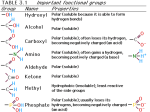
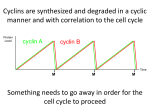
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Translation of Cyclin mRNA Is Necessary for Extracts of Activated
Paracrine signalling wikipedia , lookup
Signal transduction wikipedia , lookup
Genomic library wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Biochemistry wikipedia , lookup
Genetic code wikipedia , lookup
Expression vector wikipedia , lookup
Polyadenylation wikipedia , lookup
Non-coding DNA wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Gene regulatory network wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Messenger RNA wikipedia , lookup
Biosynthesis wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Proteolysis wikipedia , lookup
Point mutation wikipedia , lookup
Epitranscriptome wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Gene expression wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Cell, Vol. 56, 947-956, March 24, 1989, Copyright 0 1989 by Cell Press Translation of Cyclin mRNA Is Necessary for Extracts of Activated Xenopus Eggs to Enter Mitosis Jeremy Minshull,’ J. Julian Blow,t* and Tim Hunt’ Department of Biochemistry University of Cambridge Tennis Court Road Cambridge CB2 1QW England tCRC Molecular Embryology Group Department of Zoology University of Cambridge Downing Street Cambridge CB2 1QW England l The cyclins are a family of proteins encoded by maternal mRNA. Cyclin polypeptides accumulate during interphase and are destroyed during mitosis at about the time of entry into anaphase. We show here that Xenopus oocytes contain mRNAs encoding two cyclins that an? major translation products in a cell-free extract from activated eggs. Cutting these mRNAs with antisense oligonucleotides and endogenous RNAase H blocks entry into mitosis in a cell-free egg extract. The extracts can enter mitosis if either of the cyclin mRNAs is left intact. We conclude that the synthesis of these cyclins is necessary for mitotic cell cycles in cleaving Xenopus embryos. Cyclins are proteins that accumulate steadily during interphase and are destroyed at the end of mitosis (Evans et al., 1983; Swenson et al., 1986; Standart et al., 1987). They were first recognized in marine invertebrate eggs as major translation products of maternal mRNA, but up to now have not been described in vertebrates. However, almost all eukaryotic cells require protein synthesis to pass from interphase into mitosis or meiosis (Hultin, 1961; Kishimoto and Lieberman, 1964; Dettlaff, 1966; Wilt et al., 1967; Wasserman and Masui, 1975; Wagenaar, 1983; Gerhart et al., 1984; Picard et al., 1985). This protein synthesis requirement could easily be explained if there were proteins that were required for entry into M phase and destroyed as a result of it. Since cyclins behave in this way, we set out to look for them in Xenopus eggs, aiming to exploit the experimental advantages and the wealth of previous work on the cell cycle dynamics of maturationpromoting factor (MPF) in this system, as reviewed briefly below. MPF is generally agreed to be the cytoplasmic agent responsible for the initiation of M phase (for review see Masui and Shibuya, 1987). MPF was originally identified t Present address: Microbiology Unit, Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, England. in unfertilized amphibian eggs by Masui and Markert (1971) and Smith and Ecker (1971) as an activity capable of inducing amphibian oocytes to enter meiosis. It has subsequently been identified in a wide variety of meiotic and mitotic cells from yeast to man (Kishimoto and Kanatani, 1976; Wasserman and Smith, 1978; Sunkara et al., 1979; Kishimoto et al., 1982; Weintraub et al., 1982; Gerhart et al., 1984; Meijer and Guerrier, 1984). MPF can also drive GP-arrested cells into mitosis (Miake-Lye et al., 1983; Newport and Kirschner, 1984; Kirschner et al., 1985). Thus, MPF is an almost universal concomitant of M phase, and is probably responsible for catalyzing the G2-M transition in all eukaryotic cells. MPF has been purified from Xenopus eggs by Lohka et al. (1988) and from starfish oocytes by Labbe et al. (1988a) and by Arion et al. (1988). All these preparations contain a 32-34 kd subunit, which by two independent criteria corresponds to the product of the Schizosaccharomyces pombe c&2+ gene (Dunphy et al., 1988; Gautier et al., 1988; Labbe et al., 1988b; Arion et al., 1988). The c&2+ gene encodes a protein kinase that is essential for the G2-M transition in fission yeast (Nurse et al., 1976; Simanis and Nurse, 1986; Lee and Nurse, 1987). Although microinjection of MPF is sufficient to initiate M phase in G2-arrested cells, newly synthesized protein(s) is normally required to activate MPF during the cell cycle (Wasserman and Smith, 1978; Ford et al., 1983; Miake-Lye et al., 1983; Gerhart et al., 1984; Ford, 1985). It is probably not necessary to synthesize MPF itself, because this protein can be detected in interphase cells as an inactive form known as pre-MPF (Cyert and Kirschner, 1988; Dunphy and Newport, 1988). Moreover, the c&2+-homologous subunit of MPF is already present in unfertilized starfish oocytes and is stable during meiosis while MPF activity levels rise and fall (Labbe et al., 1988a). It is thus probable that protein synthesis supplies an MPF activating protein that needs to be synthesized anew in each cell cycle. The behavior of cyclins makes them attractive candidates for MPF activator proteins. Direct evidence for the ability of cyclins to activate MPF has so far been limited to experiments in which frog oocytes were injected with cyclin mRNAs and scored for entry into first meiosis. Thus, synthetic mRNAs encoding clam and sea urchin cyclins are both capable of inducing germinal vesicle breakdown (Swenson et al., 1986; Pines and Hunt, 1987). Experiments of this type do not, however, distinguish between genuine MPF activators and more distal components of the maturation pathway. An alternative approach is to study cell-free extracts that require protein synthesis to enter mitosis and permit more than one round of DNA replication (Lohka and Masui, 1983, 1984; Hutchison et al., 1987; Blow and Laskey, 1988). If it could be shown that cyclin synthesis is necessary and sufficient to promote mitosis in vitro, this would provide strong support for the cyclin-MPF connection. In this paper we report the isolation and sequence of two homologous (but not identical) cyclins from Xenopus Cell 946 Xenopus cyclin Bl I MSLRVTR ~TTCCCACTTAGTGAGACGTCTCTTTCAGACTGGGGGGCTGCAGTGTGACTTGTGGGTACAAATAGTAAAGCTW\CTGCAGGTTTGTCACCAGA NMLANAENNVKTTLAGKRVVATKPGLRPRTALGDIGNKAE AACATGCTGGC~ATGCAGAAAACAATGTGAAAACCACTTTGGCTTTGGCTGGA~GAGGGTTGTTGCTACCA~CCAGGGTT~GACCTCGTA~GCCTTGGGA~~TTGG~AC~GGCA~G VKVPTKKELKPAVKAAKKAKPVDKLLEPLKVIEENVCPKP GTGAAAGTGCCAACAAAAAAGGRATTARAGCCAGCCAGCAGT~~GCTGCC~G~GGC-CCTGTTGAC~ATTGTT~AGCCTCTT~GTGATA~GA~TGTTTGCCCTA~CCT AQVEPSSPSPMETSGCLPDELCQAFSDVLI HVKDVDADDD GCTCAGGTTGAACCCAFCTCACCAAGCCCMTGGAAACATCTGGTTGCCTCCCTGATGAGCTCTGCCAGGCTTTCTCTGATGTCCTCATTCACGTTAAAGATGTTGATGCTGATGATGAT GNPMLCSEYVKDIYAYLRSLEDAQAVRQNYLHGQEVTGNM GGCAACCCRATGCTGTGCAGTGk4TATGTCAAGGACATTTATGCTTACCTGAG~GCCTTGAGGATGCA~AGCAGTCAGACA~ACTACCTTCATGGACAGGAAGTCACAGGCAACATG RAILIDWLVQVQMKFRLLQETMFMTVGI IDRFLQEHPVPK CGTGCCATTTTGATTGACTGGCTGGTCCAGGTGC~TG~TTCCGTCTACTGCAGGAGACAATGTTCATGACTGTTGGCAT~TTGACCGCTTTCTGCAGGAACATCCAGTTCCC~A NQLQLVGVTAMFLAAKYEEMYPPEIGDFTF"TDHTYTKAQ AACCAGCTACAGCTTGTGGGGGTCACGGCTATGTTCCTTGCTGCT~ATA~~~~~~~~~~~~~,~~,~.~.~.~.~~~~~TGGAGACTTTACATTTGTAACTGATCACACATACACAAAGGCTCAA IRDMEMKILRVLKFAIGRPLPLHFLRRASKIGEVTAEQHS ATTCGGGACATGGAAATGAAGATACTTAGGGTGCTAAAGTTTGCAATTGGCCGACCCTTACCCCTGCACTTTCTTCGGAGAGCTTCTAAAATTGGAW\GGTAACTGCTGCTG~CAGCATAGT LAKYLMELVMVDYDMVHFTPSQIAAASSCLSLKI LNAGDW TTAGCCAAATATTTGATGGACTTGTGATGGTGGATTATGATATGGTACATTTCACGCC?TCCC~TAGCAGCTGCTTCCTCCTGCTTGTCTCTC~AATCTT~ATGCAGGTGACTGG TPTLHHYMAYS EEDLVPVMQHMAKNII KVNKGLTKHLTVK ACCCCAACACTCCATCACTATATGGCTTACTCTGAAGAAGATCTAGTCCCTGTTATGCAGCATATGGCCAAGG NKYASSKQMKI STIPQLRSDVVVEMARPLM' AACMGTATGCTAGCAGCAAACRAATGRAGATG~~TCAG~CGATTCCACAGCTGAGGTCAGATGTTGTTGTGGAAATGGCCCGCCCACTCATGTGAAGGACTACGTGGCATTCCAATTGTGTA TTGTTGGCACCATGTGCTTCTGTAAATAGTCTAGTGTT Xenopus cyclin 82 MATRRAAIPREADNILG GAMRSK GAATTCCGGCTAGATTTTATCGGTTGGTTTTA?~CGTTATTTTACCGGAGATGGCTACTCGTCGCGCTGCTATTCCCCGT~GC~AT~TATCCTTGGGGGTGC~TGCGATCCA~G GNKVTVRGKPPAVKQSSNAVAKP VQMNSRRAALGEI SKMA TTCMATGAATAGCAGACGAGCTGCTTTGGGA~GATTGGCAACA~GTGACTGTGCGAGG~AACCACCTGCAGTA~GCAGTCTTC~TGCTGTGGCA~GCCTTC~A~TGGCAG ATKVANVKTKHVPVKPVVAEAAPKVPSPVPMDVSLKEEEL CAACTAAAGTGGCAAATGTTAAGACTAAGCATGTACCTGTGAAACCAGTTGTAGCTGAAGCTGCCCCCAAGTGCCTTCCCCTGTGCCGATGGATGTGTCETTGAAAGAGGAAGAGCTGT CQAFSDALTSVEDIDADDGGNPQLCSDYVMDIYNYLKQLE GCCAGGCATTCTCCGATGCACTGACCAGTGTTGAAGACATTGATGCAGATGATGGTGGAAACCCTCAATG VQQSVHPCYLEGKE INERMRAILVDWLVQVHSRFQLLQET TTCAACAGTCTGTACATCCTTGCTATCTTGAAGGAAAAGATTAATGAGCGTATGAGAGCTATCCTAGTTGACTGGCTTGTTCAAGTGCATTCTAGGTTTCAGCTTCTTCAGGAGACTT LYMGVAIMDRFLQVQPVSRSKLQLVGVTSLLI ASKYEEMY TATACATGGGCGTTGCR9TCATGGATCGCTTCTTACAAGTTCAGCCAGTCTCCCGCAGTAAGCTTCAGTTGGTTGGTGTTACTTCCCTACTAATTGCTTCAAAATATATGAAGAGATGTACA . . . . . *. . . . . . . . . . . . . . . . . . . . . REMEMII TPEVADFVY ITDNAYTASQI LRLLNFDLGRPLP CT,~,C~.~.~~~~TGCAGACTTTGTTTATATCACCGATAAATGATTATCCTTCGACTCCTCAACTTTGACCTTGGACGGCCATTACCTC LHFLRRASKSCSADAEQHT LAKYLMELTLI DYEMVHIKPS TCCACTTCCTCAGACGGGCTTCAAAATCTTGCAGTGCTGATGCAGAGCAACATACCCTTGCAAAATATATTTGATGGAGCTTACACTCATAGACTATG~TGGTCCACATCAAGCCTTCAG EIAAAALCLSQKI LGQGTWGTTQHYYTGYTEGDLQLIMKH AAATTGCAGCTGCTGCCCTCTGCCTATCTCAA~AATTCTTGGCCAGGGAACCTGGGGTACCACTCAGCACTATTACACAGGCTACACAG~GGTGACTTGCAACTGATCATGAAGCATA MAKNI TKVNQNLTKHVAVRNKYASSKLMKISTLPQLMAPL TGGCTAAGAACATAACCAAAGTCAACCAGAATCTAACRAAGCATGTGGCTGTGAGGAACAAGTATGGCACCCTTCCTCAGCTTATGGCTCCTCTAA ITELAASLS* TCACAGAGCTTGCTGCAAGTCTCTCTTAGAACTGTTAAGTTAAGTGACCCTTTCAAAGAGAACCACTAATTGCACTTTTAACGTTGCTGCTGGCA~TGCTGCTGTTCCTGT~ATATGTTTGTA TTTTTATTGACTCATTTGTWACTTTGAGTAATGCTTTTTTTATTT APAAAAAAAACGGAATTC Figure 1. Nucleotide and Derived Amino Acid Sequences of X. laevis Cyclins Bl and 82 The nucleotide sequences and derived amino acid sequences of the LgtiO Xenopus cyclin clones Xlcycl and XlcycP are shown. The sequences begin with an EcoRl linker, and contain the polyadenylation signal AATAAA about 16 nucleotides before a poly(A) tract at the end of the clone. Termination codons are indicated by asterisks; the single TGA marked in the 5’ untranslated leader of cyclin 81 is in the same frame as the coding region. Positions complementary to the cyclin Bl- and BP-specific antisense oligonucleotides are marked with solid underlining, and positions corresponding to the consensus oligonucleotides (antisense #426, sense #120) are marked with dotted underlining. The redundant oligonucleotide used to screen the original Ml3 libraries overlapped and included this region. Sequence data have been submitted to the GenBanWEMBL data library (accession nos. JO3166 and JO3167). oocyte cDNA libraries. We present evidence that translation of these mRNAs is necessary for entry into mitosis. Results Isolation and Sequence of Cyclin cDNA Clones Cyclins were first detected because they are major translation products of maternal mRNA in clams and sea urchins (Evans et al., 1983). Their destruction during mitosis was easily observed owing to the natural division synchrony of fertilized eggs. Similar kinds of experiments in Xenopus did not immediately reveal periodically degraded proteins (data not shown). We therefore decided to look for frog cyclin mRNA in a cDNA library made from fertilized Xenopus egg poly(A)+ RNA: Sau3A cDNA fragments were inserted into an Ml3 phage vector (see Experimental Procedures). The library was screened in parallel with a full-length sea urchin B-type cyclin cDNA clone (cyc4; Pines and Hunt, 1987) and aconsensus oligonucleotide with a sequence based on comparison between the published clam and sea urchin sequences (see Experimental Procedures). Several clones were positive for both probes. The clones fell into two classes, cyclin Bl (represented by clone R3, with 209 nucleotides) and cyclin B2 (clone A22, with 158 nucleotides). Their sequences overlapped by 133 residues and showed 88%~ identity at the nucleotide level. In the region of overlap, 27 out of 44 amino acids were identical in the open reading frame cor- t&ins and Mitosis in Xenopus (4 Frog (X. lawis) cyclin 82 ;;i + 300 200 / 3 g li Clam (S. solidissima) cyclin A Sea urchin (A. punclulala) / s .3 e$ I Eggs / 100 i-7 ' I 0 100 a0 300 400 (N MSQPFALHHDGENNGLQMQRRGKMNTRSNQVSGQKRAALGKNQQVRIQP MALGTRNMNMNLHGESKHTFNNENVSARLG MSLRVTRNMLANAENNVKTTLAGK MATRRAAIPREADNILGGA MTTRRLTRQHLLANTLGNNDENHPSNHIARAKSSLHSSENSLVNGKKATVSSTNVPKKRHALDDBSNFHNKEGVPLASKNTNVRHTTASVS cdcl3+ COIlSi=?KlSUS Clam A Sea urchin Froo Bl Fro; B2 SRAATKKSSEFNIQDENAFSVFNAKTFGQQPSQFPTSVDPTPAAPVQKAQRVHVTDIPAALTTLCLEPLTEVPGSPDIIEEEDSMESPMILDLPPEYKPL GKSIAVQKPAQRAALGNISNVVRTAQAGSKKVVKKDTRQKAMTKTKATSSLHAVVGLPVEDLPTE~STSPDVLDAMEVDQAIEAFSQQLIALQVEDIDK RVVATKPGLRPRTALGDIGNKAEVKVPTKKELKPAVKAAKKAKPVDKLLEPLKVIEENVCP~AQVEPSSPSPMETSGCLPDELCQAFSDVLIHVKDVDA MRSKVQMNSRRAALGEIGNKVTVRGKPPAVKQSSNAVAKPSKMAATKVANVKTKHVPVKPVVAEAAPKVPSPVPSPVPMDVSLKEEELCQAFSDALTSVEDIDA TRRALEEKSIIPATDDEPASKKRRQPSVFNSSVPSLPQHLSTKSHSVSTHGVDAFHKDQATIPKKLKKDVDERVVSKDIPKLHRDSVESPESQDWDDLDA dd DREAVILTVPEYEEDIYNYLRQAEMKNRAKPGYMKRQ-TDITTSMRCILVDWLVEVSEEYKLHRETLFLGVNYIDRFLSKISVLRGKLQLVGAASMFLAA DDGDNPQLCSEYAKDIYLYLRRLEVEMMVPANYLDRQETQITGRMRLILVQFIAS DDDGNPMLCSEYVKDIYAYLRSLEDAQAVRQNYLHGQEV--TGNMRAILIDWLVQVQMKFRLLQETMFMTVGIIDRFLQEHPVPKNQLQLVGVTAMFLAA DDGGNPQLCSDYVMDIYNYLKQLEVQQSVHPCYLEGKEINER--M~ILVDWLVQVHSRFQLLQETLYMGVAIMDRFLQVQPVSRSKLQLVGVTSLLIAS EDWADPLMVSEYVVDIFEYLNELEIETMPSPTYMDROKELAWK-MRGILTDWLIEVHSRFRLLPETLFLAVNIIDRFLSLRVCSLMKLOLVGIAALFIAS d p sY DI YL 1E Y MRILDWL V F Ll ET V DRFL A LoLVG KYEEMYPPEIGDFTFVTDHTYTKA~IR~MEMKILRVLKFAIGRPLPLHFLRRASKIGEVTA~QHSLAKYLMELVMVDYDMV~-FTPSQIAAASSCLSLKI KYEEMYTPEVADFVYITDNAYTASQIREMEMIILRLLNFDLGRPLPLHFLRRASKSCSADAEQHTLAKYLMELTLIDYEMVH-IKPSEIAAAALCLSQKI KYEEVMCPSVQNFVYMADGGYDEEEILQAERYILRVLEFNLAYPNPMNFLRRISKADFYDIQTRTVAKYLVEIGLLDHKLLP-YPPSQQCAAAMYLAREM KYEE P F D L L P Flrr SK PS AA L akyL E LGMEPWPQNLVKKTGYEIGHFVDCLKDLH-----KTSLGAESHQQQAVQEKYKQDKYHQVSDFSKNPVPHNLALLAL LDPETHSSWCPKMTHYSMYSEDHLRPIVQKIVQILLRDDSASQKYSAVKTKYGSSKFMKISGIAQLDSSLLKQIAQGSNE* LNAGDWTP-----------------------AKNIIKVNK* LGQGTWGT-----------------------AKNITKVNQAPLITELAASLS* LGRGPWNRNLVHYSGYEEYQLIS-------VVKKMINYLQYASKKFNKASLFVRDWYKKNSIPLGDDADEDYTFHKQKRIQHDNKDEEW L KYsK kS (W) Figure 2. Comparisons of Frog Cyclins Bl and 62 with Each Other and with Published Cyclin Sequences (A) The DIAGON program of Staden (1982) was used to compare the predicted amino acid sequence of Xenopus cyclin Bl (vertical axis) with the sequences of Xenopus cyclin 82, cyclins from the sea urchin A. punctulata (Pines and Hunt, 1987) and the fission yeast S. pombe (Booher and Beach, 1988) and cyclin A from the surf clam S. solidissima (Swenson et al., 1988). A score of 135 for a window of 11 residues was used. (B) Manual alignment of the predicted amino acid sequences of all the published cyclins. Amino acids that show identity between all five sequences are indicated as uppercase letters in the consensus sequence; lowercase letters show the cyclin B consensus. responding to the cyclin sequence. The region of overlap started with the conserved sequence DRFL and included the central portion of the “cyclin box;’ KYEEMY-PE, as would be expected from the choice of oligonucleotide probe, which corresponded to this portion of the sequence. Both clones contained single inserts flanked by Sau3A recognition sites. These two Ml3 clones were used to screen full-length Xenopus ovary cDNA libraries contained in LgtlO. Positive clones were identified and sequenced as described in Experimental Procedures. Figure 1 shows the sequences of two clones (Xlcycl and XlcycP) that contained the complete coding regions of two highly homologous cyclins. The cyclin Bl clone lacks approximately 30 residues at the 5’ untranslated region, as estimated by primer extension with mRNA; the length of the cyclin 82 leader sequence is not yet known. Neither of the clones contains an upstream AUG, and cyclin Bl contains an upstream termination codon in frame with the AUG preceding the large open reading frame. We cannot rigorously rule out the possibility that translation of cyclin 82 mRNA starts at an AUG upstream of the XlcycP sequence, although, as shown below (Figure 4), the translation product of synthetic mRNA from this clone migrates very close to that of authentic egg mRNA. Cyclin Bl polypeptide contains 398 amino acids and has a predicted molecular weight of 44,674. Subject to the the caveat mentioned above, cyclin 82 has 392 amino acids and a predicted molecular weight of 43,625. The two sequences show great similarity in the C-terminal 300 residues, but, as Figure 2 shows, they diverge considerably at the N terminus, with only small islands of conservation in the first 100 residues. In addition, Cell 950 3 4 Oocytes 5 6 7 8 9101 - 2 3 4 5 6 7 8 91011 Embryos Figure 3. Cyclin mRNA Levels during Xenopus Early Development Oocytes were staged as described by Golden et al. (1980), and embryos according to Nieuwkoop and Faber (1987). RNA was extracted from oocytes or embryos; the RNA equivalent of a single egg was hybridized with an RNA probe for cyclin Bi mRNA and analyzed by RNAase protection. comparison of the sequences of clones obtained from different libraries (from different frog colonies) showed a significant number of nucleotide substitutions. In every case, however, the differences were at third-base positions and did not alter the predicted amino acid sequences. Figure 2A compares the amino acid sequence of frog cyclin Bl with that of frog cyclin 82 and the published sequences of cyclins from the sea urchin Arbacia punctulata and the yeast S. pombe and of cyclin A from the surf clam Spisula solidissima, by means of DIAGON plots (Staden, 1982). In every case the sequences begin to match well only after about 100 residues from the N terminus of the frog polypeptide. The match with clam cyclin A is less good, although it is still impressive between residues 150 and 300. As we shall show elsewhere, the sequences can be classified into A and B types, and the frog cyclins described here belong in the B class together with sea urchin cyclin and S. pombe cdc73+. The manually aligned sequence listings in Figure 28 show that the B-type sequences contain 64 identities in the central 205 residues, of which 51 are shared with the clam A sequence. There are other short islands of homology in both ends of the molecule, but their spacing is variable with respect to the central conserved portion, and they are not present in all the B-type cyclin molecules. Cyclin Bl mFtNA Appears Early in Oogenesis and Persists Well after the Midblastula Transition Figure 3 shows the levels of cyclin Bl mRNA during oogenesis and early development of X. laevis. The mRNA is present in stage 3 oocytes (Golden et al., 1980) and persists at least to stage 11 embryos, well after the midblastula transition (Nieuwkoop and Faber, 1967) though the level declines slightly. We have not yet checked whether cyclin 82 mRNA shows a similar pattern. There are approximately 5 x 10’ copies of each cyclin mRNA in unfertilized eggs as measured by RNAase protection mapping compared with known amounts of synthetic cyclin transcript (data not shown). Cyclln 81 and 82 An? Major Translation Products of a Xenopus Egg Cell-Free System The two Xenopus cyclin clones were subcloned into pGEM vectors in order to make mRNA in vitro with T7 RNA cyclin cyclrn 82 ~ Bl i ABCDEFGHIJ Figure 4. Identification of Xenopus Cyclins in the Cell-Free Extract by Antisense Ablation An activated egg extract was incubated with sperm nuclei. Oligonucleotides were added at time zero, and [35S]methionine at 30 min. Samples (2 al) were taken into sample buffer (28 al) at 120 (lanes A, D, F, I) and 150 min (lanes B, E, G, J). Lanes A and 8, no oligonucleotide; lanes D and E, anti-cyclin 81 oligonucleotide (20 nglml); lanes F and G, anti-cyclin 82 oligonucleotide (20 pglml); lanes I and J, both anticyclin oligonucleotides (each at 10 ug/ml). Synthetic mRNAs for cyclins Bl and 82 were translated in the rabbit reticulocyte lysate. The labeled translation products (5000 cpm) were mixed with unlabeled frog extract to give the same loading as the other lanes (approximately 0.35 al of egg extract per lane). Lane C, cyclin 1; lane l-f, cyclin 2. Molecular masses of protein standards are indicated in kd. Total inhibition of protein synthesis in this experiment was 37% by anti-cyclin Bl, 14% by anti-cyclin 82, and 18% by the mixture. polymerase. The mRNA was translated in a reticulocyte lysate cell-free system, and the labeled translation products were run on SDS-polyacrylamide gels alongside [%Imethionine-labeled Xenopus egg extracts prepared according to Blow and Laskey (1988). Figure 4 shows that a cluster of at least three prominent labeled bands in the Xenopus extract appear to correspond to the reticulocyte translation products (compare lane B with lanes C and Ii). Collectively, the three polypeptides formed 8.7% of the methionine incorporation in the Xenopus extract. The identification of the polypeptides corresponding to cyclin Bl and 82 in the frog extract was confirmed by hybrid arrest of translation, relying on endogenous RNAase H to cut the RNA strand of DNA-RNA duplex molecules (Donis-Keller, 1979; Minshull and Hunt, 1988; Dash et al., 1987; Shuttleworth and Colman, 1987). Antisense oligonucleotides complementary to the underlined region8 in Figure 1 were designed to cut cyclin Bl or cyclin 82 mRNA without affecting the other: the rate, extent, and specificity of cutting were checked by RNAase protection mapping (see Figure 8). The “arrested” lanes revealed that at least two major bands were ablated by the anti-cyclin Bl oligonucleotide (Figure 6, lanes D and E), one of which comigrated with the product of synthetic mRNA (lane C). ;;ylins and Mitosis in Xenopus Phase contrast Eggs Hoechst fluorescence (B) Anti-cyclin Bl and B2 Figure 5. Nuclear Envelope Breakdown and Chromosome tion in the Presence of Anti-Cyclin Oligonucleotides Condensa- Extracts treated with anticyclin oligonucleotides (see Figure 4) were examined under phase-contrast and fluorescence microscopy after 6 hr. (A) Anti-cyclin 81 oligonucleotide alone at 20 @g/ml. (Essentially identical results were obtained with 20 @g/ml anti-cyclin 82 oligonucleotide, a control oligonucleotide at 20 ug/ml, and no oligonucleotide.) (B) Anti-cyclin Bl and anticyclin 82 oligonucleotides, each at 10 uglml. (C) Edeine at 50 uM. DNA fluorescence is due to 1 frgglml Hoechst 33256. The anti-cyclin 82 oligonucleotide ablated one major band, which corresponded to a minor product of the synthetic mRNA (compare lanes B, G, and H in Figure 6). We suspect that the bands that run more slowly than the primary reticulocyte translation products represent posttranslational modifications of cyclin, as has previously been observed with sea urchin and starfish cyclins (Pines and Hunt, 1987; Standart et al., 1967). Ablation of Cyclins Bl and B2 Blocks the Cell Cycle in a GP-like State Lysolecithin-extracted sperm nuclei were added to the Xenopus egg extracts in order to monitor their ceil cycle state. The nuclei rapidly formed nuclear envelopes and performed one round of DNA synthesis (see Figures 5 and 7). After 2 or 3 hr the nuclear envelope broke down and the chromosomes condensed into a compact mass, indicating that the extract had switched from interphase into mitosis (Figure 5A; see Blow and Laskey, 1986,1988). Protein synthesis continued for more than 1 hr in these extracts and was strongly inhibited by edeine, showing that reinitiation of translation occurred (data not shown). The protein synthesis “window” for mitosis was determined by addition of cycloheximide or edeine at various times and scoring for nuclear envelope breakdown 4-6 hr later. Between 10 and 20 min of protein synthesis was required for subsequent entry into mitosis. Figure 6 shows that the antisense oligonucleotides described above caused complete cutting of their complementary cyclin mRNAs within 5 min and could therefore be used to determine whether cyclin synthesis accounted for the protein synthesis requirement for entry into mitosis. Extracts were incubated with antisense oligonucleotides directed against cyclin Bl, cyclin 82, a mixture of both, or a control “sense” oligonucleotide and were assayed for chromosome condensation and nuclear envelope breakdown as indicators of entry into mitosis. A tube with no oligonucleotide served as an additional control. Ablation of either cyclin Bl or cyclin 82 alone caused some delay in mitosis but did not prevent it (Figure 5A). When both cyclin mRNAs were destroyed, however, the chromosomes did not fully condense, and the nuclear envelope remained intact (Figure 58). This was very similar to the effect of the protein synthesis inhibitor edeine (Figure 5C). In other experiments, we used the consensus oligonucleotide indicated by the dotted underlines in Figure 1, and its complement as a”sense”control. In every case the antisense oligonucleotide inhibited mitosis while the sense oligonucleotide did not, and the only change in the pattern of protein synthesis was a diminution of the cyclin bands similar to that shown in lanes I and J of Figure 4. We therefore interpret the inhibitory effect of antisense oligonucleotides on mitosis as being due to their inhibition of cyclin synthesis and not to inhibition of synthesis of other proteins. This does not mean, however, that the synthesis of other proteins is unnecessary for mitosis in these extracts. Ablation of Cyclins Limits DNA Synthesis to a Single Round As a further check that preventing cyclin synthesis blocked the cell cycle at the G2-M transition, we tested whether DNA re-replication could occur in nuclei that had been incubated in an extract lacking cyclin mRNAs. As shown previously, nuclei that have undergone a single round of replication in the type of extract described here can only re-replicate their DNA when transferred to fresh extract if they have passed through a mitosis-like state (Blow and Laskey, 1988). Thus, the presence of cycloheximide in the first incubation permits a single round of DNA synthesis but prevents re-replication (Harland and Laskey, 1980). Figure 7 shows that cyclin mRNA scission has an effect on DNA replication similar to that of cycloheximide. Cyclins Bl and 82 were cut with the consensus antisense oligonucleotide indicated by the dotted underlines in Figure 1. The first round of DNA synthesis proceeded normally Cell 952 cyclin 82 probe +.$@ Q ,9?+@ \o (8 & 3 6 anti-B1 Q cd d --- 622 /-cyclin Bl probe / anti-B2 anti-B1 + 82 I, * 0 527 - cyclin 82 - cyclin Bl - cyclin 82 - cyclin 82 ^. , _ 3’rragment of cut cyclin 82 - Figure 6. Antisense Oligonucleotides Specifitally and Rapidly Cut Cyclin mRNAs to Completion Samples (1 uf) were taken from the tubes described in.Fig&e 4 at 5, IO, 15, and 20 min (i.e., before addition of [35S]methiinine). The RNA was extracted and hybridized with RNA probes for cyclin Bl and 82 and cytoskeletal actin for RNAase protection mapping. Hybrids were digested with 90 U/ml RNAase Ti and analyzed by electrophoresis and fluorography. Positions of Hpall-cut pBR322 markers are indicated. Lane A, undigested probes; lane B, protected cyclin Bl probe; lane C, protected cyclin 82 probe; lane D, extract without oligonucleotides at time zero; lanes E-H, 20 uglml anti-cyclin 61 oligonucleotide; lanes I-L, 20 pglml anti-cyclin 82 oligonucleotide; lanes M-P, 10 fig/ml of each anti-cyclin oligonucleotide. Samples were taken at 5, 10, 15, and 20 min in each series with antisense oligonucleotides. cyclin 81 5’fragment of -cut cyclin Bl - ABC DEFGH 5’fragment of cut cyclin 82 IJKLMNOP in the nuclei incubated in this cyclin-depleted extract, but the nuclei did not enter mitosis. When these nuclei were transferred to a fresh extract, no second round of replication occurred as shown by the low incorporation of 3H-nucleotide and the absence of a significant “heavyheavy” peak of re-replicated DNA. Nuclei that underwent their first round of replication in an extract containing the sense version of the oligonucleotide entered mitosis and re-replicated their DNA when added to a fresh extract. Since the control oligonucleotide did not prevent rereplication, the failure to re-replicate the DNA was not a trivial consequence of addition of oligonucleotides. Thus, ablation of the cyclin mRNAs described in this paper inhibited entry into mitosis, whether a consensus antisense oligonucleotide or a mixture of specific anti-cyclin Bl and anti-cyclin 82 oligonucleotides was used. Discussion The results presented in this paper show that Xenopus oocytes and eggs contain two B-type cyclin mRNAs, which we propose to call cyclins Bl and B2. Their homology with each other is about the same as with sea urchin cyclin, clam cyclin B, and S. pombe cdc73+, whereas their match with clam cyclin A is less good. We have recently identified two more cyclin mRNAs in Xenopus ovary cDNA libraries. The predicted polypeptides show better homol- ogy with clam cyclin A than with cyclin B (unpublished data). All the B-type cyclins known so far contain the amino acid sequence FLRR-SK (see Figure 2), which is not present in the A-type cyclins. RRXSX is a potential site for CAMP-dependent protein kinase (Edelman et al., 1987), which may be significant. Targeted destruction of the Xenopus B-type cyclin messages by antisense oligonucleotides prevented a cell-free DNA replicating system from entering mitosis. Moreover, the nuclei that had been incubated in an extract specifically depleted of cyclin mRNA failed to re-replicate their DNA when added to a fresh uninhibited extract, although the antisense oligonucleotides did not inhibit the first S phase. If either of the cyclin mRNAs was ablated by itself, the extract was still able to pass from interphase to M phase. We interpret this to mean that the two cyclins described here are functionally redundant and are each alone capable of driving entry into mitosis. The gene duplication probably occurred when Xenopus became tetraploid (Kobel and Du Pasquier, 1988). It is possible that the antisense oligonucleotides inhibit mitosis by inhibiting synthesis of unidentified proteins with fortuitous homology to the particular sequences we have used. Since we used oligonucleotides complementary to two different locations on each cyclin mRNA, this is not a very strong possibility. It is equally unlikely that the inhibitory effect on mitosis was due to nonspecific inhibition of C&lins and Mitosis in Xenopus Eggs (A) control oligonucleotide 12 10 7 ? -*- first (32P) -z-- second (3H) 20 10 fraction 30 number (B) anti-cyclin oligonucleobde 12 t I 11 I( 11 0 lb - *- first (32P) - second ( 3H) 20 30 Figure 7. Nuclei Incubated in Extracts with Anti-Cyclin Oligonucleotides Cannot Perform a Second Round of DNA Replication A two-stage incubation to assess the ability of nuclei to perform a second round of DNA replication was performed as described by Blow and Laskey (1986). In the first incubation, sperm nuclei were added to egg extracts containing BrdUTP, [a-s2P]dATF! and 15 uglml of either the antisense oligonucleotide #426 or the control oligonucleotide #120. After 4 hr the nuclei were transferred to fresh extract containing BrdUTP and $H]dATP and incubated for a further 4 hr. No oligonucleotides were present in the second incubation. DNA was isolated and fractionated on CsCl density gradients. (A) Control oligonucleotide. (B) Anticyclin oligonucleotide. Filled symbols show labeling in the first incubation, and open symbols the labeling in the second incubation. The peak of re-replicated DNA (fully BrdlJ substituted) is marked H/H (heavy-heavy), the peak of once-replicated DNA is indicated by H/L (heavy-light), and unreplicated DNA is marked L/L (light-light). Total inhibition of protein synthesis in the first incubation was 25% by the antisense oligonucleotide and 11% by the sense oligonucleotide. protein synthesis. First, all experiments had a “sense” oligonucleotide control added at the same concentration as the antisense oligonucleotide, and in no case did this prevent entry into mitosis. Second, extracts that entered mitosis sometimes had much higher levels of nonspecific protein synthesis inhibition than extracts prevented from entering mitosis by loss of both cyclins (see Figure 4). Third, oligonucleotides gave essentially no inhibition of protein synthesis for the first 30 min of the incubation (data not shown). By 20 min, however, the extract had fulfilled its protein synthesis requirement for entry into mitosis. A close connection between cyclins and MPF has recently emerged from the fusion of biochemical and genetic approaches (reviewed by Murray, 1988). MPF has been purified over 3,000-fold (Lohka et al., 1988), and shown to contain the Xenopus homolog of the S. pombe cell division mutant cd& gene (Gautier et al., 1988; Dunphy et al., 1988). Genetic evidence suggests that the gene product of cd& interacts with that of cdcW (Booher and Beach, 1987), which is clearly S. pombe cyclin as judged by its homology with published cyclin sequences (Goebl and Byers, 1988; Solomon et al., 1988). The null phenotype of cdcW is failure to enter mitosis (Nurse et al., 1976; Booher and Beach, 1988), exactly like the cellfree extract lacking cyclin B mRNA. These genetic experiments do not make it clear, however, whether cyclin is required to activate MPF or whether it acts downstream of MPF and executes MPF functions. Cyclin does not appear to contain a kinase consensus sequence, but MPF is thought to exert its effects via protein phosphorylation (Maller et al., 1977; Dorbe et al., 1983; Karsenti et al., 1987; Lohka et al., 1987; Ozon et al., 1987); it therefore seems more logical to view cyclin as an activator of MPF, although there could be functions of MPF that do not require protein phosphorylation for their execution. Moreover, the protein synthesis requirement for activation of MPF is much easier to explain if cyclin is an MPF activator. Cyclin may act by displacing an inhibitor of MPF or by affecting the activity of a phosphatase, for example. However, we note that despite the seemingly absolute requirement for cyclin to allow MPF activation or function, there is at present no evidence that cyclin is what actually determines the timing of MPF activation. In fission yeast, genetic evidence identifies several other genes, such as weel+, niml+, and cdc25+, that appear to regulate this function (Russell and Nurse, 1986, 1987a, 1987b; reviewed by Lee and Nurse, 1988), but their homologs have not yet been identified in higher organisms. It may be more appropriate to view cyclin as a kind of molecular latch, a component that is capable of and necessary for holding MPF “on,” but not as the only factor involved in the activation process. This view is more compatible with the puzzling gap between the end of the protein synthesis requirement window at lo-20 min and entry into mitosis at 2 to 3 hr in the kind of extracts described here. If cyclin levels simply had to reach a critical threshald in order to activate MPF, why not enter mitosis at 20 min? Something else has to happen, and we do not know what it is. Until recently, cyclins had been detected only in the eggs and oocytes of marine invertebrates, and the results presented in this paper still leave open the possibility that cyclins are important only for the rapid cell division cycles of early cleavage. This is probably not the case, however, since the sequence homology between cyclins and the S. pombe cdcW gene (Booher and Beach, 1988; Goebl and Byers, 1986; Solomon et al., 1988) suggests that they are required for the activation or action of MPF in normal vegetative cell cycles. Furthermore, the occurrence of cyclins over this wide phylogenetic range suggests they occur in all eukaryotes. Cell 954 Experlmental Procedures cDNA Libraries A cDNA library from activated Xenopus egg mRNA was constructed in M13mp6. Poly(A)+ RNA was prepared from electrically activated eggs that had been frozen in liquid nitrogen and finely ground in a pestle and mortar. Extraction buffer (200 m M LiCI, 50 m M Tris-HCI [pH 61, 10 m M EDTA) was made 0.5% in SDS and heated until a single phase was produced. It was mixed with the frozen egg powder and shaken vigorously. A ratio of 20 ml of liquid for each gram of tissue was used to reduce the problems of emulsion formation. After two more phenol extractions followed by an ether extraction, RNA was precipitated by adding 0.25 vol of 10 M LiCI. Poly(A)+ RNA was isolated by two cycles of chromatography on oligo(dT)-cellulose (type III from Collaborative Research). First- and second-strand cDNA was synthesized as described by Pines and Hunt (1967) except that random hexanucleotides (final concentration 400 &ml) were used as primers for the first strand. The cDNA was digested with Sau3A and ligated into BamHI-cut M13mp6 RF. This was used to transform Escherichia coli strain TGl by the method of Hanahan (1965). Xenopus oocyte cDNA libraries in kg110 were the gifts of D. Melton (Harvard University), A. Colman (University of Birmingham), and C. Dingwall (University of Cambridge). Screening cDNA Libraries Filters were prepared according to Mason and Williams (1965). Duplicate filters from the Ml3 library were probed with a redundant oligonucleotide (#40, see below), chosen to cover the region of best match between clam cyclin A and sea urchin cyclin (Swenson et al., 1966; Pines and Hunt, 1967) and with the sea urchin cDNA clone cyc4 (Pines and Hunt, 1967). Ml3 clones that were positive with both probes were isolated and sequenced. Recombinants containing cyclin inserts were identified by conceptual translation of the sequence and used to probe duplicate filters from the 1, libraries. The oligonucleotide was labeled with [Y-~~P]ATP according to Maxam and Gilbert (1960). Clone cyc4 was labeled by the method of Feinberg and Vogelstein (1963). Filters were probed overnight in 5x SCP (500 m M NaCI, 150 m M Na2HP04, 5 m M EDTA [pH to 6.6 with HCI]), 2% (wVvol) skim milk, 0.5% SDS, 10 uglml boiled herring sperm DNA; DNA probes were incubated at 65%, oligonucleotide probes at 42%. Oligonucleotide-probed filters were washed three times in 4x SSC (600 m M NaCI, 60 m M sodium citrate), 0.1% SDS at room temperature. Filters probed with cyc4 were washed twice in 4x SSC and once in 3x SSC at 37%. Filters screened with primed-cut probes were washed twice in 2x SSC and twice in 0.1x SSC at 60%. All washes lasted 10 min. DNA Sequencing The “extended” dideoxynucleotide sequencing method designed for modified T7 DNA polymerase (Tabor and Richardson, 1967) was used with the Klenow fragment of E. coli DNA polymerase as described in BRL Focus (Summer 1967). Inserts from lgtl0 libraries were subcloned into a plasmid (usually one of the Gemini transcription vectors pGEM-1 or pGEM-2) and sequenced by the “shotgun” sonication and random-selection approach of Bankier et al. (1967). The Ml3 subclones selected for sequencing were identified by screening with the purified cyclin insert. In Vitro l?anscription and Translation Synthetic mRNAs were transcribed from cDNA subcloned into pGEM transcription vectors from Promega Biotec. Capped transcripts were synthesized using SP6 or T7 RNA polymerases (SP6 according to the protocol of Melton et al. [I9641 and Krieg and Melton [1987]; T7 as described by Pines and Hunt [19m). RNAs were translated in the mRNAdependent reticulocyte lysate system as described by Jackson and Hunt (1963). Analysis of Labeled Pmtelns on SDS-Polyacrylamlde Gels Analysis of protein products was performed on lo%-20% gradient SDS-polyacrylamide gels after dilution with 5-10 vols of SDS gel sample buffer (Anderson et al., 1973). The reticulocyte lysate translation reactions were terminated by addition of 5 m M EDTA and 50 pglml RNAase for 10 min at 20% before addition of sample buffer. RNAase Protection Mapping RNAase protection was based on the protocol described by Krieg and Melton (1967). Inserts from Ml3 shotgun clones were subcloned into pGEM transcription vectors, and RNA probes were made with T7 RNA polymerase. Reaction conditions were as described by Pines and Hunt (1967) except that no cap analog was used; ATR GTR and CTP concentrations were each 1 mM, and the only UTP in the 5 ul reaction was 2.5 ul of label (PB10163 from Amersham International), giving a final concentration of 12.5 pM UTR For probes longer than 250 bases, addition of unlabeled UTP at 375 uM increased full-length transcripts by at least IO-fold. The labeled probes were gel purified (Krieg and Melton, 1967). RNA for analysis was prepared from oocytes, embryos, and extracts by freezing the sample (one to five embryos or up to 5 ul of extract) on dry ice. The sample was thawed and homogenized by vigorous pipetting in 125 ul of extraction buffer (300 m M NaCI, 50 m M Tris-HCI [pH 7.51, 1 m M EDTA, 1% SDS). RNA was extracted with phenol-chloroform, ethanol precipitated, and stored at -60%. RNA was hybridized with probes as described by Krieg and Melton (1967). RNAase concentrations were titrated for each probe. In general, 35 U/ml of BRL RNAase Tl and 100 nglml Sigma RNAase A were used. Digestion and RNA analysis were performed as described by Krieg and Melton (1967). Dligonucleotides Oligonucleotides were made by Margaret Franklin or Colin Denston in the Department of Biochemistry on a Biosearch Inc. Cyclone DNA Synthesizer donated by the Wellcome Trust. The redundant oligonucleotide #40 originally used to screen the Ml3 library was B’TCTGG[A/G]GG[A/G]TA[TIC]ATCTC[T/C]TC[A/G]TATTT-3’ and, like the anticyclin 82 oligonucleotide 5’-GGACACATCCATCGGCAC-3’, was purified by several ether extractions followed by ethanol precipitation. The anti-cyclin Bl oligonucleotide 5’CCATTGGGCTTGGTGAGC3’ was purified on an Applied Biosystems OPC column according to the manufacuterer’s instructions. Oligonucleotide #426 had the sequence 5’.ACCTCTGG[A/G]GTGTACATCTCTTC-3’ and is complementary to both cyclin 81 and 82, as indicated by dotted underlining in Figure 1; oligonucleotide #120 is the complement of #426. Cell-Free Egg Extract Cell-free DNA replication systems were prepared and analyzed according to the protocols described by Blow and Laskey (1966, 1966) based on the method of Lohka and Masui (1963, 1964). For analysis of newly synthesized proteins, [35S]methionine (Amersham SJ 1515) was added to a final concentration of 2 mCi/ml. Acknowledgments We are grateful to Jon Pines, John Gerhart, Mike Wu, Andrew Murray, Fred Wilt, Eric Rosenthal, and Alan Colman for their collaboration in the early stages of these experiments, and for the facilities they provided for making oocytes, eggs, and RNA. We thank John Shuttleworth, Colin Dingwall, and Doug Melton for samples of their cDNA libraries, Tim Mohun for his actin clone, David Judge for help with sequencing software, Pat O’Farrell, Joan Ruderman, and Will Whitfield for letting us see their clam and fly cyclin sequences, Ron Laskey for laboratory facilities and encouragement, and Phil Garrett for technical support. Oligonucleotide synthesis was made possible by a grant from the Wellcome Trust. This work was supported by an SERC studentship to J. M., and by the CRC and MRC. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 16 U.S.C. Section 1734 solely to indicate this fact. Received November 2, 1966; revised December 15, 1966. References Anderson, C. W., Baum, R R., and Gesteland, R. F. (1973). Processing of adenovirus P-induced proteins. J. Virol. 72, 241-252. $;lins and Mitosis in Xenopus Eggs Arion, D., Meijer, L., Brizuela, L., and Beach, D. (1986). cdc2 is a component of the M phase-specific histone HI kinase: evidence for identity with MPF. Cell 55, 371-378. Hultin, T. (1961). The effect of puromycin on protein metabolism and cell division in fertilized sea urchin eggs. Experientia 77, 410-411. Bankier, A. T., Weston, K. M., and Barrell, B. G. (1987). Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. M&h. Enzymol. 755, 51-93. Hutchison, C. J., Cox, R., Drepaul. R. S., Gomperts, M., and Ford, C. C. (1987). Periodic DNA synthesis in cell-free extracts of Xenopus eggs. EMBO J. 6, 2003-2010. Blow, J. J.. and &key, Ft. A. (1988). Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47; 577-587. Jackson, R. J., and Hunt, T. (1983). Preparation and use of nucleasetreated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Meth. Enzymol. 96, 50-73. Blow, J. J., and Laskey, R. A. (1988). A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332, 546-548. Karsenti, E., Bravo, R., and Kirschner, M. (1987). Phosphorylation changes associated with the early cell cycles in Xenopus eggs. Dev. Biol. 779, 442-453. Booher, Ft., and Beach, D. (1987). Interaction between cdcW and cdc2+ in the control of mitosis in fission yeast: disassociation of the Gl and G2 roles of the cd& protein kinase. EMBO J. 6, 3441-3447. Kirschner, M., Newport, J., and Gerhart, J. (1985). The timing of early developmental events in Xenopus. Trends Genet. 1, 41-47. Booher, R., and Beach, D. (1988). Involvement of cdc73+ in mitotic control in Schizosaccharomyces pombe: possible interaction of the gene product with microtubules. EMBO J. 7, 2321-2327. Cyert, M. S., and Kirschner, vitro. Cell 53, 185-195. M. (1988). Regulation of MPF activity in Dash, P, Lotan, I., Knapp, M., Kandel, E. R., and Goelet, I? (1987). Selective elimination of mRNAs in viva: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc. Natl. Acad. Sci. USA 84. 7896-7900. Dettlaff, T. A. (1968). Action of actinomycin and puromycin upon frog oocyte maturation. J. Embryol. Exp. Morphol. 76, 183-195. Donis-Keller, H. (1979). Site-specific Acids Res. 7, 179-192. enzymic cleavage of RNA. Nucl. Dome, M., Peaucellier, G., and Picard, A. (1983). Activity of the maturation-promoting factor and the extent of protein phosphorylation oscillate simultaneously during meiotic maturation. Dev. Biol. 99, 489-501. Dunphy, W. G., and Newport, J. W. (1988). Mitosis-inducing factors are present in a latent form during interphase in the Xenopus embryo. J. Cell Biol. 706, 2047-2056. Dunphy, W. G., Brizuela, L., Beach, D., and Newport, J. (1988). The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 54, 423-431. Edelman, A. M., Blumethal, D. K., and Krebs, E. G. (1987). Protein serinelthreonine kinases. Annu. Rev. Biochem. 56, 567-614. Evans, T., Rosenthal, E. T., Youngblom, J., Distel, D., and Hunt, T. (1983). Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33, 389-396. Feinberg, F. f?, and Vogelstein, B. (1983). A technique for radiolabeling DNA restriction endonuclease fragments to high specificity. Anal. Biothem. 732, 6-13. 1 is involved in Xenopus oocyte maturation. Nature 294, 358-359. Kishimoto, S., and Lieberman. I. (1964). Synthesis of RNA and protein required for the mitosis of mammalian cells. Exp. Cell Res. 36, 92-103. Kishimoto, T, and Kanatani, H. (1976). Cytoplasmic factor responsible for germinal vesicle breakdown and meiotic maturation in starfish oocytes. Nature 227, 273-274. Kishimoto, T., Kuriyama, R., Kondo, H., and Kanatani, H. (1982). Generality of the action of various maturation-promoting factors. Exp. Cell Res. 737, 121-126. Kobel, H. R., and Du Pasquier, L. (1986). Genetics of polyploid Xenopus. Trends Genet. 2, 310-315. Krieg, l? A., and Melton, D. A. (1987). In vitro RNA synthesis with SP6 RNA polymerase. Meth. Enzymol. 755, 397-415. Labbe, J. C., Lee, M. G., Nurse, P, Picard, A., and Doree, M. (1988a). Activation at M-phase of a protein kinase encoded by a starfish homologue of the cell cycle control gene cd@. Nature 335, 251-254. Labbe, J. C., Picard, A. Karsenti, E., and Do&e, M. (1988b). An M-phase-specific protein kinase of Xenopus oocytes: partial purification and possible mechanism of its periodic activation. Dev. Biol. 727, 157-169. Lee, M.. and Nurse, l? (1987). Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature 327; 31-35. Lee, M., and Nurse, P (1988). Cell cycle control genes in fission yeast and mammalian cells. Trends Genet. 4, 287-290. Lohka. M. J., and Masui, Y. (1983). Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science 220, 719-721. Lohka, M. J., and Masui, Y. (1984). Rolesof cytosol and cytoplsmic particles in nuclear envelope assembly and sperm pronuclear formation in cell-free preparations from amphibian eggs. J. Cell Biol. 98, 1222-1230. Ford, C. C. (1985). Maturation promoting factor and cell cycle regulation J. Embryol. Exp. Morph. 89 (Suppl.), 271-284. Lohka, M. J., Kyes, J. L., and Mailer, J. L. (1987). Metaphase protein phosphorylation in Xenopus leevis eggs. Mol. Cell. Biol. 7, 760-768. Ford, C. C., Wall, V. M., and Smith, C. (1983). DNA synthesis in the first cell cycle of Xenopus. Cell Biol. Int. Rep. 7, 545-546. Lohka, M. J., Hayes, M. K., and Mailer, J. L. (1988). Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc. Natl. Acad. Sci. USA 85, 3009-3013. Gautier, J.. Norbury, C., Lohka, M. J., Nurse, P., and Mailer, J. (1988). Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cd@. Cell 54, 433-439. Gerhart, J., Wu, M., and Kirschner, M. (1984). Cell cycle dynamics of an M-phase specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol. 98, 1247-1255. Goebl, M., and Byers, B. (1988). Cyclin 739-740. in fission yeast. Cell 54, Golden, L., Schafer, U., and Rosbash, M. (1980). Accumulation of individual pA+ RNAs during oogenesis of Xenopus laevis. Cell 22, 835-844. Hanahan, D. (1985). Techniques for transformation of E. co/i. In DNA Cloning: A Practical Approach, Vol. 1. D. M. Glover, ed. (Oxford: IRL Press), pp. 109-136. Harland. R. M.. and Laskey, R. A. (1980). Regulated replication of DNA microinjected into eggs of X. laevis. Cell 27, 761-771. Huchon, D., Ozon, R., and DeMaille, J. G. (1981). Protein phosphatase- Mailer, J. L., Wu, M., and Gerhart, J. G. (1977). Changes in protein phosphorylation accompanying maturation of Xenopus lawis oocyies. Dev. Biol. 58, 295-312. Mason, i? J., and Williams, J. G. (1985). Hybridisation in the analysis of recombinant DNA. In Nucleic Acid Hybridisation: A Practical Approach, B. D. Hames and S. J. Higgins, eds. (Oxford: IRL Press), pp. 113-137. Masui, Y.. and Markert, C. L. (197l). Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 777, 129-146. Masui. Y., and Shibuya, E. K. (1987). Development of cytoplasmic activities that control chromosome cycles during maturation of amphibian oocytes. In Molecular Regulation of Nuclear Events in Mitosis and Meiosis, R. A. Schlegel, M S. Halleck, and f? N. Rao, eds. (Orlando, Florida: Academic Press), pp. 1-42. Maxam, A. M., and Gilbert, W. (1980). Sequencing end-labeled DNA with base-specific chemical cleavages. Meth. Enzymol. 85, 499-560. Cell 956 Meijer, L., and Guerrier, P (1984). Maturation and fertilization oocyles. Int. Rev. Cytol. 86, 129-196. in starfish Melton, D. A., Krieg, f? A., Rebagliati, M. Ft., Maniatis. T, Zinn, K.. and Green, M. R. (1984). Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucl. Acids Res. 72, 7035-7056. Miake-Lye, R., Newport, J., and Kirschner, M. (1983). Maturationpromoting factor induces nuclear envelope breakdown in cycloheximide arrested embryos of Xenopus laevis. J. Cell Biol. 97, 81-91. Minshull, J., and Hunt, T. (1986). The use of single-stranded DNA and RNase H to promote quantitative “hybrid arrest of translation” of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucl. Acids Res. 74, 6433-6451. Murray, A. (1988). A mitotic inducer matures. Nature 335, 207-208. Newport, J. W., and Kirschner, M. W. (1984). Regulation of the cell cycle during early Xenopus development. Cell 37, 731-742. Nieuwkoop, P D.. and Faber, J. (1967). The Normal Development Table of Xenopus laevis (Daudin), Second Edition (Amsterdam: NorthHolland Publishing Company). Nurse, P, Thuriaux, P, and Nasmyth, K. A. (1976). Genetic control of the division cycle of the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 146. 167-178. Ozon. R., Mulner, O., Boyer, J., and Belle, R. (1987). Role of protein phosphorylation in Xeriopus oocyte meiotic maturation. In Molecular Regulation of Nuclear Events in Mitosis and Meiosis, R. A. Schlegel, M. S. Halleck, and P N. Rao, eds. (Orlando, Florida: Academic Press), pp. 111-130. Picard, A., Peaucellier, G., le Bouffant, F., Le Peuch, C., and Dome, M. (1985). Role of protein synthesis and proteases in production and inactivation of maturation-producing activity during meiotic maturation of starfish oocytes. Dev. Biol. 709, 311-320. Pines, J., and Hunt, T. (1987). Molecular cloning and characterization of the mRNA for cyclin from sea urchin eggs. EMBO J. 6. 2987-2995. Russell, P., and Nurse, P (1986). cdc25t functions as an inducer in the mitotic control of fission yeast. Cell 45, 145-153. Russell, P., and Nurse, f? (1987a). Negative regulation of mitosis by weelf, a gene encoding a protein kinase homolog. Cell 49, 559-567 Russell, P.. and Nurse, P (1987b). The mitotic inducer niml+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell 49, 569-576. Shuttleworth. J., and Colman, A. (1987). Antisense oligonucleotidedirected cleavage of mRNA in Xenopus oocytes and eggs. EMBO J. 7; 427-434. Simanis, V., and Nurse, P (1986). The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell 45, 261-268. Smith, L. D., and Ecker, R. E. (1971). The interaction of steroids with Ram pipiens oocytes in the induction of maturation. Dev. Biol. 25, 232-247. Solomon, M., Booher, R., Kirschner, M., and Beach, D. (1988). Cyclin in fission yeast. Cell 54, 738-739. Staden, R. (1982). An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucl. Acids Res. 70, 2951-2961. Standart, N., Minshull, J., Pines, J., and Hunt, T. (1987). Cyclin synthesis, modification and destruction during meiotic maturation of the starfish oocyte. Dev. Biol. 724, 248-258. Sunkara, P S.. Wright, D. A., and Rao, f? N. (1979). Mitotic factors from mammalian cells induce germinal vesicle breakdown and chromosome condensation in amphibian oocytes. Proc. Natl. Acad. Sci. USA 76. 2799-2802. Swenson, K. I., Farrell, K. M., and Ruderman, J. V. (1986). The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell 47, 861-870. Tabor, S., and Richardson, C. C. (1987). DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc. Natl. Acad. Sci. USA 84, 4767-4771. Wagenaar, E. 8. (1983). The timing of synthesis of proteins required for mitosis in the cell cycle of the sea urchin embryo.‘Exp. Cell Res. 744, 393-403. Wasserman, W. J., and Masui, Y. (1975). Effects of cycloheximide on a cytoplasmic factor initiating meiotic maturation in Xenopus laevis oocytes. Exp. Cell Res. 97. 381-388. Wasserman, W. J., and Smith, L. D. (1978). The cycling behavior of a cytoplasmic factor controlling nuclear envelope breakdown. J. Cell Biol. 78, R15-R22. Weintraub, H., Buscaglia, M., Ferez, M., Weiler, S.. Boulet, A., Fabre, F., and Baulieu, E. E. (1982). Mise en evidence dune activite “MPF chez Saccharomyces cerevisiae. C. R. Acad. Sci. (Paris) Ill 295, 787-790. Wilt, F. H., Sakai, H., and Mazia. D. (1967). Old and new protein in the formation of the mitotic apparatus in cleaving sea urchin eggs. J. Mol. Biol. 27 l-7.