg(chx10:gal4; UAS:BoTxBLC-GFP)). (D) Mean frequency of bout occurrence (bouts per second, Hz) measured over a four minute trial. (E) Average bout duration (ms) across all bouts recorded spontaneously in a four minute trial. (B-E) n = 8 control and 8 BoTxBLC-GFP+ larvae. (F) Schematic and example trace of fictive slow swimming in a control (top) and a BoTxBLC-GFP+ (bottom) larva. Boxes indicate expanded regions at right. Note the reduced burst frequency in BoTxBLC-GFP+ larvae when V2as are silenced. (G) Reduction in frequency of bout occurrence (bouts per second) in BoTxBLC-GFP+ larvae compared to control siblings (n = 1265 bouts for 8 control larvae, 938 bouts for 8 BoTxBLC-GFP+ larvae). (H) Fictive bout duration (s). (I) Mean burst frequency during fictive slow swim bouts (Hz). (H-I) n = 741 bouts from 8 control larvae and 497 bouts from 8 BoTxBLC-GFP+ larvae. (J) Distribution of burst frequencies (Hz) in control larvae (grey) and BoTxBLC-GFP+ larvae (teal). Inset: cumulative distribution function. For (B), (D), (E), (G), (H), and (I): Control siblings (grey, Tg(UAS:BoTxBLC-GFP)) were compared to Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae (teal) and each point represents a single larva. Means are shown ± S.E.M. in black. p-value * <0.05, ** <0.01, *** <0.001, **** <0.0001. Student’s t-test for (D) and (G). See also Table S1, Movie S5. 128 Annex 1 Figure S1. Tg(s1020t:gal4) enhancer trap line labels most but not all motor neurons. Related to Figure 1. Image of 1 dpf Tg(HuC:GCaMP5G; s1020t:gal4; UAS:mCherry) embryo. Top: The HuC promoter labels a diverse population of interneurons and motor neurons in the spinal cord. Middle: In the Tg(s1020t:gal4) enhancer trap line, most primary motor neurons are labeled in the spinal cord. Merge: GCaMP5G and mCherry expression. Magnification: axons from motor neurons exiting the spinal cord are visible in non-overlapping populations of motor neurons from both transgenic lines. Rostral is left, dorsal is up. Scale bar is 50µm. 129 Annex1 Figure S2. Neurotransmitter specification in the spinal cord is unaffected when GABAergic CSF-cNs are silenced in Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP). Related to Figure 2. (A-C) Quantification of ventral glutamatergic, GABAergic, and chx10+ interneurons per segment in rostral (segments 3-4), mid-cord (segments 10-11), and caudal (segments 25-26) regions in 3 dpf larvae. (A) Left: quantification of ventral glutamatergic cells in control and Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP). Right: image of Tg(vglut2a:|R|-GFP; pkd2l1:gal4; UAS:BoTxBLC-GFP) at segment 10. n = 14 control siblings , n = 13 BoTxBLC-GFP+. (B) Left: quantification of ventral GABAergic cells in control and Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP). Right: image of Tg(gad1b:|R|-GFP; pkd2l1:gal4; UAS:BoTxBLC-GFP) at segment 10. n = 12 control siblings, n = 12 BoTxBLC-GFP+. (C) Left: quantification of chx10+ V2as in control and Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP). Right: image of Tg(chx10:|R|-GFP; pkd2l1:gal4; UAS:BoTxBLC-GFP) at segment 10. n = 10 control siblings, n = 10 BoTxBLC-GFP+. (D) Schema of regions for quantification. (E) Number of BoTxBLC-GFP+ CSF-cNs per segment. Dotted line indicates average number of pkd2l1+ CSF-cNs in the spinal cord per segment. * p < 0.05. Student's t-test. All comparisons were performed per region between control and BoTxBLC-GFP+ conditions. Table S1. List of stable transgenic lines used and generated in this study. Related to Figures 1-4. 130 Annex 1 Name used in text Long form or alternate name Labeling Reference Tg(pkd2l1:gal4) Tg(pkd2l1:gal4)icm10 CSF-cNs Fidelin et al., 2015 [S1] Tg(s1020t:gal4) Et(-0.6hsp70l:Gal4-VP16)s1020t Motor neurons, CSF-cNs,VeLDs Scott et al., 2007 [S2] Tg(SAGFF73:gal4) SAGFF(nedd4b1):gal4FF Ubiquitous Asakawa et al., 2008 [S3] Tg(chx10:|R|-GFP) Tg(chx10:lox:DsRed:lox:GFP) V2a interneurons Kimura et al., 2006 [S4] Tg(chx10:gal4) Tg(chx10:gal4) V2a interneurons Kimura et al., 2013 [S5] Tg(UAS:BoTxBLC-GFP) Tg(Nk(UAS:zBoTxBLC-GFP)34b) N/A This study (see Auer et al., 2015; Böhm et al., 2016) [S6,S7] Tg(UAS:ChR2-mCherry) Tg(UAS:Cr.ChR2_H134R-mCherry) N/A Schoonheim et al., 2010 [S8] Tg(UAS:GCaMP5G) Tg(UAS:GCaMP5G)icm08 N/A Fidelin et al., 2015 [S1] Tg(vglut2a:gal4) Tg(vglut2a:gal4) Glutamatergic cells expressing vGluT2 Satou et al., 2013 [S9] Tg(vglut2a:|R|-GFP) Tg(vglut2:lox:DsRed:lox:GFP) Glutamatergic cells expressing vGluT2 Satou et al., 2013 [S9] Tg(gad1b:|R|-GFP) Tg(gad1b:lox:DsRed:lox:GFP) GABAergic cells Satou et al., 2013 [S9] Tg(mnx1:GCaMP5G) Tg(mnx1:GCaMP5G)icm25 Motor neurons This study Tg(HuC:GCaMP5G) Tg(elavl3:GCaMP5G) Most neurons Ahrens et al., 2013 [S10] Supplemental Experimental Procedures Zebrafish husbandry All procedures were approved by the Institut du Cerveau et de la Moelle épinière (ICM) and the National Ethics Committee on E.U. legislation or were in accordance with institutional and national guidelines and regulations at the National Institute of Genetics, Japan. All experiments were performed on Danio rerio embryos and larvae of AB, TL, TU, or mixed background. For some experiments, mitfa -/- animals were used. Embryos and larvae were raised in an incubator at 28.5°C under a 14/10 light/dark cycle until the start of experimentation. Experiments were performed at room temperature (22-26°C) on 1 to 5 dpf larvae. Generation of plasmids for conditional expression of Botulinum Toxin Light Chain (BoTxLC) in zebrafish embryos Plasmids encoding published bacterial BoTxLC proteins A, B, C, D, E, F and G were obtained in vector pQE3 (3416 bp) (a gift to KK). In parallel, cDNA sequences encoding full BoTxLC serotypes A (1398bp), B (1350 bp), C (1401 bp), and E (1320 bp) were constructed in silico based on entries from NCBI, then modified in-house for optimal translation in zebrafish, and finally purchased from GenScript (New Jersey, USA). Synthetic cDNAs were blunt-end cloned at the EcoRV site of pUC57, and in most cases subsequently amplified by PCR using high-fidelity PfuUltra II Fusion HS DNA 131 Annex1 polymerase, TOPO cloned in pCR8GW (Invitrogen, Carlsbad, California) and fully sequenced thereafter to confirm that no mutations had been introduced by PCR. To generate GFP reporter plasmids that express BoTxLC under Gal4/UAS control, we first generated a custom Tol2 transgenesis and expression vector containing the Gal4-target sequence, 5x UAS, followed by an atg-less GFP. A Gatewaycompatible vector pT2UASMCSGW-R1R2 (5419 bp) was digested with SalI/XhoI to introduce an XhoI/SalI GFP fragment (740 bp). The resulting plasmid was digested with EcoRI/XhoI to ligate either a bacterial or zebrafish-codon optimized BoTxLC cDNA fragment containing 5’ EcoRI and 3’ XhoI compatible ends. The resulting plasmids pT2UAS:BoTxLC-GFP or pT2UAS:zBoTxLC-GFP encode an in-frame fusion of GFP at the C-terminus of BoTxLC (see supplemental material in [S6, S7]). Transient expression of botulinum toxin serotypes and behavioral scoring Groups of n > 30 were raised for each injected group and examined at 36 or 48 hours post fertilization (hpf). Embryos were placed in 5 cm plastic petri dishes filled with E3 media and allowed to settle for 5 minutes before recording touch-evoked motor behaviors. Embryos were firmly touched at the tail, head, and yolk region at least 3 times with a fine syringe needle, and those that displayed no motion at all (escape response or swimming) were considered paralyzed. Paralysis was confirmed in 500 fps high-speed video recordings (Photron, United Kingdom). Coiling Assay in transgenic lines expressing the optimized BoTxBLC-GFP Embryos expressing BoTxBLC-GFP under the control of either Tg(pkd2l1:gal4), Tg(s1020t:gal4), or Tg(chx10:gal4) were screened for fluorescence and dechorionated. Coiling was evaluated between 24 and 25 hpf for 1 minute. All experiments were performed simultaneously with sibling embryos not expressing BoTxBLC-GFP as controls. All images were obtained using a uEye camera (IDS Imaging Development Systems GmbH, Obersulm, Germany). Fluorescent imaging of transgenic lines A Nikon AZ100 Multizoom microscope system was used for all images of whole embryos or larvae. Stacks of spinal neurons in diverse transgenics lines were obtained using a 20x or 40x water immersion objective on a spinning disk confocal microscope or 2-photon laser scanning microscope using a 20x water immersion objective (3i Intelligent Imaging Innovations, Inc., Denver, CO, USA). Calcium imaging in zebrafish embryos Adults of homozygous Tg(UAS:BoTxBLC-GFP) were crossed to double transgenic Tg(s1020t:gal4; UAS:GCaMP5G) and Tg(s1020t:gal4:UAS:GCaMP5G) were crossed to wild type AB adults for agematched controls. Embryos were maintained at 28.5°C and staged according to [S12], dechorionated manually, then embedded in 1.5% low melting point agarose. All imaging was performed following injection in caudal axial muscle with approximately 2 nL of 500 µM α-bungarotoxin (Tocris Biosci132 Annex 1 ence, Bristol, United Kingdom). Calcium imaging was performed at 4 or 5 Hz with a spinning disk confocal (3i Intelligent Imaging Innovations, Inc., Denver, CO, USA) for 4 or 8 minutes. Images were acquired using Slidebook software (3i Intelligent Imaging Innovations, Inc., Denver, CO, USA) and reconstructed online using Fiji (fiji.sc/). ROIs were manually selected based on a standard deviation Z-projection stack and dorsolateral position in the spinal cord. Peak detection was performed using an open-source signal processing toolbox [S12]. Individual ROI peak frequencies were averaged within embryos to determine coiling frequency from ΔF/F calculated using custom scripts written in MATLAB (The Mathworks, Natick, MA, USA). Electrophysiology Whole cell recording were performed in 3 dpf larvae in artificial CSF (concentrations in mM: 134 NaCl, 2.9 KCl, 1.2 MgCl2, 10 HEPES, 10 glucose, 2 CaCl2; 290 mOsM +/- 3 mOsm, pH adjusted to 7.8 with NaOH). Larvae were pinned through the notochord with 0.025 mm tungsten pins. Larvae were decapitated to prevent visual light response, and skin and muscle from two segments between segments 8 and 18 were dissected using a glass suction pipette. A MultiClamp 700B amplifier, a Digidata series 1440A Digitizer, and pClamp 10.3 software (Axon Instruments, Molecular Devices 446 Sunnyvale, CA, USA) were used for acquisitions. Patch pipettes (1B150F-4, WPI, Sarasota, FL,USA) with a tip resistance of 8-11 MΩ were filled with internal solution (concentrations in mM: K-gluconate 115, KCl 15, MgCl2 2, Mg-ATP 4, HEPES free acid 10, EGTA 0.5, 290 mOsm, adjusted to pH 7.2 with KOH with Alexa 488 at 10 μM final concentration). Holding potential was - 85 mV, away from the calculated chloride reversal potential (- 51 mV). Liquid junction potential in our experiments was - 19 mV, stated values for holding potential are not corrected as it did not affect the outcome of the experiments. Gabazine (Tocris Bioscience, Bristol, United Kingdom) was applied in the bath for a final concentration of 10 µM. Recordings with gabazine in the bath were initiated at least 2 minutes after bath application. Analysis of electrophysiological data was performed offline using Clampex 10 software (Molecular Devices, California, USA). Cell selection for electrophysiology For experiments in Tg(pkd2l1:gal4; UAS:ChR2-mCherry), larvae were pre-screened for fluorescence to identify motor neuron somas encircled by CSF-cN axons expressing ChR2-mCherry. For experiments in Tg(pkd2l1:gal4; UAS:ChR2-mCherry; UAS:BoTxBLC-GFP), larvae were pre-screened for fluorescence to identify motor neurons encircled by presynaptic CSF-cNs expressing both ChR2 and BoTxBLC-GFP. After recordings, motor neuron identity was confirmed by visualization of Alexa 488 dye and post hoc imaging of ChR2-mCherry. One cell per larva was used for data analysis. Optogenetic stimulation Activation of CSF-cNs was achieved by whole field stimulation through a 40x objective for 5 ms with a blue LED (UHP-Mic-LED-460, Prizmatic Ltd., Israel, power = 3.88 mW / mm2) through a Digital 133 Annex1 Micromirror Device with all mirrors in the “on” position. All experiments were run as sets of 10 trials. Two sets of experiments were performed for each cell. Behavioral recordings and analysis of acoustic escape responses from freely swimming larvae Depending on the severity of the defects, embryos that could not hatch on their own were dechorionated manually at 2 dpf, as well as their control siblings. 5 dpf larvae were placed individually in dishes with 2.2 cm inner diameter and 3.5 cm outer diameter in 400 µL of fish facility water on a homogeneous illumination plate (light intensity 0.78 mW / cm2 Phlox, ref. LEDW-BL-200/200-LLUB-Q-1R24). Only undamaged, healthy-looking larvae were selected for testing. Larvae were habituated for 10 minutes on the light source at room temperature not lower than 22°C and kept at room temperature during all recordings. For the acoustic startle assay, following acclimation, larvae were given a 500 Hz acoustic stimulus for 10 ms at frame 130 and recorded for a total of 1 second at 650 frames per second with a high-speed camera (Basler AG, ac12040-180km) placed above the setup. Synchronization of the camera and the stimulus was achieved by a custom built Arduino setup. For each group of four dishes, 10 trials were recorded with an inter-trial interval of 2-5 minutes. The acquired images had pixel dimensions of 2000 x 544 and an exposure time of 500 µs. Behavioral recordings were acquired between 1:00 and 6:00 P.M. The behavior setup used here was described in [S7]. To determine the distance traveled in each trial, using custom-written MATLAB, videos were analyzed to detect the center of mass of the larva at each frame and to determine the distance traveled between successive frames, which was summed over the trial. Behavioral recordings and analysis of acoustic escape responses from head-embedded larvae Due to failure to inflate the swim bladder in Tg(chx10:gal4; UAS:BoTxBLC-GFP) larvae, a common problem in larvae with locomotor defects, we utilized the head-embedded configuration to record high-speed kinematics in upright larvae. Recordings were performed in an identical way to freely swimming acoustic-induced escape assay (above) with the exception that larvae were embedded in 2% low-melting temperature agarose and the agarose surrounding the tail was manually removed. Tracking was performed with custom written MATLAB software. A skeleton of the tail was extracted using 10 segments. Depending on the quality of the tracking, traces were individually manually excluded from the dataset in cases of tracking failure, and parameters were manually adjusted to achieve excellent tracking. Kinematic parameters were extracted for each trial including: escape duration, escape latency, the number of tail oscillations, the average tail-beat frequency over the escape bout, and the maximum tail bend angle. Behavioral recordings and analysis of spontaneously freely swimming larvae 5 dpf larvae in the same wells and on the same setup as in freely swimming acoustic escape experiments above were recorded following a minimum 10 minute acclimation period with no stimulation 134 Annex 1 provided by the experimenter. Videos were recorded at 60 Hz for four minutes at 2000 x 1088 pixel resolution. Videos were scored manually and frames where swim bouts started or ended were recorded. To determine the distance traveled, the same custom MATLAB code as above was employed on the first minute of the four minute acquisition sequence. Fictive ventral nerve root (VNR) recordings The fictive locomotion protocol was based on published procedures ([S1,S13]). Thin-walled, borosilicate glass capillaries (Sutter Instruments, Novato, CA, USA) were pulled and fire-polished from a Flaming/Brown pipette puller (Sutter Instruments, Novato, CA, USA) to obtain peripheral nerve recording micropipettes. Pipettes were filled with artificial CSF and positioned next to the preparation using motorized micromanipulators under the microscope. Light suction was applied when the pipette reached the muscle region located at the vicinity of intermyotomal junctions, ventral to the axial musculature midline. The larva was laterally mounted and the otic vesicle was stimulated by a jet of water delivered by a glass pipette and powered by a picospritzer (WPI, Sarasota, FL, USA) on the same side of the body as the VNR pipette. VNR signals were acquired at 10 kHz in current clamp IC=0 mode using a MultiClamp 700A amplifier, a Digidata series 1322A digitizer, and pClamp 8.2 software (Molecular Devices–Axon Instruments, Sunnyvale, CA, USA). Recordings were considered for analysis when the background noise did not exceed 0.05 mV amplitude and signal to noise ratio for fictive locomotor events detection was above three. VNR recordings were analyzed offline using custom MATLAB scripts. For analysis of fast evoked locomotion, the phase 1 portion of elicited fast responses consisted of low frequency bursts between 10 and 20 Hz, lower frequencies than the typical slow swimming frequency range (between 20 to 30 Hz). Because our assay was designed to induce fast locomotor frequencies, we did not perform double VNR recordings to determine if the initial and variable phase 1 slow component corresponds to fictive struggles characterized by locomotor activity back propagating caudal to rostral ([S14]). Since these bursts were always followed by fast frequencies (above 40 Hz) and were part of induced responses, we included them in frequency analysis because it did not affect interpretation of results with respect to the generation of fast locomotion. Fictive VNR recordings combined with calcium imaging of motor neurons Calcium imaging was performed at 10 Hz with a spinning disk confocal microscope (3i Intelligent Imaging Innovations, Inc., Denver, CO, USA) in either Tg(mnx1:GCaMP5G) or Tg(mnx1:GCaMP5G; chx10:gal4; UAS:BoTxBLC-GFP) larvae at 4 dpf. Fictive recordings were performed as described above with otic vesicle stimulation. Up to 12 trials total were performed per larva. Trials in which the fictive recording had at least four bursts in response to otic vesicle stimulation were included for analysis. ROIs were manually selected based on a standard deviation of a Z projection stack computed over few optical sections. For experimental larvae expressing BoTxBLC-GFP in V2a interneurons and GCaMP5G in motor neurons, only neurons with obvious nuclear-excluded GCAMP5G were included 135 Annex1 in analysis to ensure that background BoTxBLC-GFP expression did not impact ΔF/F amplitude analysis. Cell counts in Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP) Tg(pkd2l1:gal4; UAS:BoTxBLC-GFP) adults were crossed to Tg(vglut2:IRI-GFP), Tg(gad1b:IRIGFP), or Tg(chx10:IRI-GFP) to obtain triple transgenic larvae. Siblings expressing only Tg(vglut2:IRI-GFP), Tg(gad1b:IRI-GFP), or Tg(chx10:IRI-GFP) were used as controls. Larvae were embedded in agar and anesthetized in 0.02% tricaine for imaging. From each larva, separate stacks from three regions were obtained: segments 3-4, 10-11, and 25-26. Segment boundaries were identified with a transmitted image, and cells were counted in Fiji (fiji.sc/). Statistics For larval behavioral and fictive data, statistical results were generated from a linear mixed effect models (LME) estimated with R version 3.2.3, where the individual fish was considered as random in order to take into account repeated measurements. When non-LME tests were appropriate, statistical tests were used as specified in figure legends. Supplemental References S1. Fidelin, K., Djenoune, L., Stokes, C., Prendergast, A., Gomez, J., Baradel, A., Del Bene, F., and Wyart, C. (2015). State-dependent modulation of locomotion by GABAergic spinal sensory seurons. Curr. Biol. 26, 103109. S2. Scott, E.K., Mason, L., Arrenberg, A.B., Ziv, L., Gosse, N.J., Xiao, T., Chi, N.C., Asakawa, K., Kawakami, K., and Baier, H. (2007). Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4, 323-326. S3. Asakawa, K., Suster, M.L., Mizusawa, K., Nagayoshi, S., Kotani, T., Urasaki, A., Kishimoto, Y., Hibi, M., and Kawakami, K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA. 105, 1255-1260. S4. Kimura, Y., Okamura, Y., and Higashijima, S. (2006). alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J. Neurosci. 26, 5684-5697. S5. Kimura, Y., Satou, C., Fujioka, S., Shoji, W., Umeda, K., Ishizuka, T., Yawo, H., and Higashijima, S. (2013). Hindbrain V2a neurons in the excitation of spinal locomotor circuits during zebrafish swimming. Curr. Biol. 23, 843-849. S6. Auer, T.O., Xiao, T., Bercier, V., Gebhardt, C., Duroure, K., Condordet, J-P., Wyart, C., Suster, M., Kawakami, K., Wittbrodt, J., Baier, H., and Del Bene, F. (2015). Deletion of a kinesin 1 motor unmasks a mechanism of homeostatic branching control by neurotrophin-3. eLife 4, e05061. S7. Böhm, U.L., Prendergast, A., Djenoune, L., Nunes Figueiredo, S., Gomez, J., Stokes, C., Kaiser, S., Suster, M., Kawakami, K., Charpentier, M., Concordet, J-P., Rio, J-P., Del Bene, F., and Wyart, C. (2016). CSFcontacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nat. Commun. 7, 10866. 136 Annex 1 S8. Schoonheim, P. J., Arrenberg, A. B., Del Bene, F., and Baier, H. (2010). Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J. Neurosci. 30, 7111-7120. S9. Satou, C., Kimura, Y., Hirata, H., Suster, M.L., Kawakami, K., and Higashijima, S. (2013). Transgenic tools to characterize neuronal properties of discrete populations of zebrafish neurons. Development 140, 3927-3931. S10. Ahrens, M.B., Orger, M.B., Robson, D.N., Li, J.M., and Keller, P.J. (2013). Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413-20. S11. Kimmel, C.B., Ballard W.W., Kimmel S.R., Ullmann, B., and Schilling, T.F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. S12. O’Haver, T.C. (1995). A pragmatic introduction to signal processing with applications in scientific measurement. https://terpconnect.umd.edu/~toh/spectrum/PeakFindingandMeasurement.htm. S13. Masino, M.A., and Fetcho J.R. (2005). Fictive swimming motor patterns in wild type and mutant larval zebrafish. J. Neurophysiol. 93, 3177-3188. S14. Liao, J.C., and Fetcho J.R. (2008) Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. J. Neurosci. 28, 12982-1299 137 138 Annex 2 Annex 2 Manuscript Mechanosensory feedback to spinal circuits enhances speed of locomotion 139 1 2 3 4 5 6 7 8 9 10 11 12 Title 13 Affiliations 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 Mechanosensory feedback to spinal circuits enhances speed of locomotion Authors Steven Knafo1,2,3,4,5, Kevin Fidelin1,2,3,4, Andrew Prendergast1,2,3,4, Alexandre Parrin1,2,3,4, Po-En Tseng1,2,3,4, Charles Dickey1,2,3,4, Urs Lucas Böhm1,2,3,4, Sophie Nunes Figueiredo1,2,3,4, Olivier Thouvenin1,2,3,4, Hugues Pascal-Moussellard1,2,3,4,5, Claire Wyart1,2,3,4,* 1 Institut du Cerveau et de la Moelle épinière (ICM), INSERM UMRS 1127, 3 CNRS UMR 7225, 4 Université Pierre et Marie Curie, Sorbonne Universités, F-75005, 5 Assistance Publique - Hôpitaux de Paris, CHU Pitié-Salpêtrière, F-75013, Paris, France 2 * Corresponding author Dr. Claire Wyart, ICM, Hôpital de la Pitié-Salpêtrière, 47 bld de l’hôpital, F-75013, Paris. Phone: +33-(0)1-57-27-43-10 ; Email:[email protected] Classification BIOLOGICAL SCIENCES: Neurosciences Keywords mechanosensory feedback; spinal cord; speed modulation; zebrafish; GFP-aequorin 1 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 Abstract The neural basis for the contribution of mechanosensory feedback to locomotion remains elusive. Major challenges remain in recording the activity of identified neurons in the spinal cord of moving animals and quantitatively analyzing their impact on kinematics. Here, we address these issues using optical methods for monitoring neuronal activity and performing kinematic analysis in zebrafish larvae both with and without sensory feedback. First, we measure the selective recruitment of spinal motor neurons during active locomotion by genetically targeting the bioluminescent calcium sensor GFP-aequorin. We demonstrate that mechanosensory feedback enhances spinal motor neuron recruitment in motion compared to both chemically- and genetically-induced paralysis. Second, by silencing the output of glutamatergic mechanosensory neurons, we show in freely moving animals a marked decrease in locomotor speed associated with an early termination of the fast component of the escape behavior. Altogether, we demonstrate that glutamatergic mechanosensory feedback provides an excitatory drive to the premotor network sustaining fast locomotion and gates the transition between fast and slow modules. The effect we unravel here provides a general explanation for the slowdown of locomotor rhythms observed throughout vertebrates during fictive locomotion. Significance statement Although mechanosensory feedback can alter posture and locomotion, its contribution to the control of movement kinematics cannot be investigated with regular electrophysiology during motion. By targeting the bioluminescence sensor GFP-aequorin to spinal motor neurons, we achieved non-invasive monitoring of motor pools in moving animals. We show that fast movements involve the recruitment of larger pools of spinal motor neurons and that mechanosensory feedback increases overall activity in spinal motor neurons. Silencing the output of these mechanosensory neurons during escape behavior markedly decreased the speed of locomotion by gating an early transition from fast to slow swimming regime. Our results explain how mechanosensory feedback speeds up oscillations underlying the recruitment of spinal motor neurons during active locomotion. Introduction The basic motor rhythm controlling muscle contraction can be produced by spinal central pattern generators (CPGs) in the absence of sensory inputs (1). However, there is converging evidence that mechanosensory feedback modulates the activity of spinal locomotor CPGs (2-5). Experiments using stimulation of peripheral afferents in paralyzed decerebrate cats revealed a phase-dependent reorganization of sensorimotor reflex pathways during fictive locomotion (6). In addition these studies showed the potential of sensory pathways to entrain the locomotor rhythm and to modify the amplitude and timing of motor output by interacting with premotor or motor circuits in a state-dependent manner (7-9). Genetic ablation of proprioceptive neurons in mice triggered defects in motor coordination (4) and locomotor recovery after injury (10) suggesting that sensory feedback shapes the activity of spinal circuits controlling the pattern of locomotion in walking animals. However, the contribution of mechanical sensory feedback to ongoing locomotion, as a function of speed in particular, still remains elusive. 2 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 Classical electrophysiological recordings of fictive locomotor activity from preparations in which muscles are paralyzed or dissected out do not allow investigators to probe the contribution of mechanosensory feedback (11). In such preparations, the locomotor rhythm is unusually slow, typically five to ten times slower in spinal preparations than normal locomotion (12-14). The few studies comparing active and fictive locomotion within the same animals showed a massive reduction in frequency of the locomotor rhythm in fictive conditions (15). This “slowdown” effect may be due to the suppression of excitatory inputs originating from proprioceptive afferents associated with muscle contraction. These results suggest that sensory feedback could modulate spinal circuits controlling the speed of locomotion. To probe the dynamic contribution of mechanosensory feedback during locomotion, we had to overcome multiple obstacles: one must record neurons in freely moving animals as well as achieve specific silencing of mechanosensory inputs and quantitatively analyze the impact of this silencing on locomotion kinematics. Here, taking advantage of recent optical techniques, we probed the contribution of mechanosensory feedback to spinal motor neuron recruitment and movement kinematics during ongoing locomotion. We developed an innovative bioluminescence-based method for monitoring genetically-targeted populations of spinal neurons in moving animals and combined this approach with calcium imaging and quantitative kinematic analysis. We show that glutamatergic mechanosensory neurons enhance the recruitment of spinal motor neurons during motion in freely moving zebrafish larvae, increasing locomotor frequency, and consequently, the speed of locomotion. Quantitative kinematic analysis revealed that mechanosensory feedback selectively enhances the fast component over the slow component of escape behavior, thereby gating the transition between fast and slow locomotion. Our results demonstrate the critical role of mechanosensory feedback in modulating fast and slow spinal motor circuits during active locomotion. Results Monitoring the recruitment of spinal motor neurons in behaving animals The bioluminescent calcium sensor GFP-aequorin (16, 17) has been used as a tool for monitoring calcium activity emitted by identified neuronal populations in freely moving animals (18, 19). To investigate the recruitment of motor and mechanosensory neurons in motile and immotile zebrafish larvae, we codon-optimized the aequorin sequence and targeted its expression to specific populations of spinal neurons. We recorded bioluminescence signals emitted from spinal motor neurons during active behaviors elicited by an acoustic stimulus in Tg(mnx1:gal4, UAS:GFP-aequorin-opt) zebrafish larvae at 4 days post-fertilization (dpf) (see Methods and Fig. 1A, B). We verified both in live animals and after GFP immunostaining (Supplementary Video 1) that there was absolutely no expression in muscles, as this would lead to an obvious confound due to the massive changes in intracellular calcium associated with muscle contraction. We observed typical escape responses characterized by a large initial C-bend usually followed by a subsequent swim and rarely slow swims (20) (Fig. 1C, n = 10 larvae; Supplementary Video 2, 3). Each behavior was associated with distinct bioluminescence signals (Fig. 1D; Supplementary Video 2, 3). While time-to-peak and time decay of the bioluminescence signals was invariant across maneuvers, escapes generated larger bioluminescence amplitudes than did slow swims (Fig. 1E). Bioluminescence signal amplitude correlated with the maximal angle of the tail bend (Fig. 1F), suggesting that spinal motor neuron recruitment is increased during behaviors with larger tail bends and faster locomotor frequencies. Yet, such global bioluminescence monitoring lacks the single cell 3 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 resolution to explain whether these increased bioluminescence signals result from the recruitment of additional motor neurons during fast locomotion or from increased signals in the same pool of active cells. Calcium imaging reveals specific recruitment patterns of spinal motor neurons To achieve this single cell resolution, we performed calcium imaging during spontaneous fictive slow swims and elicited escapes in Tg(mnx1:gal4, UAS:GCaMP6f;cryaa:mCherry)(21) (Fig. 2A, 2B). The fictive locomotor burst frequency ranged between 20-30 Hz for slow swims (22) (left panel, Fig. 2C) and 20-80 Hz for escapes (right panel Fig. 2C). During slow swims, a small fraction of ventrally-located motor neurons was active (23.2%, Fig. 2D-E, left panel, 2F, Supplementary Video 4). In contrast, the majority of motor neurons, including large dorsal motor neurons, was recruited during escapes (88.4%, Fig. 2D-E, right panel, 2F, Supplementary Video 5). Most dorsal motor neurons showed larger calcium transients than ventral ones (Fig. 2D, 2F), and the averaged ΔF/F across all recruited motor neurons was higher during escapes than slow swims (Fig. 2G). The greater spinal motor neuron recruitment during escapes compared to slow swims likely results therefore from the combination of two effects: a larger pool of active cells and larger calcium transients per active neuron. Mechanosensory feedback enhances the recruitment of spinal motor neurons To test whether the global recruitment of spinal motor neurons differed in actively moving versus immotile animals, we recorded bioluminescence before and after paralysis induced by bath application of cholinergic blocker pancuronium bromide (23)(Fig. 3A). Across all Tg(mnx1:gal4, UAS:GFP-aequorin-opt) larvae, mean bioluminescence amplitude was markedly decreased in paralyzed compared to motile animals (Fig. 3B,C; n = 10). Within each larva, the mean normalized bioluminescence amplitude was decreased seven-fold (0.056 +/- 0.08 versus 0.36 +/- 0.18, p < 0.001). Since this effect could be partly explained by the inhibition of acetylcholine receptors located on targets of command reticulospinal neurons, such as the Mauthner cell(24), we conducted similar experiments in immotile relaxed (cacnb1ts25) mutant zebrafish(25). The mean bioluminescence amplitude was markedly decreased in the immotile Tg(cacnb1ts25/ts25, mnx1:gal4, UAS:GFP-aequorin-opt) larvae compared to control motile siblings (Fig. 3D,E). Altogether these results demonstrate that spinal motor neuron recruitment is enhanced in active locomotion, suggesting that mechanosensory feedback increases spinal motor output. Silencing mechanosensory neurons decreases speed of locomotion We used the isl2b promoter to target glutamatergic sensory neurons known to be mechanosensitive and responsive to touch: Rohon-Beard neurons in the spinal cord, dorsal root ganglia and trigeminal ganglia (Fig. 4A, note the bundle of Rohon Beard and DRG axons ascending in the spinal cord in Fig. 4B). To probe the contribution of these mechanosensory neurons in freely moving animals, we blocked their synaptic release using selective transgenic expression of the zebrafish-optimized Botulinum toxin (26) (see Methods). Quantitative behavioral analysis in freely swimming Tg(isl2b:gal4, cmlc2:eGFP, UAS:BoTxBLC-GFP) larvae revealed alteration of several kinematics parameters compared to control siblings (Fig. 4C-E, n = 436 larvae). No significant differences were observed for distance traveled (Fig. 4F) and number of oscillations (Fig. 4G). In contrast, escape latency (Fig. 4H), escape duration (Fig. 4I), tail beat frequency (Fig. 4J) and speed (Fig. 4K) were markedly reduced in 4 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 larvae deprived of mechanosensory feedback. While the amplitude of the C bend was not affected in these animals (Fig. 4L), the subsequent tail bends on the same side were increased suggesting a mild modulation of direct motor output. Mechanosensory feedback times the transition between fast and slow motor regimes The distribution of tail bend amplitude as a function of tail beat frequency indicated that the initial C bend was not affected but the rest of the distribution (color coded per cycle) was shifted towards lower locomotor frequency when mechanosensory feedback was silenced. We performed a fine kinematic analysis on tail angle oscillations by distinguishing the slow from the fast regimes of the acoustic escape response with a 30 Hz cutoff (27, 28) (Fig. 5B). We observed a striking speed-dependent effect of mechanosensory feedback. The reduction of speed and locomotor frequency observed in silenced animals was due to a reduction of the fast component (Fig 5C-F), which was characterized by shorter duration, smaller number of bends, and lower locomotor frequency and speed. In contrast, this reduction of the fast component was associated with an increase of the slow component, characterized by longer duration and larger number of bends without any frequency or speed modulation (Fig 5C’-F’). This kinematic analysis demonstrates that glutamatergic mechanosensory feedback enhances speed of locomotion by providing excitatory inputs to the fast locomotor CPG, thereby increasing locomotor frequency, number of oscillations and duration of the fast regime and postponing the transition to the slow CPGs. Discussion Monitoring bioluminescence of neural populations in the moving spinal cord Here, by taking advantage of the transparency and genetic accessibility of the zebrafish larva, we provide an innovative approach to achieve non-invasive recording of genetically targeted spinal motor and sensory neurons during active locomotion. However, bioluminescence monitoring is technically challenging. Achieving GFP-aequorin expression in a highly selective yet strong enough manner is essential for obtaining specific signals from neurons that correlate with kinematic parameters. We combined a codon-optimized version of GFPaequorin for zebrafish together with the GAL4/UAS transactivation system to improve the expression of the sensor. The interpretation of bioluminescence signals in vivo is another challenge. In particular, it is unclear whether changes of signal amplitude reflect the dynamics of neuronal activity. Bioluminescence assays in single pyramidal neurons ex-vivo showed that the recorded number of photons increased exponentially with the number of action potentials elicited (29). However, one major difficulty in vivo is that the recorded bioluminescence integrates signals from many cells with different levels of expression and variable patterns of activity. Therefore, one technique to resolve the overall signal at the cellular level is to compare bioluminescence and calcium imaging data in similar experimental conditions. Altogether, GFP-aequorin proves to be a useful tool to monitor dynamics of neural activity at the population level in motion. Mechanosensory sensory feedback enhances speed of locomotion In our study, we demonstrate that glutamatergic mechanosensory feedback increases speed of locomotion. First, we used a zebrafish codon-optimized GFP-aequorin as a reporter of motor pool activity in freely moving animals. As zebrafish larvae engage in stronger movements, we observe an increase of the amplitude of bioluminescence signals, suggesting 5 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 that GFP-aequorin is sensitive enough to detect the dynamics of motor neurons during different locomotor behaviors, including slow locomotion where only few motor neurons are recruited. To understand whether variations of signal amplitude reflect changes in patterns of motor neurons recruitment, we used calcium imaging to resolve individual motor neurons. GCaMP imaging revealed that increases of GFP-aequorin signals were indicative of more motor neurons recruited with higher activity levels. We then tested the contribution of sensory neurons recruited during the execution of acoustic escapes by comparing GFP-aequorin signals in motor neurons either in actively moving larvae or in paralyzed or immotile mutant larvae. We observed a decrease of the amplitude of bioluminescence signals in animals deprived of mechanosensory feedback. This observation suggests that inputs from mechanosensory neurons during locomotion enhance the recruitment of motor neurons. Yet, from this data, we could not tell whether the diminution of bioluminescence signal from motor neurons upon silencing of sensory neurons was associated with a reduction of bending amplitude, duration or locomotor speed during the escape response. In order to precisely assess the contribution of mechanosensory feedback, we silenced sensory neurons with a codon-optimized botulinum toxin that efficiently blocks synaptic release in vivo (Sternberg, Severi et al., in revision) and analyzed the kinematics of acoustic escape responses. This quantitative analysis performed over hundreds of escapes revealed that selectively silencing glutamatergic mechanosensory feedback reduced locomotor frequency, and therefore locomotion speed, without affecting distance traveled. By discriminating fast and slow regimes with a 30Hz cutoff (27, 28, 30), we show that mechanosensory feedback enhanced specifically the fast regime by increasing duration, number of bends and frequency as well as it timed the transition to the slow regime. Our study suggests that some of the mechanosensory neurons we targeted, namely Rohon Beard (RB), trigeminal ganglion (TG) and dorsal root ganglion (DRG) neurons, act as detectors of muscle contraction during active locomotion. Previous reports in zebrafish have shown that RB and TG neurons respond to touch (31), while very little is known about DRG neuron sensory capability at the larval stage. During muscle contraction associated with active swimming in zebrafish larva, the recruitment of these neurons is unknown. Although RB and TG neurons are known to trigger escape responses by integrating tactile stimuli, whether and how these mechanosensory neurons could also modulate ongoing locomotion is unknown. Interestingly, we noticed that the axonal projection originating from RBs and DRGs (32) matches the position of the soma of dorsal interneurons (dorso-ventral index ~ 0.5(28), Fig. 4B,) such as glutamatergic V2a premotor interneurons as well as sensory interneurons (CoPAs) known to receive inputs from RB neurons (31). Previous reports have shown that V2a interneurons are recruited along the dorso-ventral axis as a function of locomotor frequency, with dorsal pools being recruited for faster fictive locomotion(28). V2a interneurons in turn selectively project on motor neurons recruited along the dorso-ventral axis for different speeds of locomotion (33, 34). Further work will investigate whether RB neurons project onto dorsal V2a interneurons or CoPAs to explain the result reported here of an enhancement of the fast CPG by mechanosensory feedback. We show here that the transition from the fast to the slow regime is gated by mechanosensory feedback. A recent study showed evidence that the fast module actively inhibits the slow module during fictive locomotion (35). The timing of the fast to slow transition during an escape could therefore be due to the gradual reduction of excitation onto the fast module, which would disinhibit the slow module. Results of our kinematic analysis indicate that the modulation operated by mechanosensory feedback is exclusive to the fast module, as the locomotor frequency during the slow regime is not affected. Our results enable to predict a model in which mechanosensory inputs feed back onto the fast premotor pool of 6 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342 343 344 345 346 347 V2a in order to enhanced locomotor frequency and speed, and would postpone indirectly the transition to slow regime by providing additional excitation to the fast module. Other atypical mechanosensory neurons, identified as local GABAergic sensory neurons, have been shown to detect spinal curvature and to enhance locomotor frequency(21, 36). The relevance of glutamatergic and GABAergic sensory pathways to the modulation of locomotor speed remains to be investigated in mammals. Speed-dependent recruitment of premotor interneurons has been linked in mammals to left-right coordination. Many mammalian species switch gaits from left-right alternation during walking at low speed to left-right synchrony during hopping at higher speed depending on the recruitment of V0V/D interneurons (37). Mechanosensory feedback may contribute to these change of gaits, as suggested with pioneering experiments of Thomas Graham Brown on decerebrate cats walking on a treadmill (38). Altogether, the results obtained here in a genetic model organism characterized by a relatively simple locomotion demonstrate that mechanosensory inputs provide a crucial feedback that enhances speed of locomotion by selecting fast over slow premotor networks. Our study emphasizes the importance of studying closed-loop behaviors in order to understand the dynamics of locomotor networks in vivo. Methods Zebrafish care, generation and characterization of transgenic lines All procedures were approved by the Institutional Ethics Committee at the Institut du Cerveau et de la Moelle épinière (ICM), Paris, France, the Ethical Committee Charles Darwin and received subsequent approval from the EEC (2010/63/EU). Monitoring neuronal activity with bioluminescence Embryos were dechorionated and soaked at 26°C in 100 µL of blue water with a final concentration of 60 µM of coelenterazine-h (Biotium, Hayward, USA). Coelenterazine-h was renewed at 2 days post-fertilization (dpf). All experiments were performed at 4 dpf. All GFPaequorin expressing larvae were tested at 4 dpf. In all experiments, one larva was headembedded in 1.5% low melting agarose with the tail free to move in a circular (2 cm diameter) 3D-printed arena (Sculpteo, France). The same larvae used for the active assay were subsequently paralyzed by bath application of pancuronium bromide (Sigma, P1918) at 0.6 mg/mL final concentration and stimulation intensity was adjusted to the lowest value that elicited a bioluminescent signal. For Fig. 3, cacnb1ts25/ts25 and control siblings were tested alternatively on the same day and compared to each other. In non-moving animals (i.e. paralyzed or cacnb1 mutants), the intensity was progressively increased until stimuli elicited fictive responses. A higher intensity of the acoustic stimulus was often needed after addition of pancuronium bromide, possibly due to modulation of cholinergic arousal brain circuitry (39). As negative controls, bioluminescence assays of wild type animals or Tg(mnx1:gal4, UAS:GFP) (40) where motor neurons express GFP only revealed no signal (n = 3 wild type larvae with 30 trials each, n = 5 Tg(mnx1:gal4, UAS:GFP) larvae with 30 trials each). Animals deprived of GFP-Aequorin did not produce any signals above baseline noise level during escape responses. Calcium imaging of spinal motor neurons and ventral nerve root recording (VNR) 7 348 349 350 351 352 353 354 355 356 357 358 359 360 361 362 363 364 365 366 367 368 369 370 371 372 373 374 375 376 377 378 379 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 4 dpf Tg(mnx1:gal4; UAS:GCaMP6f,cryaa:mCherry) double transgenic larvae were screened for dense labeling and good expression of GCaMP6f in spinal motor neurons. Larvae were immobilized by injecting 0.1-0.3 nL of 0.5 mM α-Bungarotoxin (Tocris, UK) in the ventral axial musculature. Zebrafish larvae were imaged using a custom spinning disk microscope (Intelligent Imaging Innovation, Denver, USA) equipped with a set of water-immersion objectives (Zeiss 20X, 40X, NA=1). Recordings were acquired using Slidebook® software at 20 Hz at 488nm. VNR signals were acquired at 10kHz in current clamp IC = 0 mode using a MultiClamp 700A amplifier (Molecular Devices–Axon Instruments, USA), a Digidata series 1322A digitizer (Axon Instruments, USA) and pClamp 8.2 software (Axon instruments, USA). Recordings were considered for analysis when the background noise did not exceed 0.05 mV amplitude and signal to noise ratio for fictive locomotor events detection was above three. VNR recordings were analyzed offline and aligned to calcium imaging data using custom-made MATLAB scripts. Behavioral analysis of freely moving BoTxBLC larvae Zebrafish larvae Tg(isl2b:gal4, cmlc2:eGFP; UAS:BoTxBLC-GFP) were screened at 3 dpf for expression. At 5 dpf, larvae were tested 4 by 4: each larva was positioned in a separate dish (2 cm diameter) and illuminated from below, freely moving. Escapes were elicited by delivering a 500 Hz stimulus for 1 ms using 20W speakers. Each trial consisted of a 200 ms pretrial window followed by a 1 ms stimulus at 500 Hz and 800 ms subsequent recording. 5 trials were performed in succession with 2 minutes intertrial rest. Behavior was recorded at 650 fps with a high-speed camera (Basler acA2000-340km) and analyzed using a tracking algorithm (ZebraZoom, (41)) and a custom Matlab script (R2012b, Mathworks, USA). Hemiperiods were calculated as the interval between two consecutive peaks, and subsequently used as a determinate to extract fast and slow components of the escape with a cutoff of 650/60 frames (30 Hz) (27, 28). Peaks which alternated between the cutoff value after the first appearance of a slow peak were excluded. Statistical analysis SPSS 20 (IBM, USA) was used to perform all statistical analyses. Mixed linear model analysis with repeated measures using an auto-regressive covariance structure was performed. Statistical significance is represented in the graphs as *** for p < 0.001, ** for p < 0.01, * for p < 0.05, corrected for multiple comparisons when needed. All data are provided in the figures and text as means +/- standard error of the mean (SEM). See SI Methods for detailed Materials and Methods. Acknowledgements We would like to thank Dr. Ludovic Tricoire (University Pierre et Marie Curie, Paris, France) for providing the original GFP-aequorin sequence, Dr. Michael Granato (University of Pennsylvania, USA), Dr. Paul Brehm (Vollum Institute, USA) for providing the Relaxed (cacnb1ts25) mutants, Dr. Maximilliano Suster and Prof. Koichi Kawakami for sharing the Tg(UAS:BoTxBLC-GFP) transgenic line, Dr. Tom Auer and Dr. Filippo Del Bene for providing the mnx1 and isl2b constructs and Tg(isl2b:gal4, cmlc2:eGFP). We are indebted to RD Vision for developing the custom API to synchronize photon collection and video acquisition. We thank Bogdan Buzurin and Natalia Maties for the excellent fish care. SK 8 398 399 400 401 402 403 404 405 406 407 408 409 410 411 412 received a PhD fellowship from Inserm and Assistance Publique – Hôpitaux de Paris. AEP received a postdoctoral fellowship from the Mairie de Paris Research in Paris programme and from the EMBO, KF from UPMC and UB from Ecole des Neurosciences de Paris (ENP). This work received financial support from the Institut du Cerveau et de la Moelle épinière with the French program “Investissements d’avenir” ANR-10-IAIHU-06, the ENP Chair d’excellence, the Fondation Bettencourt Schueller (FBS), the City of Paris Emergence program, the ATIP/Avenir junior program from INSERM and CNRS, the International Reintegration Grant from Marie Curie Actions Framework Program 6 #277200, the Human Frontier Science Program (HFSP) Research Grant RGP0063/2014 and the European Research Council (ERC) starter grant “OptoLoco” #311673. 1. Delcomyn F (1980) Neural basis of rhythmic behavior in animals. Science 210(4469):492–498. 413 414 2. Marder E, Bucher D (2001) Central pattern generators and the control of rhythmic movements. Curr Biol 11(23):R986–96. 415 416 3. Rossignol S, Dubuc R, Gossard J-P (2006) Dynamic sensorimotor interactions in locomotion. Physiol Rev 86(1):89–154. 417 418 4. Akay T, Tourtellotte WG, Arber S, Jessell TM (2014) Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci USA 111(47):16877–16882. 419 420 5. Windhorst U (2007) Muscle proprioceptive feedback and spinal networks. Brain Research Bulletin 73(46):155–202. 421 6. McCrea DA (2001) Spinal circuitry of sensorimotor control of locomotion. J Physiol 533(Pt 1):41–50. 422 7. Lundberg A (1979) Multisensory control of spinal reflex pathways. Prog Brain Res 50:11–28. 423 424 8. Grillner S, Rossignol S (1978) On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res 146(2):269–277. 425 426 9. Schomburg ED, Petersen N, Barajon I, Hultborn H (1998) Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res 122(3):339–350. 427 428 10. Takeoka A, Vollenweider I, Courtine G, Arber S (2014) Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159(7):1626–1639. 429 430 11. Grillner S (2003) The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci 4(7):573–586. 431 432 12. Bouvier J, et al. (2015) Descending Command Neurons in the Brainstem that Halt Locomotion. Cell 163(5):1191–1203. 433 434 13. Bellardita C, Kiehn O (2015) Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 25(11):1426–1436. 435 436 437 14. Chung B, Bacqué-Cazenave J, Cofer DW, Cattaert D, Edwards DH (2015) The effect of sensory feedback on crayfish posture and locomotion: I. Experimental analysis of closing the loop. Journal of Neurophysiology 113(6):1763–1771. 438 439 15. Wallén P, Williams TL (1984) Fictive locomotion in the lamprey spinal cord in vitro compared with swimming in the intact and spinal animal. J Physiol 347:225–239. References 9 440 441 16. Shimomura O, Johnson FH, Saiga Y (1962) Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol 59:223–239. 442 443 17. Baubet VV, et al. (2000) Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc Natl Acad Sci USA 97(13):7260–7265. 444 445 18. Naumann EA, Kampff AR, Prober DA, Schier AF, Engert F (2010) Monitoring neural activity with bioluminescence during natural behavior. Nat Neurosci 13(4):513–520. 446 447 19. Rogers KL, et al. (2007) Non-Invasive In Vivo Imaging of Calcium Signaling in Mice. PLoS ONE 2(10):e974. 448 449 20. Budick SA, O'Malley DM (2000) Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol 203(Pt 17):2565–2579. 450 451 21. Böhm UL, et al. (2016) CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits. Nature Communications 7:10866. 452 453 22. Masino MA, Fetcho JR (2005) Fictive swimming motor patterns in wild type and mutant larval zebrafish. Journal of Neurophysiology 93(6):3177–3188. 454 455 23. Panier T, et al. (2013) Fast functional imaging of multiple brain regions in intact zebrafish larvae using selective plane illumination microscopy. Front Neural Circuits 7:65. 456 457 458 24. Koyama M, Kinkhabwala A, Satou C, Higashijima S, Fetcho J (2011) Mapping a sensory-motor network onto a structural and functional ground plan in the hindbrain. Proc Natl Acad Sci USA 108(3):1170– 1175. 459 460 25. Granato M, et al. (1996) Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123:399–413. 461 462 26. Auer TO, et al. (2015) Deletion of a kinesin I motor unmasks a mechanism of homeostatic branching control by neurotrophin-3. Elife 4. doi:10.7554/eLife.05061. 463 464 27. Severi KE, et al. (2014) Neural control and modulation of swimming speed in the larval zebrafish. Neuron 83(3):692–707. 465 466 28. Mclean DL, Fan J, Higashijima S-I, Hale ME, Fetcho JR (2007) A topographic map of recruitment in spinal cord. Nature 446(7131):71–75. 467 468 29. Drobac E, Tricoire L, Chaffotte A-F, Guiot E, Lambolez B (2010) Calcium imaging in single neurons from brain slices using bioluminescent reporters. J Neurosci Res 88(4):695–711. 469 470 30. Mclean DL, Masino MA, Koh IYY, Lindquist WB, Fetcho JR (2008) Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci 11(12):1419–1429. 471 472 31. Pietri T, Manalo E, Ryan J, Saint-Amant L, Washbourne P (2009) Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Devel Neurobio 69(12):780–795. 473 474 32. Prendergast A, et al. (2012) The metalloproteinase inhibitor Reck is essential for zebrafish DRG development. Development 139(6):1141–1152. 475 476 33. Bagnall MW, McLean DL (2014) Modular organization of axial microcircuits in zebrafish. Science 343(6167):197–200. 477 478 34. Ampatzis K, Song J, Ausborn J, Manira El A (2014) Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron 83(4):934–943. 479 480 35. Song J, Ampatzis K, Ausborn J, Manira El A (2015) A Hardwired Circuit Supplemented with Endocannabinoids Encodes Behavioral Choice in Zebrafish. Curr Biol 25(20):2610–2620. 10 481 482 36. Jalalvand E, Robertson B, Wallén P, Grillner S (2016) Ciliated neurons lining the central canal sense both fluid movement and pH through ASIC3. Nature Communications 7:10002. 483 484 37. Talpalar AE, et al. (2013) Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500(7460):85–88. 485 486 38. Clarac F (2008) Some historical reflections on the neural control of locomotion. Brain Research Reviews 57(1):13–21. 487 488 39. Yokogawa T, et al. (2007) Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. Plos Biol 5(10):e277. 489 490 40. Zelenchuk TA, Brusés JL (2011) In vivo labeling of zebrafish motor neurons using an mnx1 enhancer and Gal4/UAS. Genesis 49(7):546–554. 491 492 41. Mirat O, Sternberg JR, Severi KE (2013) ZebraZoom: an automated program for high-throughput behavioral analysis and categorization. Frontiers in Neural …. doi:10.3389/fncir.2013.00107/abstract. 493 494 495 496 497 498 499 500 501 502 503 504 505 506 507 508 509 510 511 512 513 514 515 516 517 518 519 520 521 522 523 524 525 Figure legends Figure 1. Bioluminescence from spinal motor neurons during active locomotion reveals distinct patterns of activity in slow and fast locomotion. (A) Design of the bioluminescence setup, (B) Live fluorescent image (upper panel) and immunohistochemistry for GFP (lower panel) in 4 dpf Tg(mnx1:gal4, UAS:GFP-aequorinopt) zebrafish larva show a selective expression of GFP in spinal motor neurons only (arrowheads: dorsal primary and arrows: ventral secondary motor neurons), with no expression in muscle fibers, (C) Automated categorization classified maneuvers into escapes (86.7% ot total maneuvers) or swims (7.7% or total maneuvers) (n = 10 larvae and 283 trials), (D) Example traces of typical bioluminescence signals and kinematic parameters observed for each category, (E) Mean bioluminescence amplitude was higher for escapes (28.4 +/- 0.9 photons / 10 ms; normalized amplitude per larva = 0.41 +/- 0.18) than swims (3.9 +/- 1.4 photons / 10 ms, p < 0.001; normalized amplitude = 0.06 +/- 0.02, p < 0.001), (F) Correlation between bioluminescence signal amplitude and maximum tail angle amplitude (R = 0.4, p < 0.001). Figure 2. Calcium imaging reveals specific recruitment patterns of motor neurons during slow and fast locomotion (A) Design of the calcium imaging setup. (B) Pattern of expression of GCaMP6F in 4 dpf Tg(mnx1:gal4, UAS-GCaMP6F) larvae. (C) VNR traces for each fictive behavior illustrates typical spontaneous fictive slow swims and induced fictive escapes, (D) GCaMP6F signals from individual motor neurons; pie charts represent the proportion of active cells in each behavior: 16/69 cells across 27 swims versus 61/69 cells across 12 escapes (n = 3 larvae), (E) Dorsoventral (D-V) position of cells recruited during each behavior shows dorsal motor neurons only active during escapes (the dorso-ventral axis within the spinal cord is normalized to 0 at the ventral limit and 1 the dorsal limit; mean D-V position for escapes = 0.41 +/- 0.02 versus 0.25 +/- 0.01, p < 0.001, n = 78 cells in n = 3 larvae), (F) Mean ΔF/F 11 526 527 528 529 530 531 532 533 534 535 536 537 538 539 540 541 542 543 544 545 546 547 548 549 550 551 552 553 554 555 556 557 558 559 560 561 562 563 564 565 566 567 568 569 570 571 572 573 574 575 amplitude was higher during escapes compared to spontaneous swims across larvae (91.2 +/4.7% versus 25.9 +/- 3.8%, p < 0.001, 12 escapes and 27 swims in n = 3 larvae). Figure 3. Enhanced recruitment of spinal motor neurons in the presence of mechanosensory feedback (A) To compare the recruitment of spinal motor neurons when mechanosensory feedback was present (“active locomotion”) or suppressed (“fictive locomotion”), we conducted bioluminescence assays in 4 dpf Tg(mnx1:gal4, UAS:GFP-aequorin-opt) zebrafish larvae before and after paralyzing with pancuronium bromide, and in immotile in 4 dpf Tg(mnx1:gal4, UAS:GFP-aequorin-opt, cacnb1ts25/ts25) mutants compared to their motile siblings, (B) Bioluminescence signals in actively moving and paralyzed larvae revealed a marked decrease in bioluminescence amplitude after paralysis, (C) Quantification of the change in mean bioluminescence amplitude (before paralysis: 26.6 +/- 0.9 photons / 10 ms; after paralysis: 11.1 +/- 0.4 photons / 10 ms, n = 10 larvae in each group, 30 trials per larva, p < 0.001), (D) Similarly, averaged bioluminescence signals were markedly decreased in immotile Tg(cacnb1ts25/ts25, mnx1:gal4, UAS:GFP-aequorin-opt) mutant larvae when compared with motile siblings, (E) Mean bioluminescence amplitude in motile siblings (37.6 +/- 1.4 photons / 10 ms) compared to immotile mutants (9.8 +/- 0.4 photons / 10 ms, n = 300 trials in 10 larvae for each group, p < 0.001). Figure 4. Silencing mechanosensory neurons decreases speed of locomotion (A) In vivo fluorescence image and (B) immunohistochemistry for GFP under the isl2b:gal4 driver in 4 dpf Tg(isl2b:gal4, cmlc2:eGFP, UAS:GFP-aequorin-opt) triple transgenic zebrafish larva show selective expression of GFP-aequorin in mechanosensory neurons (trigeminal ganglia, Rohon-Beard spinal neurons and ascending axons (*) and dorsal root ganglia), as well as expression in the retina and heart, with no expression in muscle fibers (n = 4). (C-E) Superimposed tracking (left) and tail angle over time (right) of a typical escape elicited by an acoustic stimulus in a 5 dpf freely swimming control sibling (D) and same for a typical escape in Tg(isl2b:gal4; cmlc2:eGFP, UAS:BoTxBLC-GFP) larva (E). Distance traveled (F) and number of oscillations (G) were unchanged BoTxBLC+ and control larvae (10.2 +/- 0.2 mm versus 10.6 +/- 0.2 mm, p = 0.1 and 6.1 +/- 0.2 versus n = 6.5 +/- 0.2 oscillations, p = 0.07) (H) Escape latency was significantly increased in BoTxBLC+ larvae compared to control siblings (10.0 +/- 0.2 msec versus 8.8 +/- 0.2 msec, p < 0.001). (I) Escape duration was increased in BoTxBLC+ larvae (182 +/- 3.0 msec versus 168.1 +/- 2.4 msec, p < 0.001). (J) BoTxBLC larvae showed a decreased tail-beat frequency (TBF, 34.2 +/0.4 Hz versus 37.8 +/- 0.3 Hz, p < 0.001), (K) and accordingly a decreased speed of escape responses (56.9 +/- 1.1 versus 64.1 +/- 0.8 mm/s, p < 0.001). (L) Amplitude of C bend was not affected, but the second and third bends were larger in BoTxBLC+ larvae on the side of the initial C bend mainly. For all parameters: n = 304 larvae and 890 trials. Figure 5. Mechanosensory feedback times the transition between fast and slow motor regimes (A-A’) Distribution of the amplitude of the tail bends during the escape as a function of tailbeat frequency (TBF) confirmed correlation and showed a right-shifted distribution over time 12 576 577 578 579 580 581 582 583 584 585 586 587 588 (bend numbers are color-coded from red to blue). (B) We analyzed separately the fast regime of the escape response (cycles with TBF > 30 Hz) and the slow regime (cycles with TBF ≤ 30 Hz) as shown for control and BoTxBLC+ larvae. (C1-C4) Within the fast regime of the escape, silencing mechanosensory feedback reduces duration (C1, 67.2 +/- 2.0 msec versus 60.0 +/- 2.2 msec, p = 0.02), number of oscillations (C2, 2.9 +/- 0.1 versus 3.6 +/- 0.1 bends, p < 0.001), TBF (C3, 48.4 +/- 0.4 Hz versus 45.7 +/- 0.4 Hz, p < 0.001) and speed (C4, 112.6 +/- 1.6 versus 104.3 +/- 1.8 mm/s, p = 0.001) ; (D1-D4) Within the slow regime of the escape, silencing mechanosensory feedback increases duration (D1, 141.5 +/- 3.6 versus 96.7 +/- 3.5 msec, p < 0.001, interaction: p < 0.001) and number of oscillations (D2, 2.8 +/- 0.1 versus 1.9 +/- 0.1, p < 0.001, interaction: p < 0.001) but had no effect on tail-beat frequency (D3, 25.4 +/- 0.1 Hz versus 25.3 +/- 0.1 Hz, p = 0.5, interaction: p < 0.001) and speed (24.6 +/- 0.4 versus 24.9 +/- 0.4 mm/s, p = 0.5, interaction: p < 0.001). For all parameters: n = 304 larvae, n bends fast components = 2984; slow components = 2031. 13 Figure 1 Figure 2 Figure 3 Figure 4 Figure 5 Supplementary Methods Zebrafish care, generation and characterization of transgenic lines Adult AB and and Tüpfel long fin (TL) strains of Danio rerio were maintained and raised on a 14/10 hour light cycle and water was maintained at 28.5°C, conductivity at 500 µS and pH at 7.4. Embryos were raised in blue water (3 g of Instant Ocean® salts and 2 mL of methylene blue at 1% in 10 L of osmosed water) at 28.5°C during the first 24 hours before screening for GFP expression. All procedures were approved by the Institutional Ethics Committee at the Institut du Cerveau et de la Moelle épinière (ICM), Paris, France, the Ethical Committee Charles Darwin and received subsequent approval from the EEC (2010/63/EU). The Tg(mnx1:gal4)icm11 line was based on the injection of the mnx1 construct kindly provided by Dr. T. Auer and Dr. F. Del Bene (Institut Curie, Paris, France) (39). The original sequence for GFP-aequorin kindly provided by Dr. L. Tricoire (Université Pierre et Marie Curie, Paris, France) was subsequently codon-optimized for expression in zebrafish and subcloned into the PT2 14xUAS plasmid kindly provided by Pr. K. Kawakami (National Institute of Genetics, Mishima, Japan). Injection of this construct in the Tg(mnx1:gal4) allowed the generation of the Tg(UAS:GFP-aequorin-opt)icm09 line with selective expression of the GFP-aequorin in all spinal motor neuron populations: more prominently primary dorsal motor neurons but also intermediate and ventral secondary motor neurons (Fig. 1B) without any expression in the muscles and only very limited expression in the brain and hindbrain. The transgenic line Tg(UAS:GCaMP6f,cryaa:mCherry)icm06 (21) was generated by subcloning GCaMP6f (40) into pDONR221 and then assembled into the final expression vector in a three-fragment Gateway reaction using p5E-14XUAS, pME-GCaMP6f, p3E-poly(A) and pDestCryAA:mCherry Relaxed mutants (cacnb1ts25/ts25) (25) were kindly provided by Pr. Paul Brehm (Oregon Health and Science University, Portland, USA). In homozygous cacnb1ts25/ts25 mutants, a mutation of the skeletal muscle dihydropyridine receptor β1a subunit interferes with the calcium release and mutant larvae are immotile (41). The Tg(isl2b:gal4, cmlc2:eGFP) line (26) driving expression in trigeminal and Rohon-Beard neurons was kindly provided by Dr. T. Auer, Dr. F. Del Bene (Institut Curie, Paris, France) based on the injection of the isl2b construct (42). Immunochemistry on GFP confirmed that there was no expression in muscle fibers but GFP expression could also be seen in the retina, heart and blood vessels. The chicken anti-GFP primary antibody was used at 1:500 dilution (Abcam, Cambridge, UK). The secondary antibody used was the Alexa Fluor 488 donkey anti-chicken IgG (1:1000 dilution, Life Technologies). Immunostaining specificity was established by omitting the primary specific antibody, no immunoreactive signal was observed. Monitoring neuronal activity with bioluminescence Embryos were dechorionated and soaked at 26°C in 100 µL of blue water with a final concentration of 60 µM of coelenterazine-h (Biotium, Hayward, USA). Coelenterazine-h was renewed at 2 days post-fertilization (dpf). All experiments were performed at 4 dpf. All GFPaequorin expressing larvae were tested at 4 dpf. In all experiments, one larva was headembedded in 1.5% low melting agarose with the tail free to move in a circular (2 cm diameter) 3D-printed arena (Sculpteo, France). The arena was then placed in a lightproof box (Fig. 1A) and attached to a small speaker (2 Ohm). Each trial consisted of a 500 ms baseline followed by a 10 ms acoustic stimulus and 1990 ms subsequent recording. Assays consisted of 30 trials with 1-minute inter trial intervals to reduce habituation. Sinusoidal stimuli (5 cycles, 500Hz) were delivered through a wave generator (Agilent, 33210-A) and audio amplifier (Lepai, LP2020A). Intensity was adjusted to the lowest value that reliably elicited an escape response (between 0.5 and 5 Vpp). The same larvae used for the active assay were subsequently paralyzed by bath application of pancuronium bromide (Sigma, P1918) at 0.6 mg/mL final concentration and stimulation intensity was adjusted to the lowest value that elicited a bioluminescent signal. For Fig. 3, cacnb1ts25/ts25 and control siblings were tested alternatively on the same day and compared to each other. In non-moving animals (i.e. paralyzed or cacnb1 mutants), the intensity was progressively increased until stimuli elicited fictive responses. A higher intensity of the acoustic stimulus was often needed after addition of pancuronium bromide, possibly due to modulation of cholinergic arousal brain circuitry (43). As negative controls, bioluminescence assays of wild type animals or Tg(mnx1:gal4, UAS:GFP) (39) where motor neurons express GFP only revealed no signal (n = 3 wild type larvae with 30 trials each, n = 5 Tg(mnx1:gal4, UAS:GFP) larvae with 30 trials each). Animals deprived of GFP-Aequorin did not produce any signals above baseline noise level during escape responses. Infrared light illumination for monitoring larval behavior was provided by an 850 nm LED (Effisharp, Effilux, France) mounted with 2 long-pass 780 and 810 filters (Asahi ZIL0780 and Asahi XIL0810, respectively) and a diffuser (Thorlabs, DG10-120B). Video acquisition was performed at 1000 Hz using a high-speed infrared sensitive camera (Eosens MC1362, Mikrotron, Germany; objective Nikkor 50 mm f/1.8D, Nikon, Japan) at 320x320 pixels resolution controlled by the software Hiris (RD Vision, France). Photons were counted with a PMT (Hamamatsu H7360-02) located under the larva arena and sent to an acquisition card (NI PCI 6602, National Instruments, USA). A band-pass filter (Carl Zeiss 525 nm / 50 nm, ref. 489038-8002) and a short-pass filter (Asahi 670 nm, XVS0670) were placed between the larva and the PMT. A custom application-programming interface developed in collaboration with R&D Vision synchronized the video acquisition with the photon count and the stimulus delivery using 30 trials batched TTL chronogram (EG Chrono, RD Vision). Calcium imaging of spinal motor neurons and ventral nerve root recording (VNR) 4 dpf Tg(mnx1:gal4; UAS:GCaMP6f,cryaa:mCherry) double transgenic larvae were screened for dense labeling and good expression of GCaMP6f in spinal motor neurons under a dissecting microscope equipped with an epifluorescence lamp (Leica, Germany). Larvae were anaesthetized in 0.02% Tricaine-Methiodide (MS-222, Sigma-Aldrich, St-Louis, USA) diluted in fish facility water and mounted on their lateral side in 1.5% low-melting point agarose in glass-bottom dishes filled with external solution ([NaCl]=134mM, [KCl]=2.9mM, [MgCl2]=1.2mM, [HEPES]=10mM, [glucose]=10mM and [CaCl2]=2.1mM; adjusted to pH 7.7-7.8 with NaOH and osmolarity 290mOsm). Larvae were immobilized by injecting 0.1-0.3 nL of 0.5 mM α-Bungarotoxin (Tocris, UK) in the ventral axial musculature. A portion of agar was removed using a razor blade in order to expose 2 to 3 segments. To achieve a strong signalto-noise ratio during fictive locomotion recordings, the skin overlying these segments was removed using suction glass pipettes. Zebrafish larvae were imaged using a custom spinning disk microscope (Intelligent Imaging Innovation, Denver, USA) equipped with a set of waterimmersion objectives (Zeiss 20X, 40X, NA=1). Recordings were acquired using Slidebook® software at 20 Hz at 488nm. Gain and binning were optimized to maximize signal to noise ratio. Z projection stacks showed full pattern of expression using Fiji (44). Positions of cells along the D-V axis were computed using Fiji and Matlab. Calcium signals were extracted online using custom scripts. Regions of interest (ROIs) were manually designed and calcium signals time series were extracted as the mean fluorescence from individual ROIs at each time point of the recording. We observed that out-of-focus signals varied between animals, from dorsal to ventral spinal cord regions in a behavior-dependent manner. To estimate the contribution of out-of- focus signals we systematically picked two background ROIs, one placed below the ventral limit of the spinal cord to capture out-of-focus signals at the level of ventral motor neurons during slow swimming, the second in the dorsal-most part of the spinal cord to capture out-offocus signals in the dorsal spinal cord during the escape. We estimated the maximum out-offocus signals observed during each behavior and used this value as a threshold for discriminating active from silent motor neurons. Thin-walled, borosilicate glass capillaries (Sutter Instruments, Novato, USA) were pulled and fire-polished from a Flaming/Brown pipette puller (Sutter Instruments, Novato, USA) to obtain peripheral nerve recording micropipettes. Pipettes were filled with external solution and positioned next to the preparation using motorized micromanipulators under the microscope. Light suction was applied when the pipette reached the muscle region located at the vicinity of intermyotomal junctions, ventral to the axial musculature midline. VNR signals were acquired at 10kHz in current clamp IC = 0 mode using a MultiClamp 700A amplifier (Molecular Devices–Axon Instruments, USA), a Digidata series 1322A digitizer (Axon Instruments, USA) and pClamp 8.2 software (Axon instruments, USA). Recordings were considered for analysis when the background noise did not exceed 0.05 mV amplitude and signal to noise ratio for fictive locomotor events detection was above three. VNR recordings were analyzed offline and aligned to calcium imaging data using custom-made MATLAB scripts. Behavioral analysis of freely moving BoTxBLC larvae Zebrafish larvae Tg(isl2b:gal4, cmlc2:eGFP; UAS:BoTxBLC-GFP) were screened at 3 dpf for expression. At 5 dpf, larvae were tested 4 by 4: each larva was positioned in a separate dish (2 cm diameter) and illuminated from below, freely moving. Escapes were elicited by delivering a 500 Hz stimulus for 1 ms using 20W speakers. Each trial consisted of a 200 ms pretrial window followed by a 1 ms stimulus at 500 Hz and 800 ms subsequent recording. 5 trials were performed in succession with 2 minutes intertrial rest. Behavior was recorded at 650 fps with a high-speed camera (Basler acA2000-340km) and analyzed using a tracking algorithm (ZebraZoom, (45)) and a custom Matlab script (R2012b, Mathworks, USA). Hemi-periods were calculated as the interval between two consecutive peaks, and subsequently used as a determinate to extract fast and slow components of the escape with a cutoff of 650/60 frames (30 Hz) (27, 28). Peaks which alternated between the cutoff value after the first appearance of a slow peak were excluded. Statistical analysis SPSS 20 (IBM, USA) was used to perform all statistical analyses. Comparisons of bioluminescence signals parameters was conducted using a t-test for paired samples for repeated measures within subjects (i.e. active versus paralyzed data). Mixed linear model analysis with repeated measures using an auto-regressive covariance structure was performed to compare the bioluminescence amplitude between movement categories in active assays, and between moving and immotile larvae in active versus fictive assays. Bioluminescence decay coefficients (tau) were included if the goodness of fit r-square value was > 0.95. A Pearson test was used to assess correlations for parametric data. Statistical significance is represented in the graphs as *** for p < 0.001, ** for p < 0.01, * for p < 0.05, corrected for multiple comparisons when needed. All data are provided in the figures and text as means +/- standard error of the mean (SEM). Table S1. Stable transgenic lines used or generated in this study. Name Tg(mnx1:gal4)icm11 Tg(isl2b:gal4, cmlc2:eGFP) Tg(UAS:GFP-aequorin-opt)icm09 Tg(UAS:GCaMP6f;cryaa:mCherry)icm06 Tg(UAS:GCaMP5)icm08 Relaxed (cacnb1ts25) Original reference Böhm et al., 2016 Auer et al., 2015 This paper Böhm et al., 2016 Fidelin et al, 2015 Granato et al., 1996 Tg(UAS:BoTxLCB-GFP) Auer et al. 2015; Sternberg et al., in revision. Movie S1: Expression of GFP in Tg(mnx1:gal4; UAS:GFP-aequorin-opt) revealed by immunohistochemistry on whole mount larvae. Movie S2: Bioluminescence signals emitted by Tg(mnx1:gal4; UAS:GFP-aequorin-opt) free-tailed transgenic larvae and corresponding kinematics during escape. Movie S3: Bioluminescence signals emitted by Tg(mnx1:gal4; UAS:GFP-aequorin-opt) free-tailed transgenic larvae and corresponding kinematics during swim. Movie S4: Calcium imaging of spinal motor neurons in Tg(mnx1:gal4; UAS:GCaMP6f) during fictive slow swim. Animals were paralyzed with bungarotoxin injections. Movie S5: Calcium imaging of spinal motor neurons in Tg(mnx1:gal4; UAS:GCaMP6f) during fictive escape. Animals were paralyzed with bungarotoxin injections. Movie S6: Expression of GFP in Tg(isl2b:gal4; UAS:GFP-aequorin-opt) revealed by immunohistochemistry on whole mount larvae. Author Contributions SK and CD collected bioluminescence data from spinal motor neurons while monitoring behavior. KF collected and analyzed calcium imaging of spinal motor neurons. AP, PET and AP collected behavioral data from BoTxBLC experiments. SNF performed immunohistochemistry assays. OT built the preliminary version of the bioluminescence setup. UB and HPM gave critical appraisal. SK and CW designed research and analyzed data. SK and CW wrote the manuscript with feedback from all authors. Annex 3 Annex 3 Published article Three-dimensional spatiotemporal focusing of holographic patterns 161 ARTICLE Received 10 Nov 2015 | Accepted 12 May 2016 | Published 16 Jun 2016 DOI: 10.1038/ncomms11928 OPEN Three-dimensional spatiotemporal focusing of holographic patterns Oscar Hernandez1,w, Eirini Papagiakoumou1,2, Dimitrii Tanese1, Kevin Fidelin3, Claire Wyart3 & Valentina Emiliani1 Two-photon excitation with temporally focused pulses can be combined with phasemodulation approaches, such as computer-generated holography and generalized phase contrast, to efficiently distribute light into two-dimensional, axially confined, user-defined shapes. Adding lens-phase modulations to 2D-phase holograms enables remote axial pattern displacement as well as simultaneous pattern generation in multiple distinct planes. However, the axial confinement linearly degrades with lateral shape area in previous reports where axially shifted holographic shapes were not temporally focused. Here we report an optical system using two spatial light modulators to independently control transverse- and axial-target light distribution. This approach enables simultaneous axial translation of single or multiple spatiotemporally focused patterns across the sample volume while achieving the axial confinement of temporal focusing. We use the system’s capability to photoconvert tens of Kaede-expressing neurons with single-cell resolution in live zebrafish larvae. 1 Wavefront-Engineering Microscopy Group, Neurophotonics Laboratory, CNRS UMR 8250, Paris Descartes University, UFR Biomédicale, 45 rue des SaintsPères, 75270 Paris Cedex 06, France. 2 Institut national de la santé et de la recherche médicale (Inserm), France. 3 Institut du Cerveau et de la Moelle Épinière, UPMC, Inserm UMR S975, CNRS UMR 7225, Campus Hospitalier Pitié Salpêtrière, 47 building de l’Hôpital, 75013 Paris, France. w Present address: CNC Program, Stanford University, Stanford, California 94305, USA. Correspondence and requests for materials should be addressed to V.E. (email: [email protected]). NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 ince its first demonstration1, nonlinear two-photon (2P) microscopy has revolutionized diverse research fields including neuronal structural and functional imaging, photostimulation, laser processing and lithography. Original configurations were based on scanning a tightly focused pulsed laser beam across a defined sample region with galvanometric mirrors, acousto-optic deflectors2–4 or resonant scanners5. Recently, applications including lithography, uncaging, optogenetics and fast functional imaging have motivated scanless 2P-excitation method development, enabling simultaneous illumination of laterally extended regions of interest while preserving micrometre axial confinement. This was achieved by axially confining large spots generated by low-numerical aperture (NA) Gaussian beams with temporal focusing (TF)6,7. In TF, a diffraction grating conjugated to the sample plane diffracts the different spectral frequencies comprising an ultrashort excitation pulse towards different directions. The various frequencies thus propagate towards the objective focal plane at different angles, such that spatiotemporal coupling effects8 lead to temporal pulse broadening above and below the focal plane, which remains the only region irradiated at peak powers efficient for 2P excitation. In this way, TF enables axial confinement equivalent to that of line-scanning microscopy that is independent of the excitation beam’s lateral extent. Investigators have applied TF to imaging9–13, functional imaging14,15, super-resolution imaging16, lithography17 and neuronal photostimulation18,19 by generating circular-spot shapes only. As a more flexible way to elaborate light patterning, phasemodulation approaches using liquid-crystal spatial light modulators, such as computer-generated holography (CGH)20,21 and generalized phase contrast (GPC)22 have demonstrated efficient light sculpting in two-dimensional (2D) user-defined shapes. Combined with TF to axially confine the shapes, CGH and GPC can sculpt 2P-excitation volumes with the micrometre precision needed to illuminate small structures, such as neuronal dendrites22,23. These methods also enable 2P optogenetic stimulation with high temporal resolution and robustness to scattering23,24. CGH can also sculpt light in three dimensions (3D)25, a feature used to generate multidiffraction-limited traps for optical tweezers26,27 and 3D glutamate uncaging28,29. Although not yet demonstrated with 2P excitation, adding lens-phase modulations to 2D-phase holograms also enables remote axial displacement and 3D positioning of laterally shaped targets27,30,31. Combining CGH’s 3D light-shaping capability with 2P excitation can significantly broaden the range of possible applications. For example, simultaneous multiplane holographic pattern generation can enable fast 2P multiplane imaging, photostimulation and photo-polymerization methods. In addition, remote axial displacement of holographic targets can couple holographic illumination with a second imaging or stimulation channel, providing independent control of their respective focal planes32, as well as remote volume scanning. However, previous 3D CGH optical configurations could not be implemented with TF because axially shifted excitation planes cannot be simultaneously imaged on the TF grating. This shortcoming restricted CGH-TF to 2D patterns focused at the objective focal plane21. Here we demonstrate a unique optical system overcoming this limitation. The system achieves remote axial displacement of temporally focused holographic beams as well as multiple temporally focused planes by shaping the incoming wavefront in two steps using two spatial light modulators (SLMs). A first SLM laterally shapes the target light distribution that is focused on the TF grating, while a second SLM, positioned after the S 2 grating, controls the axial position(s) of the spatiotemporal focal plane(s) in the sample volume. By integrating phase profiles that minimize optical aberrations and intensity compensation protocols, we generate spatiotemporally focused patterns with uniform light distribution throughout the entire accessible volume, and demonstrate generation of laterally shaped targets across an unprecedented axial range. We apply axially confined multiplane light illumination to photoconvert in vivo Kaede-protein-expressing neurons in zebrafish larvae. The system enables cellular resolution photoconversion of tens of spinal cord neurons occupying separate axial planes. Results Optical system. In the original optical system we designed (Fig. 1, see Methods), the output of a Ti:Sapphire laser was expanded to illuminate a first liquid crystal on silicon SLM (LCOS-SLM; SLM1) used to generate 2D holographic light patterns at the focal plane of a first lens where a blazed grating (G) was placed for TF. TF enables 2D CGH pattern generation with the axial confinement of a line-scanning microscope, practically decoupling axial confinement from lateral extent21 (Supplementary Fig. 1). Two telescopes (L2, L3; L4, OBJ1) relayed the holographic pattern on the sample plane. The phase holograms addressed on SLM1 were calculated using a Gerchberg and Saxton (GS)-based algorithm33. A second LCOS-SLM (SLM2) was placed at the confocal plane of the first telescope and imaged at the back focal plane of the microscope objective through the second telescope. By addressing SLM2 with single or multiple lens phases, we axially displaced single or multiple spatiotemporally focused patterns across the sample volume. In order to characterize the optical properties of the holographic patterns, we used a microscope configuration using two opposite-facing objectives20,21. By doing so, holographic patterns illuminated a thin fluorescent film and 2P-excited fluorescence was collected by a second objective (OBJ2) placed opposite to OBJ1 and imaged on a CCD (charged couple device) camera. For 3D reconstruction of illumination volumes, OBJ2 was fixed and focused on the fluorescent layer, while OBJ1 was SLM1 BE Ti:Sapphire laser CL G L1 L2 CCD L5 OBJ2 FFP OBJ1 L4 L3 SLM2 Figure 1 | Experimental set-up for 3D-CGH-TF. The output beam of a Ti:Sapphire laser is magnified using a beam expander (BE) and projected on a first SLM (SLM1). SLM1 modulates the beam’s phase so that light forms a user-defined intensity pattern on the diffraction grating (G) after passing through lens L1. The first diffraction order is collimated by the lens L2 and directed on a second SLM (SLM2). SLM2 imprints a lens-phase modulation that enables precise axial positioning of the spatiotemporal focal plane. The laser beam is then relayed and scaled by lenses L3 and L4 to the excitation objective (OBJ1) pupil size. OBJ1 is mounted on a piezo positioner so that it focuses and axially scans the excitation beam across a thin fluorescent layer. A second objective (OBJ2), always focused on the fluorescent layer, collects emitted fluorescence and forms an image on a CCD camera. Two cross-oriented cylindrical lenses (CL), with focal lengths of equal power and opposite sign, are used to suppress the zero-order spot of the first SLM64. NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 moved along the axial direction with a piezo-scanning stage20,21. For experiments on characterizing SLM’s diffraction efficiency, the holographic patterns were directly imaged on the CCD camera (image of the transmitted laser light). For the experiments of photoconversion and optogenetic stimulation in zebrafish larvae, the system was coupled to a HiLo imaging set-up (see following sections). The optical system enabled remote axial displacement of temporally focused holographic patterns as well as generation of multiple spatiotemporally focused holographic targets in distinct axial planes. In a simplified configuration where mirrors replaced the SLM2 and the grating, the system also generated 2P multiplane arbitrarily shaped non-temporally focused patterns. Remote axial shift of spatiotemporally focused patterns. First, we demonstrated remote axial displacement of a temporally focused holographic pattern by separating input-beam-phase modulation in two steps, controlling first the target lateral light distribution, and, second, its axial position. In contrast to conventional CGH, axial displacement of temporally focused holographic patterns cannot be achieved by simply adding a Fresnel lens to the phase hologram34 because the lens effect, by axially displacing the excitation plane with respect to the TF grating, would forbid the spatial and temporal focal planes to coincide35. We resolved this issue by implementing a novel, two-SLM strategy. SLM1 generated 2D CGH illumination patterns focused on the grating G, which dispersed the spectral components of the illumination pattern on SLM2. SLM2, conjugated to the objective back focal plane, was addressed with a Fresnel lens-phase profile to control the target’s axial position in the sample volume. This design enables the spatial and temporal focal planes to coincide at the grating, and to be jointly translated by SLM2 across the sample volume axial extent. The effect of spherical aberrations on targets generated out of OBJ1’s nominal focal plane (Supplementary Fig. 2) was minimized by describing the objective focal sphere36 within the approximation of small defocus and high NA. We thus addressed SLM2 with lens-phase profiles featuring spherical phase28,37: qffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi fðrÞ ¼ kDz n2 NA2 r2 ð1Þ where k is the free-space wavenumber, n is the refractive index of the immersion medium, NA is the numerical aperture of the r objective, r ¼ NAf (with feq being the equivalent focal length: eq pffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi f3 feq ¼ f4 fobj and r ¼ x2 þ y2 þ z 2 ) is the normalized pupil at the SLM2 plane and Dz the axial displacement of the holographic light pattern in the sample volume. With the above strategy, we demonstrated remote axial translation of temporally focused holographic patterns by displacing a 20-mm-diameter holographic spot throughout a ±130-mm axial range (Fig. 2a–c), the accessible field of excitation (FOEz) without hologram aliasing (Supplementary Note 1). Within this range, we could remotely displace holographic patterns while conserving target shape sharpness (Fig. 2a, bottom) and axial confinement (5–10 mm; Fig. 2b,c). The integrated 2P fluorescence intensity decreased by approximately fivefold at the edges because of the diffraction efficiency of SLM2 (Supplementary Note 1 and Supplementary Fig. 3a–c). Furthermore, we generated targets with micrometre axial confinement (10–25 mm) and sharp shapes across a broader axial range (±300 mm, that is, approximately two times greater than the FOEz). However, in this extended range, the full-width at half-maximum (FWHM) broadened (B25 mm at ±300 mm; Fig. 2b,c), fluorescence intensity decreased (B10 times at ±300 mm) and spot quality deteriorated (Supplementary Fig. 4) as a consequence of optical aberrations, SLM2 diffraction efficiency and the finite dimensions of mirrors and lenses placed after SLM2 that can vignette beam edges for highly diverging/ converging wavefronts, among other factors. For applications requiring constant illumination across the axial range, attenuating illumination intensity at axial positions near the centre of the FOE can compensate the positiondependent intensity decrease (Supplementary Fig. 3g). This can be accomplished either by reducing the incident laser power or by redirecting light into an extra spot similar to the procedure described in Supplementary Fig. 3d–f. 3D-CGH-TF spatiotemporally focused patterns. Next, we demonstrated that decoupling lateral and axial light shaping into two separate steps also enables generation of multiplane spatiotemporally focused patterns. In this configuration, SLM1 is tiled into n vertical regions (that is, parallel to the orientation of the grating lines and orthogonal to the grating linear dispersion), with n equal to the number of axial planes populated by the final illumination pattern. Each tile generates a 2D target shape focused through L1 on the TF grating (Fig. 3a, top, Supplementary Fig. 5). SLM2, vertically tiled into n independent lens-phase profiles, then axially displaces each shape into its target axial plane (Fig. 3a, bottom). In this configuration, for each of the n targeted planes, both spatial and temporal foci coincide at the grating, while the n phase-lens profiles addressed on SLM2 enable independent remote displacement of each shaped target in the sample volume (Fig. 3b,c). In agreement with previous observations38, reducing the hologram width, Dy, along the direction perpendicular to the grating’s dispersion did not compromise spot axial confinement, as the reduction of the hologram width, Dx, in the orthogonal direction would do (Fig. 3d). Moreover, tiling holograms along the x axis would create crosstalk between axially separated planes because of the dispersion at the grating that generates at SLM2 a lateral spatial overlapping of the holograms generated by SLM1, making impossible to imprint independent axial shifts to the corresponding patterns. However, vertical hologram resizing introduced a spatial filter that vertically elongated speckles (Fig. 3e). In particular, the vertical mean speckle size, estimated by calculating the vertical autocorrelation width39, sy, increased by a factor of two with respect to the original size (sy ¼ 2symin ) when the hologram vertical dimension was reduced to one-fourth of the full SLM aperture (Fig. 3f). In contrast, the horizontal autocorrelation width sx was unaffected by vertical hologram resizing (Fig. 3f). The limited size of the SLM can constitute the limiting factor defining the maximum number of axially separated planes in 3D-CGH-TF. Theoretically, the maximum number of planes is determined by the number of pixel rows on the SLM, but practically there is a tradeoff between the number of axial planes and the lateral resolution requirements for each application. As far as the spot shape is not distorted and spot’s contour remains well defined, the smoothing of the excitation holographic spot to some extent can be even beneficial21,38,40. Moreover, vertical SLM tiling induced a lateral tilt on the intensity propagation due to the asymmetric illumination of the objective back focal plane. Addressing SLM1 and SLM2 symmetrically along the vertical axis of the SLM eliminated this effect (Supplementary Fig. 6). Hologram resizing also affected the intensity of the illumination pattern at the sample plane. Specifically, holograms projected at the centre of the objective back aperture generated brighter targets than holograms projected on the side (Supplementary Fig. 7 and Supplementary Note 2). This effect adds to the position-dependent diffraction NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications 3 ARTICLE a NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 z = 100 μm 50 μm 0 μm –50 μm b –100 μm 0 Fluorescence intensity (a.u.) 1 FOEz 1.0 0.8 0.6 0.4 0.2 0.0 c y y –100 0 100 Axial position (μm) FOEz 200 300 70 CGG 25 60 20 50 40 15 30 10 5 0 x –200 30 Axial confinement FWHM (μm) z –300 20 CGH-TF –300 –200 10 –100 0 100 Axial position (μm) 200 300 0 Figure 2 | Axial displacement of spatiotemporally focused patterns. (a) Axial displacement of a 20-mm-diameter temporally focused holographic spot. Top, orthogonal maximum fluorescence intensity projection of the spot, for axial displacements of ±50 and±100 mm from the focal plane (0 mm). Bottom, corresponding x–y fluorescence intensity cross-sections. Scale bar, 20 mm. The colour bar refers to normalized intensity. (b) Axial profile of the integrated fluorescence intensity of the 20-mm-diameter holographic spot for different axial displacements. (c) Axial confinement (FWHM) of the profiles shown in b (CGH-TF; red data) compared with the axial confinement of a 20-mm-diameter holographic spot without TF (CGH; blue data). Data were fitted with a parabolic function (dashed lines) in both cases. White area in b,c represents the field of excitation (FOEz). efficiency determined by the SLM pixel size (Supplementary Note 1 and Supplementary Fig. 3). Thus, generation of homogeneous 3D-CGH-TF patterns required compensating both lateral and axial position-dependent intensity variations, which in both cases was higher at the FOE centre and falling off towards the periphery. For lateral shape generation with SLM1, we weighted target intensity input to the GS algorithm such that targets occupying low-efficiency regions were brighter than targets position at the efficient FOEx,y central zone (Supplementary Fig. 3). To achieve uniformity in the axial direction, we scaled the vertical tile size, allocating greater area to holograms projected at the objective pupil periphery with respect to the centre (Supplementary Note 2 and Supplementary Fig. 7). This enabled generation of homogeneous light patterns (Fig. 3g) across the whole excitation volume. Holographic patterns generated in distinct planes yielded fluorescence intensity distributions showing that axial confinement was also well conserved (Fig. 3g). However, an unavoidable background in the intermediate planes appeared when targets were laterally aligned (Fig. 3g, right bottom panel, and Supplementary Fig. 8). 3D computer-generated holography. We demonstrate a simplified, single-SLM system for non-temporally focused multiplane pattern generation, replacing the grating and second SLM with mirrors (Fig. 4a); we patterned multiple targets occupying separate axial planes (Fig. 4b,c, Supplementary Movie 1) with a modified GS algorithm for multiplane pattern projection41 (Supplementary Fig. 9). By using the spherical phase expression described in equation (1) to translate the various targets into different axial planes, with r ¼ NAfr eq1 ðwhere feq1 ¼ ff12 ff34 fobj Þ, we minimized the effects of spherical aberrations on targets generated out of the focal plane, improving agreement between experimental and expected axial positions for the different targets compared with 3D-CGH implemented with parabolic-lens phases42 (Supplementary Fig. 2). We compensated diffraction 4 efficiency-induced intensity variations by weighting target intensity inputs to the multiplane GS algorithm, as previously described (Supplementary Fig. 3), achieving uniform intensity shape generation throughout the accessible B240 240 260 mm3 volume (Supplementary Note 1). In vivo 3D-patterned selective photoconversion of neurons. We leveraged the axial specificity achievable with 3D-CGH-TF to photoconvert Kaede protein-expressing neurons in live Tg(HuC:Gal4; UAS:Kaede) double transgenic zebrafish larvae43. Kaede is a green photoactivable fluorescent protein that, when exposed to ultraviolet light, undergoes a photo-induced protein cleavage, red shifting its fluorescence emission spectrum44. With 2P excitation, efficient Kaede photoconversion can be obtained for wavelengths in the range of 760–800 nm45. We first performed Kaede photoconversion in spinal neurons (Fig. 5a, blue and red areas) to demonstrate the ability for single-cell resolution. Next, we demonstrated the robustness of 3D-CGH-TF to scattering by moving to the brain (Fig. 5a, dark yellow area) that is a more scattering region (Supplementary Fig. 10). To monitor photoconversion in the spinal cord, we combined multiplane patterned photostimulation with a two-colour HiLo (high/low-frequency sequential acquisition) imaging system46 (see Methods and Supplementary Fig. 11). First, a HiLo z-stack exciting green fluorescence (excitation wavelength lexc ¼ 473 nm) was acquired to localize Kaede-expressing neurons from which we selected photoconversion targets. We then photoconverted selected cells with 2P holographically patterned illumination (stimulation time 0.20–100 s; excitation power density: 0.03–4.0 mW mm 2; Methods. We note that power densities are always given relatively to the area of the spots’ surface). A second HiLo z-stack exciting red fluorescence (lexc ¼ 561 nm) was acquired after the photostimulation protocol in order to assess the photoconversion efficiency. For photoconversion experiments in the brain, scattering quickly deteriorates the quality of HiLo images. We therefore monitored the extent of NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 1 Plane A Plane A Plane B x 0 z y x Plane B Plane B SLM 2 b 0 – Plane A SLM 1 a x z y y c 1.0 0.2 Axial confinement FWHM (μm) d x e 8 12 mm 10 mm f 8 mm Δx Δy 2.0 1 0 7 6 mm 6 4 mm 2 mm Autocorrelation width FWHM (μm) z 5 2 4 6 8 10 Hologram width (mm) 12 y 1.0 0.0 –100 –50 0 50 Axial position (μm) 100 Fluorescence intensity (a.u.) 0.5 0.0 –100 –50 0 50 Axial position (μm) Fluorescence intensity (a.u.) Fluorescence intensity (a.u.) 100 0.5 0.0 –100 –50 0 50 Axial position (μm) 100 1.0 1.0 0.5 0.0 –100 4 6 8 10 12 Hologram width (mm) 1.0 1.0 1.0 0.5 2 x Fluorescence intensity (a.u.) Fluorescence intensity (a.u.) g 1.5 0.5 0 σx σy –50 0 50 Axial position (μm) 100 0.5 0.0 –100 –50 0 50 Axial position (μm) 100 Figure 3 | Multiplane spatiotemporally focused pattern generation. (a) Top, tiled phase profiles addressed to SLM1 for encoding the words ‘neuro’ (plane A) and ‘photonics’ (plane B). Bottom, Fresnel lens-phase profiles addressed to SLM2 to axially displace each holographic pattern generated by SLM1 on separated planes at þ 20 mm (plane A) and 20 mm (plane B). (b) Left, x–y 2P fluorescence intensity cross-sections at planes A and B generated by the holograms in a. Right, orthogonal maximum 2P intensity projection along x (top) and y (bottom). (c) Orthogonal fluorescence intensity projection of spatiotemporally focused patterns created by projecting 20-mm-diameter holographic spots in one, two, three and four planes at positions laterally shifted and in four planes at positions axially aligned (from left to right). Scale bars, 20 mm. (d) Axial confinement (FWHM) of a 20-mm-diameter holographic spot for different hologram widths, tested in both directions, Dx (blue data) and Dy (red data) parallel and perpendicular to grating’s dispersion, respectively. (e) x–y 2P fluorescence intensity cross-sections of a 20-mm-diameter holographic spot generated with different hologram widths Dy. (f) Autocorrelation width at the sample plane of a 20-mm-diameter holographic spot as a function of the hologram width along the x- (blue data; sx) and y directions (red data; sy). (g) Axial profile of the integrated fluorescence intensity of the holographic spots shown on c. Colour bars refer to normalized intensity. NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications 5 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 a c SLM1 BE Ti:Sapphire laser CL M L1 L2 150 L5 OBJ2 FFP OBJ1 L4 L3 b 100 M z = 130 μm z = –65 μm 50 μm CCD z = 0 μm 0 –50 z = 65 μm –100 z x –150 –50 y 0 0 μm μm z = 130 μm –50 50 Figure 4 | 3D-CGH. (a) Schematic of the optical set-up for 3D-CGH. In this case the diffraction grating G and SLM2 were replaced by mirrors. (b) 2P fluorescence images of 3D-CGH patterns, depicting the letters ‘a’, ‘b’ and ‘c’ at three different axial positions, z ¼ 130 mm, 0 and 130 mm, respectively. The phase profile used to project these patterns was calculated using a multiplane GS algorithm (Supplementary Fig. 9). Weighting of the input patterns according to their lateral and axial position (Supplementary Fig. 3) enabled diffraction efficiency correction and generated equal-intensity light patterns. (c) Volumetric reconstruction of three-dimensional distribution of 5-mm-diameter holographic spots (see also Supplementary Movie 1). photoconversion by moving the sample to a 2P galvo-based scanning imaging system in another set-up (see Methods). Green and red fluorescence in the 2P imaging system were excited at 780 nm, and scanning was performed at 0.74 Hz. In the spinal cord, we first used 2D-CGH-TF to demonstrate simultaneous photoconversion (lphot ¼ 800 nm) of multiple neurons in a single plane with cellular precision (Fig. 5a,b). Then, we used 3D-CGH-TF to photoconvert isolated neurons (Fig. 5c) or groups of multiple neurons (Fig. 5d) in distinct axial planes. The axial confinement achievable with 3D-CGH-TF enabled single-layer selectivity that would otherwise not be possible using CGH deprived of TF (Supplementary Fig. 12a). In all experiments performed, photoconversion induced a B10-fold increase in the ratio of pre- and post-photoconversion red fluorescence. To demonstrate in-depth photoconversion, we performed a second set of experiments in the brain of zebrafish larvae by photoconverting neuronal ensembles in two different axial planes using 35-mm-diameter temporally focused holographic spots separated by B80 mm (Fig. 5e), with the first plane positioned at B90 mm from the surface. Although the axial confinement deteriorates with depth because of scattering, the red fluorescence intensity profiles along the axial direction showed selective and efficient photoconversion (Fig. 5d,e), which was not achievable with 3D-CGH alone (Supplementary Fig. 12b). Strikingly, axial resolution and overall shape were also preserved despite light propagation through the zebrafish brain (Supplementary Fig. 10). In agreement with previous results in rats23,24, these results demonstrate that TF enables robust in-depth propagation of shaped patterns through scattering media. It is important to note that 2P Kaede photoconversion is a low-efficiency process and requires relatively high illumination doses (B0.04–4 mW mm 2) and long (from 200 ms to a few hundreds of seconds) stimulation protocols. Other type of applications such as activation of neurons via 2P-mediated optogenetic stimulation can work with drastically reduced 6 photostimulation time (5 pulses of 50 ms), as well as lower illumination doses (0.04–0.60 mW mm 2; Supplementary Note 3). A still open question for multiplane multispot photostimulation concerns the possible temperature rise induced by laser illumination. For the illumination doses we used in the optogenetics experiments (Supplementary Note 3), we estimated that this does not exceed few degrees Celsius. Specifically, by solving the heat diffusion equation47 with typical thermal optical parameters of tissue found in literature48–50, we estimated the mean temperature rise to be of the order of 0.06–0.25 °C, using illumination pulses of 50 ms in the range of 0.04–0.6 mW mm 2 and a 10-mm diameter spot (Picot et al., private communication). Using 10 spots of the same size distributed randomly over a FOE of 100 100 mm2 would generate a mean temperature rise of 0.9–3.7 °C. Diffusion over the tissue volume enables the temperature to return to its equilibrium value in less than 150 ms, which was the interval between illumination pulses (Picot et al., private communication). Discussion We have demonstrated a unique optical system enabling remote axial displacement of temporally focused holographic patterns, as well as generation of multiple temporally focused holographic targets occupying separate axial planes. In our two-step system, the first SLM is addressed with phase holograms controlling the transverse target light distribution. The second SLM, positioned after the TF grating, is addressed with Fresnel lens-phase functions and controls target axial position. We demonstrated that this configuration can jointly translate single or multiple spatiotemporally focused patterns across the sample volume. We demonstrated axial displacement of a single temporally focused holographic pattern across an axial range (±300 mm), roughly two times greater than the nominal accessible axial FOE, FOEz (±130 mm). For axial shifts of jDzj FOEZ =2, spot shape and axial resolution (5–10 mm) were well conserved, while fluorescence intensity decreased approximately fivefold NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 d z = 3 μm z y x x c 3D-CGH-TF z = 18 μm z = –12 μm z = 1 μm z = 9 μm z Fluorescence intensity 2D-CGH-TF Fluorescence intensity b 1.0 1.0 Fluorescence intensity 3D-CGH-TF z = –18 μm 1.0 Fluorescence intensity a 1.0 0.5 0.0 –50 50 –25 0 25 Axial position (μm) 50 –25 0 25 Axial position (μm) 50 0.5 0.0 –50 0.5 0.0 –50 y x –25 0 25 Axial position (μm) x e 3D-CGH-TF z = –34 μm 84 (μm) z = 50 μm Δz = 84 μm z y x x 0.5 0.0 –100 –50 0 50 Axial position (μm) 100 Figure 5 | 3D simultaneous 2P photoconversion of Kaede in vivo. (a) Merged brightfield and widefield fluorescence images of a double transgenic Tg(HuC:gal4; UAS:kaede) zebrafish larvae. Red and blue squares represent the approximate areas where we performed photoconversion. Scale bar, 400 mm. (b) Left, overlaid green and red HiLo fluorescence images before and after photoconversion, respectively. Right, orthogonal maximum red fluorescence intensity projection showing 14 photoconverted neurons on a single axial plane (illumination density 0.4 mW mm 2, 200 pulses of 50 ms). Scale bars, 60 mm. (c) Orthogonal maximum fluorescence intensity projection of overlaid HiLo pre- and post-photoconversion images (green and red fluorescence, respectively). Three single cells were photoconverted on separated axial planes (4.0 mW mm 2, one pulse of 200 ms). Scale bar, 60 mm. (d) Simultaneous 3D photoconversion of neural ensembles in the spinal cord. Left, overlaid HiLo pre- and post-photoconversion fluorescence images, where three 35-mm-diameter holographic spots projected at z ¼ 18 mm, 3 and 18 mm were used for photoconversion (0.03 mW mm 2, 2,000 pulses of 50 ms). Right, axial distributions of green pre- and red post-photoconversion integrated fluorescence intensity over z for the spots projected at the three different planes. Scale bars, 60 mm. (e) Simultaneous 3D photoconversion of neural ensembles in the zebrafish brain. Left, overlaid 2P-excited green- and red post-photoconversion fluorescence images, where two 35-mm-diameter holographic spots projected at z ¼ 38 mm and 50 mm were used for photoconversion (0.11 mW mm 2, 9,000 pulses of 50 ms). Scale bar, 20 mm. Middle, Orthogonal maximum 2P-excited fluorescence intensity projection of overlaid green and red-post-photoconversion images. Scale bar, 20 mm. Right, axial distributions of green pre- and red post-photoconversion integrated 2P fluorescence intensity over z for the spot at z ¼ 38 mm (solid lines) and the one at z ¼ 50 mm (dotted lines). z-values in all cases are given as distances from the focal plane of the objective, which for the spinal cord experiments was at B60 mm and for the brain at B90 mm from the fish surface (where green fluorescence was starting). Positive z-values are closer to the surface. lphot ¼ 800 nm. (consistent with the roughly twofold decrease in illumination intensity, Supplementary Fig. 3). Displacements up to twice the accessible axial FOE were also possible, although requiring compensation mechanisms to correct for broadening of axial confinement (B25 mm for Dz ¼ ±300 mm) and intensity losses. With our approach, applications requiring continuous fast scanning of a single spatiotemporally focused target will be limited by the SLM refresh rate (60–200 Hz). Replacing SLM2 with a tunable lens51 would overcome this limitation. However, it would not enable generation of multiple spatiotemporally focused targets at distinct axial planes. Alternative solutions for axial displacement of a temporally focused shape used either variable group velocity dispersion (GVD)38,52–55 or mechanical axial translation of the TF grating35. Indeed, introducing variable GVD to the input laser pulse enabled efficient axial displacement of a Gaussian beam’s temporal focal NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications 7 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 plane. However, GVD-induced axial displacement strongly depends on the autocorrelation width of the illumination patterns, which for holographic spots is small due to speckles. Therefore, in order to achieve axial displacement of temporally focused holographic patterns, spatial filtering is required to increase the correlation width. However, this degrades spatial resolution and decreases illumination intensity38. Furthermore, the GVD approach achieved axial displacements of only a few microns38. Greater scanning ranges could be obtained by combining 2D-CGH with the optical design proposed by Dana et al.35 for axial scanning of a temporally focused line. The system implemented on-axis-light propagation and mechanical axial translation of the TF grating. However, for high-magnification (M) objectives, the axial shift, d, was limited by the long required grating translation, Dpd M2. Thus, the maximum shift achieved with a 40 objective was inferior to 30 mm. In addition to limited range, neither GVD shift nor grating translation enable the generation of multiple temporally focused targets at distinct axial planes. Here we overcome this limitation with a two-step, two-SLM system, addressing a first SLM with multiple vertically tiled phase holograms, each encoding the light distribution of a single plane, and a second SLM with an equal number of Fresnel lens phases, which individually control the axial position for each plane. In this way, all targets are projected to the TF plane, which is kept at a fixed position, while the second SLM imposes the spatial wavefront curvature needed to displace each plane axially. The number of spatiotemporal focal planes that can be generated with this design is a tradeoff between the number of available pixels and spatial resolution. Here we demonstrated generation of up to four spatiotemporal focal planes. In this case, the vertical spatial resolution (that is, the average speckle size in the y axis), where tiling occurs, decreases roughly twofold because of the reduced size of the hologram at the objective back aperture. LCOS-SLM devices with increased number of pixels should increase the number of achievable planes and reduce hologram resolution deterioration. Moreover, we implemented a simpler single-SLM system to generate multiplane CGH light patterns without TF. Although this approach has a poor axial confinement with respect to 3D-CGH-TF, it could still be a powerful method for applications using sparsely expressing samples. Previous schemes for multiplane generation of laterally extended shapes through high-NA objectives reported patterns spanning a limited (tens of microns) axial range27,30,31 and used linear excitation. Here we exploited the whole excitation volume reachable with the LCOS-SLM using high-NA objectives. This was achieved by minimizing spherical aberration of targets generated out of the nominal objective focal plane by accounting for the high-NA objective in the expression of the Fresnel lens-phase profile. In addition, throughout the accessible volume, we obtained uniform light distribution among the target shapes. Weighting the amplitude of each target input to the GS algorithm according to position enabled compensation of intensity loss because of diffraction efficiency, which decreases with increasing distance from the excitation volume centre. We demonstrated the capabilities of the 3D-CGH-TF system by performing multiplane 2P photoconversion of Kaedeexpressing neurons. Previous in vivo experiments photoconverting Kaede in single neurons of zebrafish larvae used one-photon visible light illumination43,56,57. Here we demonstrated 2P-mediated single-cell resolution simultaneous photoconversion of multiple Kaede-expressing neurons. The unprecedented spatial precision achieved by our approach can, in the future, be extended to track the morphology of single neurons across the entire nervous system of the zebrafish larva. 8 For multiplane photoconversion of multiple isolated targets (for instance, in sparsely expressing samples), 3D-CGH holds an advantage over 3D-CGH-TF by enabling light shaping in a greater number of planes. However, 3D-CGH-TF enables a better axial confinement, a key parameter for experiments requiring illumination of spatially nearby multiple targets or large areas. Moreover, generation of multiplane illumination patterns with 3D-CGH also produces spurious light in the intermediate planes absent in illumination geometries produced with 3D-CGH-TF. In addition, we have previously demonstrated that TF is particularly robust to scattering making 3D-CGH-TF more suitable than 3D-CGH for applications requiring in-depth illumination through scattering media23,24. The same optical design could be used to photoswitch other proteins such as photactivatable green fluorescent protein58 or kindling fluorescent protein (KFP1)59 to precisely track the 3D position of specific cells in vivo during embryo development. Combined with optogenetics (Supplementary Note 3) or uncaging, multiplane generation of spatiotemporally focused patterns will enable simultaneous control of neurons and substructures in different planes, as well as provide a flexible mean to stimulate locations lying above or below the imaging plane. Combined with extended depth-of-field imaging60, multiplane light patterning could also improve the spatial specificity of functional voltage or calcium physiology by shaping light on cells or structures of interest61. In this study, the two-SLM system is combined with HiLo imaging. Combining this approach with 2P imaging would also enable deep photostimulation and imaging in vivo. Decoupling of lateral and axial wavefront shaping could also be adopted in optical designs different from the one presented here. For example, placing a second SLM at the Fourier plane of a fast switchable array should enable 3D-encoded multisite 2P microscopy62 or high-speed 3D holographic light patterning63. Methods Two-SLM optical set-up. The optical system, schematically depicted in Fig. 1 and Supplementary Fig. 11, was built around a commercial Olympus IX71 inverted microscope, modified in order to accommodate two opposite-facing objectives, OBJ1 and OBJ2, for excitation and fluorescence collection, respectively. To this end, the condenser lens of the microscope was substituted with a dielectric mirror and an Olympus LUMPLFL60xW/IR2, NA 0.90 objective (OBJ1). The expanded ( 10) beam of a Ti:Sapphire laser (MaiTai Deep-See, Spectra-Physics) covered the active area of a first LCOS-SLM (X10468-07, Hamamatsu Photonics; SLM1), which modulated the phase of the incoming beam to create a first image of the desired intensity pattern on the diffraction grating (830 l/mm, 53004ZD02-035R, Richardson Gratings; G) for TF through the lens L1 (f1 ¼ 500 mm). 2D-phase holograms were calculated using a standard GS algorithm20,33. The first diffraction order was subsequently collimated by lens L2 (f2 ¼ 500 mm) and impinged on a second SLM (X10468-07, Hamamatsu Photonics; SLM2), which was imaged at the back focal plane of the excitation objective, OBJ1, via a 2:1 telescope (lenses L3, f3 ¼ 1,000 mm and L4, f4 ¼ 500 mm). Suppression of the zero-order spot arising from SLM1 was achieved by using two cylindrical lenses (fL1 ¼ 1,000 mm and fL2 ¼ 1,000 mm) oriented at þ 45° and 45° with respect to the grating lines64. Holographic light patterns generated at the sample volume illuminated a thin spin-coated fluorescent layer of rhodamine-6G in polymethyl methacrylate 2% w/v in chloroform and the induced fluorescence was imaged on a CCD camera (CoolSNAP HQ2, Roper Scientific) through OBJ2 (Olympus UPLSAPO60XW, NA 1.2). For 3D reconstruction of illumination volumes, OBJ2 was fixed and focused on the fluorescent layer, while OBJ1 was moved along the axial direction with a piezo positioner of 1 mm range when working in closed loop (PI N-725.2A PIFOC). The two SLMs, the CCD camera, the piezo positioner, lasers and other electronic components of the set-up were controlled by a custom-developed interface in LabVIEW. GS-based algorithms were run in MATLAB. When the set-up was used for generation of multiplane holographic patterns (not temporally focused) a mirror replaced the diffraction grating, and SLM2 was either used in reflectance mode by applying only the flatness correction phase mask of the device or was replaced by a mirror. Both the grating and SLM2 were mounted on magnetic bases enabling fast switching between the different configurations. The multiplane GS algorithm used in this case was run in a custom-designed C þ þ software interface, Wavefront Designer20. NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 Two-colour HiLo imaging system. High-resolution multiplane fluorescence imaging of zebrafish larvae was achieved by coupling an optical set-up for two-colour HiLo microscopy46 to the Olympus IX71 microscope. Two continuous wave 473-nm (Laser Quantum, Ciel 350 mW) and 561-nm (CNI laser, MGL-N-561-500 mW) lasers were co-aligned in the same optical path with the dichroic mirror D2 (Semrock, Di02-R514) and collimated with lenses L6 (f6 ¼ 125 mm), L7 (f7 ¼ 150 mm) and L10 (f10 ¼ 35 mm) to illuminate an oscillating diffuser plate (Optotune LSR-3005-10) that was imaged through lenses L8 (f8 ¼ 75 mm) and L9 (f9 ¼ 200 mm) at the back aperture of the excitation objective, OBJ1 (Supplementary Fig. 11). The D1 dichroic mirror reflected the collected fluorescence to a CMOS camera (Hamamatsu Photonics, Orca Flash 4.0-V2) through the appropriate filter cube (FC2) for green (dichroic mirror Semrock FF495-Di02, emission filter Semrock FF01-520/35-25) or red fluorescence (dichroic mirror Semrock Di02-R561, emission filter Semrock FF595-Di02). The sectioned image was computed with custom scripts written in MATLAB65. The cutoff frequency used to merge the low- and high-frequency components was chosen such as kcE0.1 klow, where klow is the frequency of the low-pass filter applied to the uniform illumination image. With those parameters, we measured an axial resolution of 3.2-mm FWHM for the emitted fluorescence (Supplementary Fig. 11b). The axial resolution was measured using a Rhodamine-6G thin layer. First, we recorded a z-stack with uniform illumination (oscillating diffuser on) and then with speckle illumination (oscillating diffuser off). The two stacks were then processed in MATLAB to generate an axially resolved HiLo z-stack using algorithms previously described34,65. The axial resolution shown in Supplementary Fig. 11 is the axial resolution measured on the processed HiLo z-stack. 2P galvo-based scanning imaging system. 2P imaging of photoconverted zebrafish larvae in the brain performed by a mode-locked Ti-Sapphire laser source (Coherent Chameleon Vision II, pulse width 140 fs, tuning range 680–1,080 nm). The femtosecond pulsed beam was raster-scanned on the sample via a pair of xy galvanometric mirrors (3 mm aperture, 6215H series, Cambridge Technology) imaged at the back aperture of the microscope objective ( 40 W APO NIR, Nikon) through an afocal telescope (scan lens: f ¼ 100 mm, tube lens: f ¼ 300 mm). Galvanometric mirrors were driven by two servo drivers (MicroMax series 671, Cambridge Technology) controlled by a Digital/Analog converter board (PCI-6110, National Instrument). Emitted fluorescence was collected by a fibrecoupled detection scheme66. The fibre exit was imaged on two photomultiplier tubes GaAsP (H10770-40 SEL, Hamamatsu Photonics, active area 5 mm) by a set of three matching asphere lenses (f ¼ 23.5 mm, Melles Griot #LAG-32.5-23.5-C). Following the fibre exit, fluorescence light was filtered with an infrared-lightblocking filter (FF01-750sp, Semrock), split into two channels by a dichroic mirror (FF555-Di03, Semrock) and detected through two emission filters (FF01-510/84 and FF02-617/73, Semrock). The whole system was built around a commercial upright microscope (SliceScope, Scientifica). 2P imaging laser power was tuned by combining an electrically controlled liquid crystal variable phase retarder (LRC-200-IR1, Meadowlark Optics) and a polarizer cube (BB-050-IR1, Meadowlark Optics) at the exit of the laser source. Green and red fluorescence z-stacks of photoconverted Kaede in the zebrafish brain were acquired by scanning the excitation beam (780 nm) at 0.74 Hz (full frame) and averaging 10–20 frames for each plane. Photoconversion protocol. First, a HiLo z-stack in the green channel (200 200 100 mm3) was recorded to map the location of neuronal cells for photoconversion. On the basis of these images, we calculated phase holograms that produced the corresponding 2D or 3D illumination patterns. We typically used 5mm-diameter holographic spots to target single cells and 30–35-mm-diameter holographic spots to target sets of neurons. In order to quantify the efficiency of photoconversion, we also recorded the corresponding z-stack in the red channel before photoconversion. Simultaneous 2P photoconversion (lphot ¼ 800 nm) of all targets was performed while monitoring the fluorescence in the red channel. We typically observed a tenfold increase of red fluorescence in the targeted cells. Photoconversion during fluorescence imaging was minimized by keeping the total acquisition time below 2 min and laser power at the sample plane below 20 mW. To minimize thermal damage during photoconversion, we delivered trains of 50-ms pulses, low laser intensity B0.04–4.0 mW mm 2 (power densities are always given relatively to the area of the spots’ surface) for periods of time that ranged from 200 ms to a few hundred seconds depending on the laser intensity. Transgenic lines. Experiments were performed on Danio rerio larvae between 2 and 6 days post fertilization following procedures approved by the Institutional Ethics Committee Darwin in the ‘Institut du Cerveau et de la Moelle épinière’ (ICM). AB and TL strains of wild-type (WT) larvae were obtained from laboratory’s stock of adults. Embryos and larvae were raised in an incubator at 28.5 °C until shortly before recordings were performed. For photoconversion experiments, we used Tg(HuC:gal4; UAS:kaede)43 where the HuC promoter drives pan-neuronal expression of Gal4 and Kaede at the larval stage. Tg(pkd2l1:gal4; UAS:ChR2-H134R-mCherry; UAS:GCaMP5G)65,67 were used for combination of optogenetics and calcium imaging in Supplementary Data. Before performing image acquisitions, embryos were dechorionated and screened for fluorescence at 1 days post fertilization. Larvae screened for Kaede fluorescence were later embedded laterally in 1.5% agarose. Larvae were anaesthetized in 0.02% tricain (MS-222, Sigma-Aldrich, USA). Data availability. The data that support the findings of this study are available from the corresponding author upon request. References 1. Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990). 2. Salomé, R. et al. Ultrafast random-access scanning in two-photon microscopy using acousto-optic deflectors. J. Neurosci. Methods 154, 161–174 (2006). 3. Reddy, G. D., Kelleher, K., Fink, R. & Saggau, P. Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity. Nat. Neurosci. 11, 713–720 (2008). 4. Grewe, B. F., Langer, D., Kasper, H., Kampa, B. M. & Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with nearmillisecond precision. Nat. Methods 7, 399–405 (2010). 5. Nguyen, Q. T., Callamaras, N., Hsieh, C. & Parker, I. Construction of a two-photon microscope for video-rate Ca2 þ imaging. Cell Calcium 30, 383–393 (2001). 6. Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005). 7. Zhu, G., van Howe, J., Durst, M., Zipfel, W. & Xu, C. Simultaneous spatial and temporal focusing of femtosecond pulses. Opt. Express 13, 2153–2159 (2005). 8. Akturk, S., Gu, X., Bowlan, P. & Trebino, R. Spatio-temporal couplings in ultrashort laser pulses. J. Opt. 12, 093001 (2010). 9. Tal, E., Oron, D. & Silberberg, Y. Improved depth resolution in video-rate line-scanning multiphoton microscopy using temporal focusing. Opt. Lett. 30, 1686–1688 (2005). 10. Vaziri, A. & Shank, C. V. Ultrafast widefield optical sectioning microscopy by multifocal temporal focusing. Opt. Express 18, 19645–19655 (2010). 11. Isobe, K. et al. Enhancement of lateral resolution and optical sectioning capability of two-photon fluorescence microscopy by combining temporalfocusing with structured illumination. Biomed. Opt. Express 4, 2396–2410 (2013). 12. Choi, H. et al. Improvement of axial resolution and contrast in temporally focused widefield two-photon microscopy with structured light illumination. Biomed. Opt. Express 4, 995–1005 (2013). 13. Song, Q. et al. Two-dimensional spatiotemporal focusing of femtosecond pulses and its applications in microscopy. Rev. Sci. Instrum. 86, 083701 (2015). 14. Losonczy, A., Zemelman, B. V, Vaziri, A. & Magee, J. C. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 13, 967–972 (2010). 15. Dana, H. et al. Hybrid multiphoton volumetric functional imaging of large-scale bioengineered neuronal networks. Nat. Commun. 5, 3997 (2014). 16. Vaziri, A., Tang, J., Shroff, H. & Shank, C. V. Multilayer three-dimensional super resolution imaging of thick biological samples. Proc. Natl Acad. Sci. USA 105, 20221–20226 (2008). 17. Kim, D. & So, P. T. C. High-throughput three-dimensional lithographic microfabrication. Opt. Lett. 35, 1602–1604 (2010). 18. Andrasfalvy, B. K., Zemelman, B. V, Tang, J. & Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl. Acad. Sci. USA 107, 11981–11986 (2010). 19. Rickgauer, J. P., Deisseroth, K. & Tank, D. W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 17, 1816–1824 (2014). 20. Lutz, C. et al. Holographic photolysis of caged neurotransmitters. Nat. Methods 5, 821–827 (2008). 21. Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008). 22. Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010). 23. Bègue, A. et al. Two-photon excitation in scattering media by spatiotemporally shaped beams and their application in optogenetic stimulation. Biomed. Opt. Express 4, 2869–2879 (2013). 24. Papagiakoumou, E. et al. Functional patterned multiphoton excitation deep inside scattering tissue. Nat. Photon. 7, 274–278 (2013). 25. Piestun, R., Spekor, B. & Shamir, J. Wave fields in three dimensions: analysis and synthesis. J. Opt. Soc. Am. A 13, 1837–1848 (1996). 26. Curtis, J. E., Koss, B. A. & Grier, D. G. Dynamic holographic optical tweezers. Opt. Commun. 207, 169–175 (2002). 27. Sinclair, G. et al. Interactive application in holographic optical tweezers of a multi-plane Gerchberg-Saxton algorithm for three-dimensional light shaping. Opt. Express 12, 1665–1670 (2004). NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications 9 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms11928 28. Yang, S. et al. Three-dimensional holographic photostimulation of the dendritic arbor. J. Neural Eng. 8, 46002 (2011). 29. Anselmi, F., Ventalon, C., Bègue, A., Ogden, D. & Emiliani, V. Threedimensional imaging and photostimulation by remote-focusing and holographic light patterning. Proc. Natl Acad. Sci. USA 108, 19504–19509 (2011). 30. Hilario, P. L., Villangca, M. J. & Tapang, G. Independent light fields generated using a phase-only spatial light modulator. Opt. Lett. 39, 2036–2039 (2014). 31. Ma, B. et al. Generation of three-dimensional optical structures by dynamic holograms displayed on a twisted nematic liquid crystal display. Appl. Phys. B 110, 531–537 (2013). 32. Emiliani, V. et al. Wave front engineering for living cells microscopy. Opt. Express 13, 1395–1405 (2005). 33. Gerchberg, R. W. & Saxton, W. O. A pratical algorithm for the determination of the phase from image and diffraction pictures. Optik (Stuttg) 35, 237–246 (1972). 34. Zahid, M. et al. Holographic photolysis for multiple cell stimulation in mouse hippocampal slices. PLoS ONE 5, e9431 (2010). 35. Dana, H. & Shoham, S. Remotely scanned multiphoton temporal focusing by axial grism scanning. Opt. Lett. 37, 2913–2915 (2012). 36. Richards, B. & Wolf, E. Electromagnetic diffraction in optical systems. II. Structure of the image field in an aplanatic system. Proc. R Soc. Lond. A 253, 358–379 (1959). 37. Botcherby, E. J., Juskaitis, R., Booth, M. J. & Wilson, T. Aberration-free optical refocusing in high numerical aperture microscopy. Opt. Lett. 32, 2007–2009 (2007). 38. Leshem, B., Hernandez, O., Papagiakoumou, E., Emiliani, V. & Oron, D. When can temporally focused excitation be axially shifted by dispersion? Opt. Express 22, 7087–7098 (2014). 39. Dainty, J. C. Laser Speckle and Related Phenomena. Topics in Applied Physics 9 (Springer-Verlag, 1975). 40. Matar, S., Golan, L. & Shoham, S. Reduction of two-photon holographic speckle using shift-averaging. Opt. Express 19, 25891–25899 (2011). 41. Haist, T., Schönleber, M. & Tiziani, H. Computer-generated holograms from 3D-objects written on twisted-nematic liquid crystal displays. Opt. Commun. 140, 299–308 (1997). 42. Leach, J. et al. Interactive approach to optical tweezers control. Appl. Opt. 45, 897–903 (2006). 43. Scott, E. K. et al. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4, 323–326 (2007). 44. Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl Acad. Sci. USA 99, 12651–12656 (2002). 45. Isobe, K. et al. Measurement of two-photon excitation spectrum used to photoconvert a fluorescent protein (Kaede) by nonlinear Fourier-transform spectroscopy. Biomed. Opt. Express 1, 687–693 (2010). 46. Lim, D., Chu, K. K. & Mertz, J. Wide-field fluorescence sectioning with hybrid speckle and uniform-illumination microscopy. Opt. Lett. 33, 1819–1821 (2008). 47. Carslaw, H. S. & Jaeger, J. C. Conduction of Heat in Solids (Clarendon Press, 1959). 48. Elwassif, M. M., Kong, Q., Vazquez, M. & Bikson, M. Bio-heat transfer model of deep brain stimulation induced temperature changes. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1, 3580–3583 (2006). 49. Johansson, J. D. Spectroscopic method for determination of the absorption coefficient in brain tissue. J. Biomed. Opt. 15, 057005 (2010). 50. Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011). 51. Grewe, B. F., Voigt, F. F., van ’t Hoff, M. & Helmchen, F. Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens. Biomed. Opt. Express 2, 2035–2046 (2011). 52. Durst, M. E., Zhu, G. & Xu, C. Simultaneous spatial and temporal focusing for axial scanning. Opt. Express 14, 12243–12254 (2006). 53. Durst, M. E., Zhu, G. & Xu, C. Simultaneous spatial and temporal focusing in nonlinear microscopy. Opt. Commun. 281, 1796–1805 (2008). 54. Suchowski, H., Oron, D., Silberberg, Y., Suchowski Oron, D. & Silberberg, Y., H. Generation of a dark nonlinear focus by spatio-temporal coherent control. Opt. Commun. 264, 482–487 (2006). 55. Du, R. et al. Analysis of fast axial scanning scheme using temporal focusing with acousto-optic deflectors. J. Mod. Opt. 56, 81–84 (2009). 56. Hatta, K., Tsujii, H. & Omura, T. Cell tracking using a photoconvertible fluorescent protein. Nat. Protoc. 1, 960–967 (2006). 10 57. Warp, E. et al. Emergence of patterned activity in the developing zebrafish spinal cord. Curr. Biol. 22, 93–102 (2012). 58. Patterson, G. H. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002). 59. Chudakov, D. M. et al. Kindling fluorescent proteins for precise in vivo photolabeling. Nat. Biotechnol. 21, 191–194 (2003). 60. Quirin, S., Peterka, D. S. & Yuste, R. Instantaneous three-dimensional sensing using spatial light modulator illumination with extended depth of field imaging. Opt. Express 21, 16007 (2013). 61. Foust, A. J., Zampini, V., Tanese, D., Papagiakoumou, E. & Emiliani, V. Computer-generated holography enhances voltage dye fluorescence discrimination in adjacent neuronal structures. Neurophotonics 2, 021007 (2015). 62. Ducros, M., Goulam Houssen, Y., Bradley, J., de Sars, V. & Charpak, S. Encoded multisite two-photon microscopy. Proc. Natl Acad. Sci. USA 110, 13138–13143 (2013). 63. Go, M. A. et al. Four-dimensional multi-site photolysis of caged neurotransmitters. Front. Cell. Neurosci. 7, 231 (2013). 64. Hernandez, O., Guillon, M., Papagiakoumou, E. & Emiliani, V. Zero-order suppression for two-photon holographic excitation. Opt. Lett. 39, 5953–5956 (2014). 65. Lauterbach, M. A., Ronzitti, E., Sternberg, J. R., Wyart, C. & Emiliani, V. Fast calcium imaging with optical sectioning via HiLo microscopy. PLoS ONE 10, e0143681 (2015). 66. Ducros, M. et al. Efficient large core fiber-based detection for multi-channel two-photon fluorescence microscopy and spectral unmixing. J. Neurosci. Methods 198, 172–180 (2011). 67. Fidelin, K. et al. State-dependent modulation of locomotion by GABAergic spinal sensory neurons. Curr. Biol. 25, 3035–3047 (2015). Acknowledgements We thank Jenna Sternberg for help with the preparation of the biological samples, Marc Guillon for helpful discussion on the implementation of the multiplane GS algorithm, Marcel Lauterbach and Emiliano Ronzitti for discussions on the design of the HiLo set-up, Emiliano Ronzitti and Amanda Foust for building the 2P imaging system, Benoı̂t Forget and Alexis Picot for useful discussions on heating effects, Vincent de Sars for the continued development of ‘Wavefront Designer’ software and Amanda Foust for critical reading of the manuscript. V.E. and E.P. acknowledge the ‘Agence Nationale de la Recherche’ (grants ANR-12-BSV5-0011-01, Neurholog and ANR-15-CE19-0001-01, 3DHoloPAc), VE acknowledges the National Institutes of Health (NIH 1-U01-NS090501-01). O.H. acknowledges the programme ‘Nanotechnologies France-Israel’ for financial support. C.W. and K.F. acknowledge the City of Paris Emergence programme, the Human Frontier Science Program (HFSP) Research Grant #RGP0063/2014 and the European Research Council (ERC) starter grant ‘OptoLoco’ #311673. Author contributions O.H., E.P. and V.E. designed the experiments. O.H. built up the optical system, performed the experiments and analysed the data. E.P. participated in the development of the optical system and experiments. D.T. participated in 2P imaging experiments of photoconverted Kaede. K.F. prepared zebrafish samples. C.W. helped in designing the zebrafish experiments. E.P. and V.E. wrote the manuscript, with contributions from O.H. and C.W. V.E. supervised the project. Additional information Supplementary Information accompanies this paper at http://www.nature.com/ naturecommunications Competing financial interests: The authors declare no competing financial interests. Reprints and permission information is available online at http://npg.nature.com/ reprintsandpermissions. How to cite this article: Hernandez, O. et al. Three-dimensional spatiotemporal focusing of holographic patterns. Nat. Commun. 7:11928 doi: 10.1038/ncomms11928 (2016). This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ NATURE COMMUNICATIONS | 7:11928 | DOI: 10.1038/ncomms11928 | www.nature.com/naturecommunications