* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download thesis - ETDA
Development of the nervous system wikipedia , lookup
Embodied language processing wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Long-term depression wikipedia , lookup
Metastability in the brain wikipedia , lookup
Aging brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Environmental enrichment wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neurogenomics wikipedia , lookup
Basal ganglia wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Spike-and-wave wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Channelrhodopsin wikipedia , lookup
The Pennsylvania State University
The Graduate School
Graduate Program in Cellular and Molecular Biology
ABNORMAL CEREBELLAR SIGNALING INDUCES
DYSTONIA IN MICE
A Thesis in
Cell and Molecular Biology
by
Carolyn E. Pizoli
Copyright 2003 Carolyn E. Pizoli
Submitted in Partial Fulfillment
of the Requirements
for the Degree of
Doctor of Philosophy
May 2003
We approve the thesis of Carolyn E. Pizoli.
Date of Signature
____________________________________
Ellen J. Hess
Associate Professor of Neurology
Thesis Co-Advisor
Co-Chair of Committee
______________________
____________________________________
Melvin L. Billingsley
Professor of Pharmacology
Thesis Co-Advisor
Co-Chair of Committee
______________________
____________________________________
Robert Levenson
Professor of Pharmacology
Director, Cell and Molecular Biology Program
______________________
____________________________________
James Connor
Professor of Neuroscience
______________________
____________________________________
Teresa Wood
Associate Professor of Neuroscience
______________________
iii
Abstract
Dystonia is a relatively common neurological syndrome characterized by twisting
movements or sustained abnormal postures. The pathophysiology of dystonia remains
poorly understood; however, recent evidence suggests that abnormal cerebellar signaling
contributes to the expression of dystonia. To study the role of the cerebellum in dystonia,
we first analyzed neurotransmission in the cerebellum of the genetically dystonic mouse,
tottering. A deficiency in excitatory but not inhibitory neurotransmission in tottering
mice was seen after superfusion of cerebellar synaptosomes with 60mM KCl. Further
analysis of the role of cerebellar Purkinje cells in the generation of tottering dystonia was
completed through breeding a transgene responsible for post-developmental Purkinje cell
death onto the tottering mouse. Prior to Purkinje cell degeneration, transgenic tottering
mice exhibited classical tottering dystonic events; however, the same animals failed to
exhibit dystonia after Purkinje cell loss had occurred in adulthood. The loss of the
dystonic phenotype in double mutant mice indicates that Purkinje cells and the cerebellar
cortex participate in the pathogenesis of dystonia in the tottering mouse. These data
support the theory that an abnormal signal from the cerebellar cortex of tottering mice is
responsible for the dystonic phenotype. To test this theory and examine the role of the
cerebellum in dystonia, we developed a novel mouse model of dystonia. Microinjection
of low-doses of the glutamate analogue kainic acid into the cerebellum of wild type mice
elicited reliable and reproducible dystonia.
Transgenic mice lacking Purkinje cells
showed dramatically decreased dystonia after kainic acid injections, supporting the
theory that aberrant cerebellar excitation is sufficient to produce dystonia. Together these
data suggest that the cerebellum plays a role in the pathophysiology underlying dystonia.
iv
Table of Contents
Chapter
Page
List of Tables
vi
List of Figures
vii
1
Introduction/Literature Review
1.1
Dystonia in Humans
Introduction
Classification
Pathophysiology
Treatment
Paroxysmal Dyskinesias
1.2
Tottering Mouse: Animal Model of Dystonia
Introduction
Calcium Channels
Behavioral Phenotype
Cellular Pathology
1.3
Chapter Summary
1.4
Hypotheses
1
1
1
2
3
12
13
19
19
19
23
26
27
28
2
Altered Neurotransmission in the Tottering Cerebellum
Abstract
Introduction
Materials and Methods
Results
Discussion
30
30
30
35
37
40
3
Role of Purkinje Cells in the Expression of Tottering Dystonia
Abstract
Introduction
Materials and Methods
Results
Discussion
45
45
46
48
51
58
4
Role of the Cerebellum in a Novel Animal Model of Dystonia
in Wild Type Mice
Abstract
Introduction
Materials and Methods
Results
Discussion
63
63
64
65
71
86
v
5
Discussion
5.1
Mouse Models of Dystonia
The Tottering Mouse
Kainate-Induced Dystonia
5.2
The Cerebellum and Dystonia
Cerebellar Circuitry
Cerebellar Function
Pathophysiology of Dystonia
87
87
87
101
104
104
107
110
REFERENCES
118
vi
List of Tables
Table
Page
1.1
Familial dystonic syndromes for which
gene loci have been identified
3
1.2
Subgrouping of the paroxysmal dyskinesias
14
1.3
Classification of neuronal high-voltage activated
calcium channels
22
3.1
Genotypes of progeny generated in SV4+/-;+/tg X
+/+;tg/tg crosses
53
5.1
Comparison of salient features of tottering dystonia and PNKD
98
5.2
Comparison of the salient features of the kainate model of
dystonia and PKD
103
vii
List of Figures
Figure
Page
1.1
Schematic depiction of brain regions and
pathways theorized to malfunction in dystonia
9
1.2
Schematic depiction of a voltage-gated calcium channel
21
2.1
Schematic diagram of neuronal connections in the cerebellum
34
2.2
3
38
2.3
Comparison of tottering and wild type peak 3H-glutamate
release
38
2.4
3
39
2.5
Comparison of tottering and wild type peak 3H-GABA
release
39
3.1
PCR genotyping for presence of the SV40 transgene
53
3.2
Body weights of F7 generation progeny
54
3.3
Loss of dystonic phenotype in transgenic tottering mice
over time
56
3.4
Regional loss of dystonic phenotype in F7 transgenic
tottering mice
57
3.5
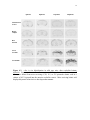
Calbindin mRNA in situ hybridization
58
4.1
Typical dystonic postures after cerebellar kainate injection
in wild type mice
71
4.2
Dose-response and dose-recovery curves after cerebellar
injection of kainic acid in wild type mice
73
4.3
c-fos in situ hybridization in wild type mice after cerebellar
kainate injection
75
4.4
Regional expression of c-fos mRNA after cerebellar kainate
injection
76
4.5
Cerebellar injection site localization
77
H-Glutamate release from cerebellar synaptosomes
H-GABA release from cerebellar synaptosomes
viii
4.6
Dystonic severity in transgenic mice lacking Purkinje cells
81
4.7
Representative EEG recordings from wild type mice
receiving cerebellar microinjections of kainic acid
83
4.8
Dystonic severity after cerebellar NBQX co-injection with
kainic acid
85
4.9
Dystonic severity after cerebellar injection of domoic acid
in wild type mice
86
5.1
Schematic diagram of pathways theorized to malfunction
in cerebellar-induced dystonia
108
5.2
Comparison of release of inhibition treating dystonia in the
basal ganglia and the cerebellum
116
Chapter 1. INTRODUCTION/Literature Review
1.1. DYSTONIA IN HUMANS
Introduction
The scientific advisory board to the Dystonia Medical Research Foundation defines
dystonia as a neurological syndrome characterized by the simultaneous co-contraction of
antagonistic muscles leading to twisting and repetitive movements or sustained abnormal
postures (Fahn S, 1987). However, a great deal of confusion exists because the term
dystonia refers to both the behavioral symptom of certain abnormal hyperkinetic
involuntary movements and to the syndrome or disease entity itself. In fact, the term
dystonia encompasses numerous heterogeneous disorders which share the characteristic
symptom of sustained contraction of antagonistic muscles. Taken together, the various
forms of dystonia represent a common neurological disorder which is the second most
commonly encountered disorder seen in movement disorder clinics after Parkinsonism
(Fahn S, 1995) and reaches an estimated prevalence of ¾ that of multiple sclerosis
(Richter A, 1998). Dystonic movements vary greatly in speed, amplitude, rhythmicity,
torsion, forcefulness, distribution, and initiating factors (Fahn S, 1988). Historically, the
wide range of characteristics involved in dystonia syndromes led to confusion and
misdiagnosis.
Terms such as seizures, convulsions, myoclonus, ballism, and
choreoathetosis were often misused to describe dystonia. Currently, clinical neurology is
readily equipped to correctly diagnose and report dystonia, but the fields of laboratory
research and animal research in particular are still slow to recognize either the symptom
or syndrome of dystonia.
2
Classification
Dystonia is an extremely heterogeneous disorder classified by age of onset,
distribution, and etiology. While age of onset and distribution are useful classifications
for prognostics and treatment strategies, etiologic classification is essential for
understanding the pathophysiology and prevention of disease (Fahn, S, 1998). Dystonia
is divided into two broad etiologic classifications, primary and secondary, each with
numerous sub-classifications.
Primary dystonia is either of a familial or sporadic
etiology while secondary dystonia occurs symptomatically from a broad range of
neurological diseases and lesions.
Secondary dystonia is of particular interest because of the concomitant insight gained
to the basis of the dystonia, particularly the brain regions involved.
Numerous
environmental factors may cause insults leading to secondary dystonia, including cerebral
palsy, encephalitis, stroke, tumors, drugs, and toxins.
Heredodegenerative diseases,
marked by neuronal loss, also may cause secondary dystonia; these include Parkinson’s
disease, Wilson’s disease, and Huntington’s disease among others (Fahn, S, 1998). A
majority of secondary dystonias result from lesions in the basal ganglia and, to a lesser
degree, the thalamus. Therefore, these regions have historically been implicated in the
pathogenesis of dystonia syndromes.
To meet the clinical definition of primary dystonia, dystonia must be the sole
neurological sign other than tremor and no other exogenous, inherited, or degenerative
cause should be identified (Bressman, 1998).
Primary dystonia accounts for 2/3 of
cases, including both the familial and sporadic forms. Currently, little is known about
sporadic dystonia, which remains the single largest category of patients.
Familial
3
dystonias are being actively researched; the genes responsible for many of the
monogenetic inherited dystonias have been mapped and some of the gene products
identified.
It is also important to note that many of these genetically determined
syndromes express symptoms in addition to dystonia and are thus classified as dystoniaplus syndromes rather than primary dystonia (Fahn, S, 1998). Table 1.1 summarizes the
dystonic syndromes, both primary and dystonia-plus for which gene loci have been
established.
Gene
DYT1
Location
9q34.1
Origin
Ashkenazi jews
and non-jewish
European decent
Syndrome
Early-onset primary
dystonia (Dystonia
musculorum deformans)
Protein
ATP-binding
protein
DYT3
Xq13
Philippines
Lubag (X-linked
Parkinsonism-dystonia)
?
DYT5
(DRD)
14q22.1
--
Dopa-responsive
dystonia (DRD)
GTPcyclohydrolase I gene, TH
Ichinose, 1994;
Knappskog,
1995
DYT6
8p21-q22
Mennonite/Amish
Mixed: Childhood/Adult
with limb or cranial
onset
?
Almasy, 1997
DYT7
18p
German kindred
Adult onset torticollis
?
Leube, 1996
--
2q33-35
Polish-American
Paroxysmal nonkinesegenic dyskinesia
? suspected Na+
channel
Fouad, 1996
Fink, 1996
Choreoathetosis/
? suspected K+
spasicity, episodic (CSE) channel
*italicized genes denote non-primary dystonia syndromes; TH, Tyrosine Hydroxylase
--
1p
German kindred
References
Ozelius, 1989;
Kramer 1990;
Kwiatkowski,
1991
Eidelberg, 1993
Auburger, 1996
Table 1.1. Familial dystonic syndromes for which gene loci have been identified.
Pathophysiology
Although the genetic basis of a few inherited dystonias, as well as the pathology
behind most secondary dystonias has been determined, little is known concerning the
pathophysiology of this disease. As etiologies continue to be discerned, research
4
elucidating central mechanisms involved in genetic, sporadic, or secondary dystonia will
provide insight to this broad and devastating group of disorders.
Medicine has traditionally viewed dystonia as a disorder of the basal ganglia. The
basis for this view rests largely in the knowledge that the basal ganglia is a common site
of pathology in many of the secondary dystonias caused by toxins, injuries, and various
heredodegenerative diseases.
Furthermore, the majority of movement disorders are
considered to have an origin in the basal ganglia until proven otherwise. However, if
dystonia is indeed a disorder of the basal ganglia, it is the least well understood basal
ganglia disorder in terms of pathophysiology (Crossman AR, 1998). Gross and histologic
examination of brain tissue from patients with primary dystonia fail to demonstrate
morphological changes in the basal ganglia in contrast to lesions seen in secondary
dystonia. Alternatively, a biochemical abnormality may exist in the basal ganglia of
primary dystonia patients.
The incidence of dystonia arising secondary to drug
treatments affecting monoamines in the striatum supports this theory (Fahn S, 1995);
however, no consistent changes have been documented. As with gross lesions, it appears
that dopaminergic dysfunction is readily discernable in secondary cases (Vidailhet M,
1999), while studies in primary dystonia fail to show consistent abnormalities in the
nigro-striatal dopaminergic pathway (Playford ED, 1993).
Numerous theories attempt to describe the pathophysiology behind dystonia; these
implicate (1) the basal ganglia, particularly the globus pallidus, and (2) the thalamus.
More recent work has also implicated (3) the sensorimotor cortex and (4) the cerebellum
as well. While these four regions may all be capable of independently causing dystonia,
the existence of a single common pathway affected by each of these regions is a more
5
likely and sought after explanation. The possibility of numerous independent pathways
resulting in various primary and secondary dystonia syndromes cannot be excluded
however.
Secondary dystonias and the dopa-responsive dystonia-plus syndrome have
historically maintained the basal ganglia at the center of dystonia research. Some of the
strongest evidence implicating the basal ganglia in dystonia comes from studies in druginduced primate models of dystonia and functional brain studies of drug-induced human
dystonia (and other hyperkinetic dyskinesias). These models implicate a causative role
for decreased basal ganglia output in dystonia.
Decreased globus pallidus (internal
segment, Gpi) and substantia nigra (pars reticulata, SNr) output result in disinhibition of
the thalamic motor nuclei and increased excitatory input to the cerebral cortex. In theory,
decreased Gpi/SNr output can result from increased direct pathway inhibition of these
structures or decreased indirect pathway excitation (Berardelli A, 1998). It is precisely
this theory expressed by Vitek and Giroux as they describe dystonia as a hyperkinetic
movement disorder. They describe firing rates of Gpi being decreased, with altered
patterns and synchronization in dystonic states (Vitek JL, 2000).
A more complex theory was presented by Crossman and Brotchie however, as they
describe dystonia as both a hypokinetic and dyskinetic disorder (Crossman AR, 1998).
Because hypokinesias result from increased Gpi/SNr output and dyskinesias result from
decreased Gpi/SNr output, the authors concluded that temporal and/or spatial fluctuations
in Gpi/SNr activity are responsible for dystonia. Alternatively, the basal ganglia may not
be central to the production of dystonia; rather, inhibitory and excitatory influences of the
basal ganglia may fluctuate as a consequence of the dystonia. Components of the motor
6
control circuitry may attempt to compensate for the activity resulting in the dystonia and
these changes are what researchers are identifying.
While lesions of the basal ganglia often result in dystonia (Munchau A, 2000;
Lehericy S, 1996; Kostic VS, 1995), other areas of the brain also induce dystonia when
lesioned, most commonly the thalamus (Lehericy S, 1996; Lee MS, 1994).
Focal,
segmental, and generalized hemi-dystonia have been described after lesions of the
thalamus. In a review of movement disorders caused by thalamic lesions, Lee and
Marsden discuss two points which suggest that the mechanism of dystonia induced by
basal ganglia disorders and thalamic dysfunction are indeed separate. First, thalamic
lesions reported to induce dystonia were often restricted to the posterior, posterolateral,
and paramedian regions (Lee MS, 1994). These areas do not overlap with those receiving
input from the basal ganglia (ventrolateral, ventromedial, and ventroanterior regions).
Rather, these areas are largely associated with somatosensory input. Lehericy et al.
defined other regions of the thalamus involved in dystonia-producing lesions and they
also determined that the striatopallidal circuit was entirely unaffected.
The ventral
intermediate and ventral caudal regions defined by the latter are involved in sensory and
cerebellar relays (Lehericy S, 1996). Thus, a direct connection cannot be drawn between
the pathway basal ganglia dysfunction affects in the thalamus and that affected in
dysfunction based within the thalamus itself. Secondly, most basal ganglia researchers
present a theory of pallidal disinhibition of the thalamus in dystonia. The resulting
excitation of the thalamus would therefore be in direct contradiction to the presumed
effect of thalamic lesion.
Therefore, the overexcitation of the thalamus through
disordered basal ganglia function, and the defective output of the thalamus due to lesions
7
result in dystonia through what appears to be separate pathways (Lee MS, 1994; Lehericy
S, 1996). Although numerous separate circuits exist within the thalamus, the thalamus
acts as an integrating relay with considerable excitatory efferents reaching the
sensorimotor cortex. As research continues to define at least two separate mechanisms of
dystonia induction involving the thalamus, perhaps study of the resulting effects on
cortical function will merge these apparently divergent pathomechanisms.
Hallett proposed a broad deficiency of cortical inhibition as the central mechanism of
dystonia from a range of causes (Hallett M, 1998). Instead of the thalamus providing
excessive excitatory input, perhaps too little inhibitory input is given, or a combination of
both. Balance between excitation and inhibition is a crucial role the basal ganglia exerts
on the motor cortex through the thalamus, which itself influences the cortex in an
excitatory manner. Inhibition of areas adjacent to those activated ("center-surround") in
the somatatopically-organized motor cortex is necessary to prevent co-contraction of
antagonistic muscle groups. It is precisely co-contraction and overflow into adjacent
muscles that characterizes dystonia on EMG recordings. Therefore, any loss of surround
inhibition in the cortex could theoretically result in dystonia (Hallett M, 1998) and does
so when GABA antagonists are applied directly to the motor cortex (Matsumura M,
1991). Defective inhibition of the cortex could be endogenous to the area or due to
altered excitatory versus inhibitory input from the thalamus or other structures. The
thalamus in turn may be the original site of malfunction or may receive aberrant signals
from the basal ganglia or even the cerebellum, which has a large input to the motor
thalamus.
8
The theory of impaired cortical inhibition also applies to repetitive-use induction of
dystonia, which results in larger cortical representation areas of the affected body parts
and that may affect inhibition (Hallett M, 1998). While many theories are currently being
discussed in the literature, very few are consistently supported by findings in dystonic
patients. This could mean that the current theories do not address the mechanisms central
to dystonia or perhaps no one common pathway is responsible for dystonic states. It is
plausible that multiple pathways could result in dystonia independently; however, it is
more likely that multiple causes merge into one common final pathway leading to
dystonia. If the latter is the case, theories concentrating on upstream components of the
generic or common pathway will not be supported by data collected from dystonic
patients or animals that have dystonic origin in another upstream branching pathway.
Thus, research on a particular cause or theory of dystonia will not show consistent results
if dystonias of different etiologies comprise the population being studied. However, a
potentially common downstream component of the dystonic pathway should consistently
show alterations in many forms of dystonia when studied. This theory of branching
etiologies is depicted schematically in figure 1.1.
9
3
MOTOR CORTEX
DYSTONIA
Somatosensory
Cortex
2
THALAMUS
1
BASAL
GANGLIA
Gpi/SNr
4
CEREBELLUM
Striatum
Figure 1.1. Schematic depiction of brain regions and pathways theorized to malfunction
in dystonia. There are four main regions currently implicated in the pathophysiology of
dystonia: (1) the basal ganglia, (2) the thalamus, (3) the sensorimotor cortex, and (4) the
cerebellum.
While theories implicating altered basal ganglia function, thalamic dysfunction and
cortical disinhibition continue to be debated, studies from animal models and patients
with dystonia will help determine the mechanistic basis of dystonia.
Despite new
evidence and the description of alternative plausible theories, such as the central broad
deficiency of cortical inhibition, the role of the basal ganglia continues to dominate the
field of dystonia. Concentration on and search for alterations in basal ganglia function in
10
patients with primary dystonia occurs perhaps at the expense of crucial new insights to
the yet undetermined pathophysiology of this disease.
Findings in animal models suggest that the main basal ganglia-thalamo-cortical
circuit is too restricted as the sole pathway examined in understanding the
pathophysiology of dystonia (Richter A, 1998). Numerous other brain regions and even
the spinal cord have been shown to be functionally altered in dystonic patients. Evidence
implicating sensory dysfunction and impaired reciprocal inhibition in dystonic patients
are two examples of consistent findings seemingly unrelated to the basal ganglia.
Perhaps the most consistent finding however comes from functional imaging studies of
dystonic patients representing a wide range of etiological origin. Blood flow analysis and
glucose utilization imaged in the CNS of dystonic patients consistently demonstrates
increased activity in the cerebellum.
Patients with familial generalized idiopathic
dystonia due to the DYT1 mutation were studied and two patterns of altered glucose
metabolism were identified. One related to dystonic movements (movement related) and
the other unrelated to movement (movement free).
The midbrain, cerebellum and
thalamus showed hypermetabolism in the movement related pattern of activity while the
lentiform nuclei, cerebellum and supplemental motor areas were hypermetabolic in nonmanifesting as well as dystonic patients carrying the DYT1 mutation in the movement
free pattern (Eidelberg D, 1998). Furthermore, the movement free pattern was also noted
during sleep, when involuntary movements are suppressed. Prior to this study, a single
patient with generalized dystonia was reported to have increased cerebral blood flow in
subcortical motor structures in addition to changes in the cerebellar blood flow.
Specifically, the was discordance between the blood flow in the right and left deep
11
cerebellar nuclei (DCN) and abnormal correlation between right cerebellar cortical and
right DCN blood flow (LeDoux MS, 1995). As more imaging studies are reported, an
enormous amount of literature is accumulating, many demonstrating functional alteration
of the cerebellum in patients with dystonia.
Patients with writer's cramp, a form of focal dystonia, repeatedly demonstrate
increased activity in the contralateral primary sensorimotor and premotor corticies and
thalamus with ipsilateral hyperactivity in the cerebellum (Odergren T, 1998; Preibisch C,
2001). These regions form the cerebrocerebellar circuit and the regions of the cerebellum
and thalamus involved indeed correspond to the areas of efferent origin and termination
from the cerebellum respectively. Such over-activation in the cerebrocerebellar circuit
may be causative in initiating dystonia or may reflect an attempt to compensate for an
otherwise initiated dystonic signal.
Similar activation of the cerebellum was also
demonstrated in patients with essential blepharospasm (EB), a focal dystonia affecting
eyelid closure. EB patients demonstrated increased metabolism in patterns analogous to
those described above for the movement-free pattern described for DYT1 dystonia. Their
movement related pattern however differed from that seen in DYT1 dystonia with
hypermetabolism in the cerebellum and pons being most notable (Hutchinson M, 2000).
Increased cerebellar perfusion was also seen in studies of patients with paroxysmal
exercise-induced dystonia with relative hypoperfusion of the frontal cortex and to a lesser
extent the basal ganglia (Kluge A, 1998).
Thus, it appears that consistent findings of
altered cerebellar function affecting the cerebrocerebellar circuit are made in dystonias of
vastly different etiologies.
12
An animal model of primary generalized dystonia, the dystonic rat shows a consistent
and necessary role of abnormal cerebellar activity in the dystonic phenotype, specifically
increased DCN output (Ledoux MS, 1995).
The wriggle mouse sagami has also
demonstrated cerebellar abnormalities, mainly histologic changes within the molecular
layer (Ikeda M, 1989) and there exist lesions of mossy fibers within the cerebellum of the
dystonia musculorum mutant mouse (Sotelo C, 1988). The dystonic hamster, a rodent
model of paroxysmal nonkinesegenic dystonia, demonstrates cerebellar (DCN) along
with red nuclear and thalamic alterations in metabolism (reviewed in Richter A, 1995).
While rodent animal models and human functional brain studies implicate a role for
the cerebellum in numerous dystonic states, the basal ganglia continues to receive the
most attention in the field of dystonia research. In fact, literature pertaining to studies of
human dystonia frequently mention the lack of suitable animal models of dystonia other
than MPTP treated monkeys, which represent a drug-induced dystonia model of basal
ganglia origin. The restricted focus of dystonia research to pathologies of the basal
ganglia may severely impede progress in the field and retard potential benefit to the
patients who suffer from dystonia. Hopefully, the findings of consistently abnormal
cerebellar function in human studies will broaden the field of dystonia research and
expand the role rodent animal models play in understanding the pathophysiology of
dystonia.
Treatment
Given the lack of understanding of the pathophysiology of dystonia and the
heterogeneity of dystonic syndromes, no single treatment is effective in all patients. In
fact, treatment for the dystonic syndromes is only moderately effective overall. Initially,
13
levodopa is administered to verify that a diagnosis of dopamine responsive dystonia has
not been overlooked (Adler CH, 2000; Fahn S, 1995). A series of drugs are reported to
have some effect in different forms of dystonia, and are administered on a trial -and-error
basis. These include anticholinergics (high-dose), baclofen (high-dose), benzodiazepines,
and anti-dopaminergics. Each of these classes of drugs works only in a minority of
patients and thus underscores the need for better understanding of the pathophysiology of
dystonia. Surgical thalamotomy is also used in severe refractive cases with variable and
often temporary success. A more recent central intervention is the use of deep brain
stimulation of the globus pallidus. This technique is in its infancy, but holds promise for
the future (Adler CH, 2000). A common treatment for focal dystonia is periodic injection
of botulinum toxin in the affected muscle groups. The majority of patients tolerate the
toxin well and benefit from the therapy. Surgical denervation of affected muscles is
sometimes used in intractable focal dystonias as well (Adler CH, 2000; Fahn S, 1995).
Paroxysmal Dyskinesias
Paroxysmal dyskinesias are a specific subgroup of dystonia defined by the
intermittent nature of the dystonic movements on a background of otherwise healthy
individuals. The episodic nature, involvement of motor behaviors, and other salient
features of this subgroup of dystonia historically resulted in misdiagnosis as reflex
epilepsy, movement-induced seizures, and subcortical epilepsy (Demirkiran M, 1995).
However, the dystonic nature of the movements, absence of any EEG correlates,
complete maintenance of consciousness, and lack of any postictal state refuted the theory
that the paroxysmal dyskinesias are a form of epilepsy (Demirkiran M, 1995).
Paroxysmal dyskinesias occupy a unique position in that they represent a crossroads
14
between dystonia and episodic neurological disease. Consequently, advancement in the
study of episodic diseases will benefit research on the paroxysmal dystonias and therefore
on dystonia as a whole.
The paroxysmal dyskinesias are rare syndromes of intermittent dystonia subdivided
into four groups based on clinical characteristics: (1) Paroxysmal kinesigenic dyskinesia
(PKD), (2) Paroxysmal non-kinesigenic dyskinesia (PNKD), (3) Paroxysmal exerciseinduced dyskinesia (PED), and (4) Paroxysmal hypnogenic dyskinesia (PHD).
Definitions and salient features of the paroxysmal dyskinesias are presented in Table 1.2.
Further consideration will only be given to the first two forms, PKD and PNKD, due to
relevance here.
Subgroup
PKD (PKC)
Characteristics
Brief duration (seconds to 5 minutes)
Always preceded by sudden initiation of movement
PNKD (PDC)
Prolonged duration (2 minutes to 4 hours)
Spontaneous or triggered by various stressors
PED
Intermediate duration (5 to 30 minutes)
Precipitated by continuous movement (not sudden)
PHD
Occurs during sleep (ADNFLE in some kindreds)
ADNFLE, Autosomal Dominant Nocturnal Frontal Lobe Epilepsy,
PKC, Paroxysmal kinesigenic choreoathetosis, PDC, Paroxysmal Dystonic
Choreoathetosis
Table 1.2. Subgrouping of the Paroxysmal Dyskinesias.
Paroxysmal dyskinesias like other forms of dystonia result from both primary and
secondary etiologies. Secondary paroxysmal dyskinesias are relatively uncommon and
have been associated with neurological diseases such as multiple sclerosis, cerebral palsy,
stroke, encephalitis, birth asphyxia, certain seizure disorders, or metabolic diseases such
as idiopathic hypoparathyroidism and thyrotoxicosis (Goodenough DJ, 1978; Demirkiran,
1995; Nardocci, 1989). In contrast to the primary paroxysmal dyskinesias, secondary
dyskinesias are accompanied by neurological findings and often abnormal EEG
15
recordings (Goodenough DJ, 1978).
Symptomatic presentation of paroxysmal
dyskinesias is similar between primary and secondary forms; however, for ease of
presentation the remainder of discussion will concern primary paroxysmal dyskinesias.
Paroxysmal Kinesigenic Dyskinesia
PKD occurs more frequently than PNKD (Nardocci, 1989; Goodenough DJ, 1978).
PKD is characterized by attacks of dystonia precipitated by sudden movement lasting
from a few seconds to 5 minutes. The majority of patients experience 1 to 10 attacks per
day (Houser MK, 1999; Goodenough DJ, 1978; Nardocci, 1989; Bhatia KP, 1999). The
most common precipitating situation is standing from a sitting or lying position. In
general, sudden movements after a period of rest most commonly cause the attacks.
Startle is also occasionally associated and predisposing factors to increased sensitivity
include alcohol and exhaustion. The distribution of the dystonia varies widely, often
restricted to one side of the body but may be generalized. Some of the attacks display
mixed hyperkinetic involuntary movements of dystonia, choreoathetosis, and ballismus
(Demirkiran M, 1995; Goodenough DJ, 1978; Bhatia KP, 1999). Sensory aura preceding
the attacks is also common and described as "tingling" or stiffness in the limbs. Most
patients have learned to avoid the induction of attacks altogether by initiating movements
slowly or warming-up prior to initiation (Goodenough DJ, 1978; Bhatia KP, 1999). No
alteration in consciousness is ever reported and no postictal state is associated with the
attacks (Houser MK, 1999; Goodenough DJ, 1978; Bhatia KP, 1999). EEG recordings
are almost invariably normal during and between attacks in PKD patients. All other
physical and neurological exams and laboratory studies are also normal, confirming the
healthy background on which this episodic disorder occurs (Goodenough DJ, 1978). The
16
age of onset is typically in early adolescence or early adulthood with a male
preponderance often noted. The vast majority of described cases are of primary etiology
with nearly 1/2 to 2/3 being sporadic and the remainder of familial origin (Houser MK,
1999; Nardocci, 1989). Transmission in families with PKD occurs in an autosomal
dominant fashion with reduced penetrance (Goodenough DJ, 1978). The frequency of
the paroxysms decreases with age after peaking sometime in adolescence (Goodenough
DJ, 1978; Nardocci, 1989).
Administration of anticonvulsants (e.g., phenytoin,
carbamazepine) causes marked reduction in attack frequency or elimination of the
paroxysms altogether.
Similar to other dystonias, the basal ganglia is also thought to be of central
importance in the paroxysmal dyskinesias because of the involuntary nature of the
movements, the absence of EEG abnormalities, and the presence of basal ganglia
pathology in conditions leading to secondary or symptomatic disease (Nardocci N, 1989).
Abnormal basal ganglia metabolism has been reported on PET scans of secondary PKD
patients during attacks and some have experienced a favorable response to levodopa
therapy (Goodenough DJ, 1978). Others have found thalamic lesions in the ventral
posterolateral nuclei in some secondary PKD cases (Sunohara N, 1884; Camac A, 1990;
Burguera JA, 1991; Nijssen PCG, 1992).
Paroxysmal Non-kinesigenic Dyskinesia
PNKD is characterized by attacks of dystonia that are spontaneous in nature. The
paroxysms are longer than those in PKD and last from 5 minutes to a few hours. Few
patients may have longer attacks but a majority last 30 minutes to one hour. Attack
frequency ranges widely as many as 2-3 per day to as few as 2-3 per year, with the
17
majority of patients experiencing a few attacks per month (Jarman PR, 2000;
Goodenough DJ, 1978; Nardocci, 1989; Bhatia KP, 1999). In general, the frequency of
attacks is higher in childhood and declines with increasing age and the duration of attacks
shortens with increasing age as well. PNKD is never associated with sudden initiation of
movement and is generally considered to be a spontaneous event. However, a number of
triggers have been identified that precipitate attacks in most patients. Common triggers
include caffeine, alcohol, stress, fatigue, hunger, cold, menstruation, intercurrent illness,
and excitement (Jarman PR, 2000; Goodenough DJ, 1978; Nardocci, 1989). In addition to
certain precipitating factors, there is a diurnal pattern with an increased frequency of
attacks in the late afternoon and evening compared to early in the day (Jarman PR, 2000).
Attacks always begin in a limb and progress to hemidystonia or generalized dystonia
consisting largely of sustained dystonic posturing (Jarman PR, 2000; Nardocci, 1989;
Bhatia KP, 1999). Sensory aura preceding the attacks is also common and described as
"tingling" in the skin or tightness in the muscles with a feeling of restlessness (Jarman
PR, 2000; Goodenough DJ, 1978; Bhatia KP, 1999). During the sensory prodrome and in
the initial portion of an attack, patients may interrupt the progression by going to sleep.
Just a few minutes of sleep will often abort the attack altogether if initiated early in the
course (Jarman PR, 2000).
No alteration in consciousness is ever reported and no
postictal state is associated with the attacks. EEG recordings are almost invariably
normal during and between attacks in patients with PNKD. The age of onset is typically
in infancy or childhood, which is earlier than PKD. As with PKD a male preponderance
is noted, especially in the more common familial PNKD (Goodenough DJ, 1978;
Nardocci, 1989; Bhatia KP, 1999). An autosomal dominant mode of inheritance is seen
18
in families with PNKD. Administration of anticonvulsants is not an effective treatment in
PNKD, clearly separating this disorder from PKD in treatment strategies.
Benzodiazepines often have a modest degree of improvement, but not in all patients
(Jarman PR, 2000; Nardocci, 1989).
Linkage analysis of several families with PNKD has localized the causative gene in
the autosomal dominant form of the disease to chromosome 2q33-35 (Fouad GT, 1996;
Fink JK, 1996). Due to the episodic nature of the disorder and the association of episodic
disease and channelopathies, a candidate ion channel in this region has been identified
(Hofele K, 1996). A second gene locus has also been identified in a German kindred
with a variant of PNKD that is triggered by physical exercise in addition to the common
PNKD triggers. In addition, this form is often associated with headaches, diplopia
(which is also a rare symptom in some severe PNKD cases), perioral paresthesias, and
spastic paraplegia in some of the patients.
The gene has mapped to an area of
chromosome 1p where several potassium channel genes reside and has been termed CSE
(choreoathetosis/spasicity, episodic) (Auburger G, 1996).
As is typical with
channelopathies, occurrence of other episodic neurological diseases in patients with
PNKD has been reported.
Migraine has most commonly been associated with
paroxysmal dyskinesias, along with epilepsy and an isolated case of episodic ataxia as
well (Hofele, 1997; Mayeux, 1982).
19
1.2.
TOTTERING MOUSE: ANIMAL MODEL OF DYSTONIA
Introduction
The use of animal models in the study of human disease has proved invaluable in
defining pathophysiologies and testing treatment strategies.
Models can be derived
through surgical, drug, or genetic manipulation as well as occur naturally through random
mutations. The tottering mouse is one such naturally occurring genetic animal model of
human disease.
As a model for absence epilepsy and more recently, paroxysmal
dystonia, the tottering mouse has been studied extensively since its discovery in 1957 at
the Roscoe B. Jackson Memorial Laboratory in Maine. An abnormal, wobbly gait was
observed in three DBA/2J mice who subsequently were shown to be homozygous for a
new recessive mutation, which was named tottering (Green, 1962). The original mice
were fertile when out-crossed to C57Bl/10JGn and F1 intercrossing produced tottering F2
in expected ratios for autosomal recessive trait. Tottering (tg) was found to be closely
linked to Oligosyndactylism (Os) with crossover occurring between Os and tg rarely, if at
all. (Green, 1962) Through positional cloning, the tottering mutation was identified as a
C to T base substitution at position 1802 in the ? 1A subunit of the P/Q-type high-voltage
dependent calcium channel. The mutation results in a proline to leucine substitution at
amino acid 601 near the P-domain of repeat II between transmembrane segments S5-S6
(Fletcher, 1996).
Calcium Channels
Structure
Calcium channels are part of the cellular mechanism that tightly regulates calcium
concentration in and around the cell. Such tight regulation is necessary for the accurate
20
and timely execution of numerous calcium-dependent functions. Excitation-contraction
coupling in muscle cells, gene regulation, and second messenger system activation are all
important functions of various cells that occur in response to changes in local calcium
concentration. In neurons, additional processes dependent on dynamic local calcium
concentration include membrane excitability and neurotransmitter release.
Neuronal
calcium channels pertinent to this discussion are the voltage-dependent calcium channels
(VDCC), comprised of a pore-forming ? 1 subunit and additional auxiliary subunits ? ,
? 2-?, and sometimes ?. Numerous genes encode ? 1 subunits, all of which share a
common structure of four repeated domains, each containing six transmembrane
segments (S1-S6). A P-domain in the extracellular space between S5-S6 transmembrane
segments is responsible for the pore environment and ion selectivity. In addition to
determining ion selectivity, the ? 1 subunit acts as the voltage sensor and determines the
kinetics of activation and inactivation. The cytoplasmic face of the protein also interacts
with G-protein ? ? subunits. The remaining auxiliary subunits of intact calcium channels
act to modulate gating and kinetics of the channel (Catterall WA, 1988; Walker D, 1998).
The subunits associate to form a complete channel as depicted in Figure 1.1.
21
?2
?2
?
extracellular
?1
intracellular
?
Figure 1.2. Schematic depiction of voltage-gated calcium channel. The ? 1 subunit is
the main pore-forming subunit while ? and ? 2- ? subunits are auxiliary.
Function
VDCC function varies depending on the subunit composition as well as the
subcellular and tissue distribution. Five genes encode neuronal high-voltage activated
Ca2+ channel pore-forming ? 1 subunits (A-E). Four encode the ? subunit (1-4), two
genes are believed to encode the ? 2-?, and one for the neuronal ? subunit. The neuronal
high-voltage activated calcium channels are classified in Table 1.3 according to their
molecular biology, pharmacology, and functional characteristics. An enormous potential
for VDCC heterogeneity exists because of the numerous combinations of ? 1, ? , ? 2-? and
? subunits that may assemble and the number of splice variants of each subunit. Highvoltage activated calcium channels involved in neurotransmitter release (P, Q, N and Rtype) are located at synaptic terminals in close approximation to the docking and release
machinery of synaptic vesicles (Westenbroek RE, 1992; Ludwig A, 1997). As the
22
depolarizing action potential reaches the axon terminal, the VDCC senses the change in
electrical potential and opens the channel pore if the potential is indeed great enough.
This allows calcium to rapidly enter the terminal by flowing down its concentration
gradient into the cell. The local calcium concentration increases tremendously and acts
as a trigger for release of synaptic vesicle contents into the synaptic cleft.
L-type
channels play an entirely different role in neurons. These channels (? 1C and ? 1D) are
located on the proximal dendrites and somata of neurons and conduct calcium in response
to membrane depolarization leading to further excitability, gene transcription, and
activation of second messenger cascades (Ludwig A, 1997; Hell JW, 1993). Due to the
slight redundancy of VDCC subtype functions, it is plausible that one subtype may
compensate for altered activity of another similar subtype. For this sort of compensation
to occur however, a second subtype would have to be expressed in that region already or
expression would have to begin de novo.
Subunit
? 1A-a
Subtype
P
Pharmacology
? -agatoxin IVA
Location
cerebellum, hippocampus,
inferior colliculus olfactory
bulb, spinal cord
Function
Neurotransmitter
release
? 1A-b
Q
? -conotoxin MVIIC
? 1B
N
? -conotoxin MVIIA, ? conotoxin GIVA
diffuse, hippocampus
Neurotransmitter
release
? 1C
L
diffuse, olfactory bulbs,
hippocampus, superior
colliculus, cerebellum
Electrical excitability,
gene transcription
L
dihydropyridines,
benzothiazepines,
and phenylalkylamines
? 1D
? 1E
R
cadmium, nickel
olfactory bulb, habenula,
Neurotransmitter
cortex, hippocampus,
release
cerebellum
(Hillman D, 1991; Tanaka O, 1995; Westenbroek RE, 1992; Stea A, 1994; Ludwig A, 1997)
Table1.3. Classification of neuronal high-voltage activated calcium channels.
23
Behavioral Phenotype
As with many disorders caused by mutations in ion channels, tottering mice display
several phenotypes and some are characterized as episodic or intermittent in nature.
Diseases caused by mutations in ion channel genes, termed channelopathies, are of
growing interest as they demonstrate causative roles in episodic neurological and
neuromuscular diseases such as migraine, ataxia, epilepsy, dystonia, and paralysis
(Ophoff RA, 1996; Grosson CLS, 1996; Browne DL, 1994; Fouad GT, 1996; Auburger
G, 1996; Steinlein OK, 1995). These diseases often share an overlapping clinical spectra
of symptoms, suggesting common pathomechanisms involving the ion channel gene
defects.
Co-occurrence of multiple episodic phenotypes in a single disorder (e.g.,
migraine headache and hemi-paresis in Familial Hemiplegic Migraine) is common and
suggests a unique pathomechanism in channelopathies. It is likely that the expression of
mutated channels in multiple regions results in the seemingly unrelated concomitant
phenotypes, but the reason for the intermittent expression of the phenotypes is less clear.
Tottering mice display three distinct behavioral phenotypes as part of their neurological
syndrome, including polyspike discharges, ataxia and paroxysmal dystonia. Both the
polyspike discharges and the dystonic attacks are episodic in nature, while the ataxia is
always present.
Polyspike Recordings
Initial studies were aimed at defining an epileptiform activity associated with the
bizarre characteristic motor episodes of the tottering mouse that are described below
under 'intermittent dystonic attacks.'
Instead of defining motor seizures however,
researchers discovered an unexpected phenotype of abnormal bursts of bilaterally
24
synchronous and symmetrical spike waves, six to seven per second, over the cerebral
hemispheres in tottering mice at rest. This activity constitutes ~10% of resting EEG
activity and is always accompanied by sudden arrest in movement, staring and often
twitching of the jaw. The bursts are 200-400? V in amplitude and last from 0.3-10
seconds. The spike-wave abnormality is fully developed in the 4-week old animal,
although the wave component decreases substantially with age (Noebels, 1979). These
polyspike bursts occurred paroxysmally during waking hours and within motor episodes,
but reliably during drowsiness.
Sometimes a spike-wave appearance was appreciated,
resembling human absence seizures (3/s spike-wave activity) and postictal EEG
depression was never present, similar to absence epilepsy (Kaplan, 1979).
This
serendipitous finding has led to the use of the tottering mouse as a genetic animal model
of absence epilepsy.
Ataxia
The most easily observed phenotype of the tottering mouse is that for which it was
named, an ataxic gait. Original reports on Os/tg stock at Jackson laboratories describe
increased toeing-out of the hind feet at 2-3 weeks and soon thereafter the trunk is held
closer to the ground and the mouse may lean while walking (Green, 1962). Analysis of
tottering gait patterns revealed decreased stride and step lengths and increased gait angle
compared to controls (Campbell DB, 1999).
Although the ataxic phenotype of the
tottering mouse is useful in differentiating tottering animals from control littermates, little
research has been aimed at defining the basis for this finding.
25
Intermittent dystonic attacks
Tottering mice display striking episodic attacks of severe motor dysfunction that were
originally described as seizures. Quite stereotypical in nature, the attacks typically begin
with a hind limb being held tight against the trunk or abducted with some paddling in the
air. Forelimb involvement follows with the limb again being held tight against the trunk
or with paddling. Progression to include both hindlimbs, abducted at the hip and knee
into the air, results in the abdomen resting on the cage bottom and the trunk flattens. The
back next becomes stiff and arched such that the perineum is pressed against the cage
bottom. As forelimbs become more prominently involved in the next phase, the neck
also flexes severely and the ears fall flat against the back and the jaw and eyelids may
move repetitively (Green, 1962). The attacks typically last 30-60 minutes and occur
spontaneously or in response to several known stressors and pharmacological agents with
no discernable refractory period. Although the described attack is most common, there
are other characteristic postures adopted by dystonic tottering mice and attacks do vary in
course, duration, and severity within and between mice. There is no reliable abnormal
EEG recording during these attacks (Kaplan, 1979). Therefore, the term ‘seizure’ is
rapidly falling out of favor as the preferred descriptor of this behavior. Convulsions,
motor seizures, motor episodes, and myoclonic-like movement disorder are all still used
in the literature to describe this phenomenon but we believe that the best and most
accurate descriptor is paroxysmal dystonia. The remainder of this work will deal almost
exclusively with the phenotype of intermittent dystonia and defining the neuronal
networks responsible for its occurrence.
26
Cellular Pathology
Locus ceruleus hyperarborization
Using the tottering mouse as a model for absence epilepsy, researchers investigated
catecholaminergic innervation in the mutant.
Histochemical analysis showed a
significant increase in the number of noradrenergic axons in terminal fields innervated by
the nucleus locus ceruleus (LC) when compared to wild type. A concomitant 100-200%
rise in norepinephrine (NE) levels is found in the same areas, including hippocampus,
cerebellum, and dorsal lateral geniculate. These changes were indeed due solely to
hyperarborization as the size and number of LC neuronal somata were unchanged (Levitt,
1981). 6-hydroxydopamine lesioning of noradrenergic fibers innervating the neocortex
and hippocampus resulted in loss of the polyspike phenotype (Noebels JL, 1984). In
these experiments the dystonic phenotype and LC innervation to cerebellar and brainstem
structures remained however. Therefore, locus ceruleus axons were lesioned with the
neurotoxin, DSP-4 in the tottering mouse to test the hypothesis that the noradrenergic
hyperinnervation (to the cerebellum in particular) was responsible for the intermittent
dystonic phenotype. After successful lesioning of LC innervation to the cerebellum,
tottering dystonic attacks remained unchanged further supporting the independence of
these two phenotypes (Campbell DB, 1999).
Aberrant Tyrosine Hydroxylase Expression
The discovery of noradrenergic hyperinnervation also led researchers to examine the
expression patterns of the rate-limiting enzyme in catecholamine synthesis, tyrosine
hydroxylase (TH). TH mRNA and protein expression is normal in the major
catecholaminergic nuclei, however, expression was altered in the cerebellum. TH mRNA
27
and protein are transiently expressed in Purkinje cells (PC) of normal and heterozygous
mice during development at postnatal days P21-P35. Expression is aberrantly maintained
in posterior cerebellar PC and expressed de novo in the anterior cerebellum throughout
adulthood in tottering mice, indicating compromised gene regulation in the mutant mouse
(Hess EJ, 1991; Fureman, 2001).
Purkinje Cell abnormalities
Determination of the direct effect the tottering mutation has on the P/Q-type VDCC
has been achieved using electrophysiological analysis. Dissociated mutant PC from 1830 day old tottering pups show a 40% reduction in total calcium current density
compared to wild type without changes in cell size. Recombinant tottering channel
protein expressed in baby hamster kidney cells also showed decreased current density
(Wakamori M, 1998). Thus, the mutation does indeed alter the basic function of the P/Qtype calcium channel as expected.
1.3. CHAPTER SUMMARY
Dystonia is a relatively common neurological disorder with an estimated prevalence
of 30 per 100,000 in the population (Nutt JG, 1988). Despite the discovery of genes
involved in a few of the monogenetic inherited dystonias and knowledge of the pathology
present in secondary dystonias, the pathophysiology resulting in dystonia remains poorly
understood.
Although knowledge of the neuronal basis of dystonia has grown
tremendously, conflicting results and heavy reliance on human studies underscore the
need for better utilization and discovery of animal models. Animal models provide
unique opportunities to study and manipulate in vivo neurological systems, allowing great
strides in the understanding of the pathophysiological basis of disease. Current rodent
28
animal models are limited in usefulness because the primary gene defect resulting in the
phenotype is unknown. The tottering mouse, therefore has a unique advantage as a
genetic rodent model of dystonia because of the well-defined nature of the genetic
mutation and a growing understanding of the resultant behavioral and cellular
phenotypes. Discovery of the neuronal basis of tottering mouse dystonia can further
define the neuronal networks capable of producing dystonia of other etiologies. The next
step in the use of animal models of dystonia would then be to replicate the dystoniaproducing signals defined in the tottering mouse in genetically normal animals.
Replication of the abnormal dystonic activation in wild type animals acts to eliminate
confounding variables introduced by the wide spread neurological effects in the tottering
mouse. This rationale guided the study of the cerebellar role in tottering dystonia and the
subsequent discovery of a novel animal model suited to the study of neuronal networks
capable of causing dystonia in wild type mice.
1.4 HYPOTHESES
The cerebellum plays a key role in the expression of dystonia in a mouse model with a
defined genetic background, tottering, and in a novel model using kainic acid in wild type
mice. Together, this information supports the theory that aberrant cerebellar cortical
activity leads to profound dystonic attacks.
1. Neurotransmission in the cerebellum of tottering mice is altered compared to controls.
Tottering mice harbor a mutation in the ? 1A pore-forming subunit of P/Q-type
calcium channels that function at presynaptic terminals to trigger neurotransmitter
release. Cerebellar granule and Purkinje cells in particular have a high density of P/Q-
29
type calcium channels; therefore, it is theorized that tottering cerebella may have
disrupted neurotransmitter release as a functional consequence of the mutated gene.
2. Purkinje cells are a necessary component in the expression of tottering dystonia.
Cerebellar Purkinje cells contain a high density of P/Q-type calcium channels, the
channel subtype mutated in tottering mice. The cerebellum is intensely activated during
tottering dystonic attacks and the Purkinje cell is the sole output source for the cerebellar
cortex. The role of the Purkinje cell in the cerebellar circuitry, the high level of P/Q-type
channel expression and the degree of cerebellar activation during tottering dystonia all
support the theory that the cerebellar Purkinje cell is central to the expression of tottering
dystonia.
3. Specific localized excitation of the cerebellum with kainic acid in wild type mice
causes dystonia.
Aberrant activation of the cerebellar cortex in tottering mice is involved during
initiation of dystonic attacks. Exogenous excitation of the cerebellar cortex in wild type
mice will also induce dystonia.
4. Kainic acid excitation in mice lacking Purkinje cells fails to cause dystonia.
The cerebellar cortex is able to activate pathways in excess, leading to dystonic
attacks. As is the case with the tottering mouse, it is theorized that the Purkinje cell is an
essential component of these pathways in the kainate-induced dystonia model and
dystonia cannot be elicited in animals lacking Purkinje cells.
30
Chapter 2. ALTERED NEUROTRANSMISSION IN THE
TOTTERING CEREBELLUM
Abstract
P/Q-type calcium channels are involved in several neuronal functions including
neurotransmitter release.
Tottering mice have a mutation in the ? 1A pore-forming
subunit of P/Q-type calcium channels.
This calcium channel subtype is expressed
abundantly in the cerebellum. Furthermore, the tottering mouse behavioral phenotypes of
generalized ataxia and intermittent dystonic episodes are largely cerebellar in origin.
Because P/Q-type calcium channels have been implicated in calcium-dependent
neurotransmitter release, glutamate and GABA release was investigated in the tottering
mouse cerebellum as a functional consequence of the channel mutation. Cerebellar
synaptosomes from wild type and tottering mice were preloaded with 3H-glutamate or
3
H-GABA and then superfused with Earle’s balanced salt solution. Neurotransmitter
release was induced by depolarization with 60mM KCl. Potassium-stimulated release of
3
H-glutamate and 3H-GABA in wild type and tottering mouse cerebellar synaptosomes
was calcium-dependent. Potassium stimulated calcium-dependent 3H-glutamate release
was significantly decreased in tottering cerebella compared to controls while 3H-GABA
release remained unchanged. These data indicate a deficiency in excitatory but not
inhibitory neurotransmission in the tottering cerebellum.
Introduction
Tottering mice harbor a mutation in the ? 1A pore-forming subunit of P/Q-type
voltage dependent calcium channels (VDCCs). P/Q-type calcium channels are highvoltage activated channels located at presynaptic terminals where influx of calcium
31
through P/Q-type channels triggers neurotransmitter release. Therefore, it is theorized
that tottering mice may have disrupted neurotransmitter release in regions of high P/Qtype calcium channel expression as a functional consequence of the mutated gene.
Furthermore, alterations in neurotransmitter release may in part be responsible for the
tottering mouse phenotypes of six- hertz polyspike discharges (absence seizures), ataxia,
and paroxysmal dystonia. In effort to examine neurotransmitter release in the tottering
mouse, numerous researchers have employed various techniques to investigate
neurotransmission in this mutant.
The tottering neuromuscular junction (NMJ) was investigated by Plomp et al. because
P-type calcium channels are expressed at the NMJ and these synapses are relatively easy
to study. Miniature endplate potential (MEPP) and low frequency evoked endplate
potential (EPP) amplitudes were not different between tottering homozygotes, tottering
heterozygotes, or wild type controls.
However, EPP amplitude run down was
significantly increased in tottering mice and tottering muscles were paralyzed at lower
concentrations of the acetylcholine receptor antagonist, tubocurine, than were controls.
These data indicate a smaller 'safety factor' in tottering mice, which is the ratio between
EPP size and the depolarization necessary for muscle firing. The safety factor is typically
substantial in the NMJ to prevent small changes in acetylcholine release from failing to
elicit the all-or-none requirement for muscle activation. A second change noted in the
tottering NMJ was an increase in spontaneous MEPP frequency in both tottering homoand hetero-zygotes (Plomp JJ, 2000).
Although it is difficult to extrapolate the
significance of these NMJ changes on CNS neurotransmission, the increased spontaneous
32
release of quanta and the increased run down of release after high frequency stimulation
suggest a deficit in the control of neurotransmitter release in tottering mice.
In the CNS, neurotransmission studies are usually separated into excitatory and
inhibitory categories with glutamate and GABA being representative of each group
respectively.
Ayata et al. showed decreased glutamate and GABA release in the
frontoparietal cortex (neocortex) of adult male and female tottering mice using in vivo
microdialysis after 100mM KCl stimulation. A 2-fold decrease in glutamate release and
a 3-fold decrease in GABA release was measured in tottering mice compared to controls
(Ayata C, 2000).
Different results were obtained in thalamus when Caddick et al.
measured excitatory post-synaptic potentials (EPSPs) and inhibitory post-synaptic
potentials (IPSPs) using whole cell recordings of single neurons in the somatosensory
thalamus (ventrobasal nucleus) of P14-28 male tottering mice. Stimulus-evoked EPSPs
recorded after electrical stimulation of adjacent neurons revealed significantly smaller
EPSPs in tottering animals compared to wild type controls. In contrast, tottering and
control maximal evoked IPSPs were not significantly different. These data support a
defect in excitatory neurotransmission in tottering thalami with no change in inhibitory
neurotransmission (Caddick SJ, 1999).
Tottering mouse neurotransmission at the hippocampal Schaffer collateral synapse
was studied by Qian and Noebels. Both Ca2+ influx and field excitatory post-synaptic
potentials (fEPSPs) were recorded after electrical stimulation. Although Ca2+ influx and
fEPSPs were at normal levels, tottering animals have an increased requirement for N-type
calcium channel function compared to controls (Qian J, 2000). Similar results were
obtained by Jun et al. working with the ? 1A subunit knockout mouse (Jun K, 1999). In
33
these animals, neurotransmission at the hippocampal Schaffer collateral synapse was
solely dependent on N-type channels in comparison to controls, which rely on both N-,
and P/Q-type channels. Barium currents were also studied in these knockout animals in
the excitatory cerebellar granule cells and in inhibitory Purkinje cells (PCs). Total
current was decreased in both cell types; however, only PCs showed a compensatory
increase in current through N- and L- type channels. Campbell and Hess reported the
original evidence for alternative calcium channel subunit compensation for deficits in the
tottering mouse, with increased L-type calcium channel expression in the tottering
cerebellum (Campbell DB, 1999). All of these data suggest that some regions of the
nervous system may better be able to compensate for impaired P/Q-type calcium channel
function than other areas. Regions with both N- and P/Q-type channel dependent release
may more easily compensate for the P/Q-type deficiency than neurons where N-type (or
R-type) channels are not typically expressed.
In fact the ability to compensate for
decreased P/Q-type dependent release may not only be regionally restricted, but also
restricted by neuronal sets, such as excitatory versus inhibitory.
The 6-Hz polyspike phenotype of the tottering mouse is often studied as a model for
absence epilepsy. Therefore, brain regions tested for altered neurotransmission often are
chosen due to their relationship to seizure generation. A less studied phenotype of the
tottering mouse is the characteristic intermittent dystonic attacks. The cerebellum has
been associated with tottering dystonic attacks through in situ studies of c-fos activation
following an attack and through genetic ablation of cerebellar PCs in double mutant
pcd/tottering mice (Campbell DB, 1998; Campbell DB, 1999). In addition, the calcium
channel subunit mutated in tottering mice, ? 1A, is abundantly expressed in cerebellar
34
PCs and cerebellar granule cells. Together these data suggest that the cerebellum is a
likely site for functional alterations from the tottering mutation and such changes may
directly relate to dystonic attacks in this mutant.
We therefore examined neurotransmitter release in the cerebellum, a region not yet
studied.
GABA and glutamate are the most prevalent neurotransmitters in the
cerebellum.
Mossy fibers and climbing fibers are the extracerebellar input to the
cerebellar cortex and these connections excite granule cells and PCs, respectively,
through glutamatergic synapses. Glutamate is also released by granule cells to stimulate
Purkinje, basket, stellate, and golgi cells. The inhibitory neurotransmitter, GABA, is
released by Purkinje, basket, stellate, and golgi cells to their respective targets as
illustrated in Figure 2.1. Superfusion of cerebellar synaptosomes preloaded with tritiated
neurotransmitters was used to test the hypothesis that neurotransmitter release is altered
in the cerebellum of the tottering mouse as a functional consequence of the mutation in
the P/Q-type calcium channel.
Extracerebellar
Nuclei
Climbing
Fiber
Mossy
Fiber
GC
PC
DCN
B
S
G
Figure 2.1. Schematic diagram of neuronal connections in the cerebellum. Arrowheads
indicate excitatory neurotransmission of glutamate and flat bars represent GABAergic
inhibitory neurotransmission. GC, granule cell; PC, Purkinje cell; G, golgi cell; S,
stellate cell; B, basket cell; DCN, deep cerebellar nuclei.
35
Materials and Methods
Mice
Originally obtained from Jackson laboratories, tottering mice and control C57Bl/6J
mice were maintained at the Pennsylvania State University College of Medicine vivarium
on a 12-hour light cycle with access to food and water ad libitum. Tottering progeny
were rapidly identified by lack of oligosyndactylism from crosses between tottering
heterozygotes carrying the oligosyndatylism allele in repulsion to the tottering allele.
Male and female mice used in these experiments were between 8-10 weeks of age.
Synaptosome Preparation
Mice were sacrificed by carbon dioxide asphyxiation followed by decapitation and
brains were removed on ice. Cerebella were homogenized in 10 volumes of 0.32 M
sucrose. The homogenate was centrifuged for 10 minutes at 3,300 rpm at 4oC. The
supernatant was then centrifuged for 45 minutes at 13,500 rpm at 4oC to yield a pellet
containing the crude synaptosomal fraction. The pellet was resuspended in 1.2 ml of
balanced Earles salt solution (1.8mM CaCl2, 5.3mM KCl, 0.8mM MgSO4, 117mM NaCl,
26mM NaHCO3, 1mM NaH2PO4-H2O, 5.6mM glucose). The synaptosomal preparation
was equilibrated to 37oC for 10 minutes. Tritiated glutamate or GABA was added to a
concentration of 150 nM or 200 nM respectively. The synaptosomes were incubated for
20 minutes at 37oC to allow incorporation of the exogenous neurotransmitter into
synaptic vesicles.
Release Assay
200? l of the preloaded synaptosomes were aliquoted into a 12-chamber Brandel
superfusion apparatus between two GF/C filters (Brandel, Gaithersburg, MD).
The
36
synaptosomes were rinsed with Earles buffer without CaCl2 for 45 minutes at 0.5 ml/min.
The buffer was continually oxygenated with 95%oxygen/5%carbon dioxide gas. Three
baseline fractions were collected for 3 minutes each before the perfusate was changed to
the stimulation buffers containing 60 mM KCl with or without 1.8 mM CaCl2 for 2 min.
The buffer was returned to basal Earles buffer without CaCl2 for the remainder of the
fraction collection. Following collection, water perfused the synaptosomes to release any
remaining neurotransmitter by osmotic lysis. Three milliliters of ScintiVerse (Fisher,
Pittsburgh, PA) was added to each of the 1.5 ml fractions (3 min collection at 0.5 ml/min)
and mixed prior to liquid scintillation spectroscopy at an efficiency of 35-45%.
Data Analysis
The neurotransmitter collected in each fraction is expressed as a percentage of the
total neurotransmitter available in that chamber at the time of fraction collection. Percent
fractional release is calculated using the following formula (Snyder DL, 1992):
% Fractional =
Release
DPM of Fraction
(Total DPM – DPM collected
in prior fractions)
x
100
This conversion allows comparison between samples that may contain differing amounts
of tritiated neurotransmitter. The release in each of the two fractions following KCl
stimulation was subtracted from baseline and summed for every chamber.
Each animal
provided 2 experimental (60mM KCl, 1.8mM CaCl2) and 2 control (60mM KCl; 0mM
CaCl2) samples. The duplicate samples from each animal were then averaged after
correction for baseline to generate a peak %-fractional release value per animal. The
peak % fractional release was then averaged and compared between genotypes and tested
for statistical significance using the Student's t-test. Data greater than two and a half
37
standard deviations from the mean were excluded from analysis. Occasionally a given
chamber failed to show release of any kind indicated by extremely low DPM upon liquid
scintillation spectroscopy; these chambers were excluded from further analysis.
Results
3
H-Glutamate release from cerebellar synaptosomes
Synaptosomes exposed to stimulation buffer containing 60mM KCl and 1.8mM
CaCl2 released 3H-glutamate in the two fractions following stimulation. Synaptosomes
exposed to 60mM KCl in the absence of CaCl2 did not release 3H-glutamate, verifying
the calcium-dependence of release in these experiments (Figure 2.2). The average peak
%-fractional release of 3H-glutamate from tottering synaptosomes was 33% reduced
compared to controls representing a significant reduction (p<0.005) in excitatory
neurotransmission (Figure 2.3).
3
H-GABA release from cerebellar synaptosomes
Cerebellar synaptosomes from wild type and tottering animals released 3H-GABA
after perfusion with stimulation buffer containing 60mM KCl only in the presence of
1.8mM CaCl2.
Samples exposed to 60mM KCl in the absence of CaCl2 did not
demonstrate neurotransmitter release, again indicating the calcium dependence of release
in this assay (Figure 2.4). No difference was observed in the peak % fractional release
between wild type and tottering mice (Figure 2.5).
Together these data suggest a
decrease in excitatory neurotransmission in the tottering cerebellum with no change in
inhibitory neurotransmission.
38
40
Averege % Fractional Release
35
wt +K/+Ca
tg +K/+Ca
wt +K/-Ca
tg +K/-Ca
30
25
20
15
10
5
0
1
2
3
4
5
Fraction
6
7
8
9
Figure 2.2. 3H-Glutamate Relesase From Cerebellar Synaptosomes. Buffers were
perfused over cerebellar synaptosomes loaded with 3H-glutamate at a rate of 0.5ml/min
and collected in 9 three-minute fractions. The horizontal line in fraction 3 indicates time
of stimulation buffer perfusion containing 60mM KCl/1.8mM CaCl2 in closed symbols
and 60mM KCl without CaCl2 in open symbols. Wild type animals (n=9) are represented
by squares and tottering animals (n=9) by triangles.
Average Peak % Fractional Release
50
45
40
**
35
30
25
20
15
10
5
0
wt
tg
Figure 2.3. Comparison of Tottering and Wild Type Peak 3H-Glutamate Release.
Percent fractional release was summed over two fractions after correction for basal 3Hglutamate release for every animal. The peak release was then averaged for the 9 animals
in each group and compared using the Student's t-test. Data represent mean %-fractional
release + SEM. ** indicates significant decrease in tottering release, p<0.005.
39
50
45
wt +K/+Ca
wt +K/-Ca
tg +K/+Ca
tg +K/-Ca
% Fractional Release
40
35
30
25
20
15
10
5
0
1
2
3
4
5
6
7
8
9
Fraction
Figure 2.4. 3H-GABA Relesase From Cerebellar Synaptosomes. Buffers were perfused
over cerebellar synaptosomes loaded with 3H-GABA at a rate of 0.5ml/min and collected
in 9 three-minute fractions. The horizontal line in fraction 3 indicates time of stimulation
buffer perfusion containing 60mM KCl/1.8mM CaCl2 in closed symbols and 60mM KCl
without CaCl2 in open symbols. Wild type animals (n=6) are represented by squares and
tottering animals (n=6) by triangles.
60
Average Peak % Fractional Release
55
50
45
40
35
30
25
20
15
10
5
0
wt
tg
Figure 2.5. Comparison of Tottering and Wild Type Peak 3H-GABA Release. Percent
fractional release was summed over two fractions after correction for basal 3H-GABA
release for every animal. The peak release was then averaged for the 6 animals in each
group and compared using the Student's t-test, p>0.05. Data represent mean %-fractional
release + SEM.
40
Discussion
Multiple VDCC subtypes regulate neurotransmission in the mammalian CNS,
including P/Q-, N- and R-type channels. Co-expression of VDCC subtypes occurs within
the same neuron and within the same terminal where several VDCCs are involved in
neurotransmission. The proportions of VDCC subtypes expressed in a given terminal
vary depending on the region of the CNS and the type of neuron (Dunlap K, 1995).
Many studies of neurotransmitter release reveal marked inhibition of release after
application of a VDCC subtype-specific antagonist and further decrease in release with
the addition of a second antagonist specific to a second VDCC subclass (Takahashi T,
1993; Luebke JI, 1993). These studies support the participation of multiple independent
classes of VDCCs in triggering neurotransmitter release in the same neurons. Some
recent studies have demonstrated however, that VDCCs can also act cooperatively to
induce release rather than independently. In studies of dopamine release from rat striatal
synaptosomes, antagonists from two VDCC classes failed to reduce release when applied
separately but caused a marked reduction in release when co-administered. These data
also support the presence of multiple channel types within the same neurons and
furthermore, suggest that a single channel subtype can supply sufficient Ca2+ to support
maximal release under conditions of strong depolarization (Turner TJ, 1993). It is not yet
clear why VDCCs are coexpressed when one can support maximal secretion. Perhaps
selective pressure in environments with subclass specific VDCC toxins, differential
control of secretion under various conditions of stimulation, and subtype specific
modulation of activity all require co-expression of VDCC subtypes involved in
41
neurotransmission (Dunlap K, 1995). In the case of calcium channel mutations, this
redundancy may be the key to viability.
While multiple VDCCs involved in neurotransmitter release may be expressed
within the same neuron, differential expression and regulation of VDCCs occurs between
neurons. Within a given region of the brain, different VDCCs participate in triggering
neurotransmitter release. Patterns of subtype specific VDCC release may be further
subdivided within regions in excitatory versus inhibitory neurons.
For instance, in the
rat cerebellum, parallel and climbing fibers form excitatory synapses with PCs and these
fibers rely on P/Q/N- type currents for neurotransmitter release. Alternatively, basket and
stellate cells forming inhibitory synapses on PCs rely on ‘resistent,’ or R-type VDCCs for
neurotransmitter release (Doroshenko PA, 1997). Studies in CA1 hippocampal neurons
demonstrate reduction of GABAergic inhibitory transmission by 85-90% with application
of the N-type VDCC antagonist ? -conotoxin-GVIA, with only 65-70% blockage of
excitatory glutamatergic transmission (Horne AL, 1991). These data suggest that within
this region, N-type VDCCs affect inhibitory transmission to a greater extent than
excitatory transmission.
While the aforementioned studies were performed in wild type rodents, these
concepts also apply to the tottering mouse. In fact, recent studies in tottering mice have
demonstrated compensation of P/Q-type deficient release with other VDCCs coexpressed in the same neurons. Excitatory neurotransmission in the hippocampus occurs
at normal levels in the tottering mouse due to compensation for the deficient P/Q-type
current by the endogenous, co-expressed N-type VDCC (Qian J, 2000). These results are
not at all surprising due to the overlapping expression patterns of these channels in the
42
hippocampus and the redundancy of their functions. Earlier discovery of increased Ltype calcium channel expression in the tottering mouse cerebellum (Campbell DB, 1999)
was somewhat surprising because P/Q-type and L-type channels do not have redundant
functions.
L-type channels affect electrical excitability and other processes, however, they are
not traditionally involved in neurotransmitter release.
Therefore, compensation of
deficient P/Q-type function directly by L-type channels seems unlikely. Some studies do
implicate L-type VDCCs in neurotransmitter release however.
Momiyama and
Takahashi demonstrated decreased frequency of miniature inhibitory post-synaptic
currents (mIPSCs) after high-potassium stimulation in cerebellar DCN by 49% in the
presence of the L-type channel blocker, nicardipine. Frequency was also decreased by
83% in the presence of the P-type blockers, but unaffected by N-type blockade. Together
these data suggest that both L- and P-type VDCCs contribute to GABA release from
cerebellar Purkinje cells under potassium-stimulated conditions (Momiyama A, 1994).
In the studies presented here, potassium-stimulated glutamate release from tottering
cerebellar synaptosomes was decreased compared to control C57Bl/6J mice. In contrast,
GABA release was unchanged in tottering mice. Together these results suggest a defect
in excitatory but not inhibitory neurotransmission in the cerebellum of tottering mice.
These results are analogous to those obtained by Caddick et al. (1999) in the thalamus of
tottering mice and by Ayata et al. (2000) in the neocortex of leaner mice (allelic ? 1A
mutation).
As mentioned above, excitatory synapses within the cerebellum, including parallel
and climbing fiber inputs to PC, rely on P/Q- and N-type VDCCs although P-type
43
channels play a predominant role (Doroshenko PA, 1997). Therefore, the decreased
glutamatergic release seen here suggests that full compensation of the deficient P/Q-type
dependent release does not occur in these cell types.
Inhibitory synapses however
probably rely on R-type channels at basket and stellate cell terminals (Doroshenko PA,
1997) and are therefore, likely to be unaffected in the tottering mouse. Inhibitory PC
input to DCN relies predominantly on P/Q-type channel function, with reports of N-, R-,
and L-type expression and release as well (Volsen SG, 1995; Momiyama A, 1994; Chung
YH, 2000; Stea A, 1994; Tanaka O, 1995). The up-regulation of L-type channels in the
tottering cerebellum (Campbell DB, 1999) and the effect of L-type antagonists on mIPSC
frequency in DCN after potassium stimulation (Momiyama A, 1994) suggests that
compensatory mechanisms may maintain PC neurotransmission in tottering mice. The
necessity of the PC in expression of tottering dystonia (Campbell DB, 1999; Chapter 3
Section 2) refutes this theory however. Therefore, a functional deficit in PC inhibitory
neurotransmission may still exist, and the whole cerebellar synaptosome preparation used
here simply diluted the PC effects with the GABA released from molecular layer
inhibitory synapses.
The mutation of the P/Q-type VDCC and the resultant decreased Ca2+ current density
(Wakamori M, 1998) suggest that P/Q-type dependent neurotransmitter release may be
altered functionally in the tottering mouse. The abundant expression and function of
P/Q-type VDCCs in the CNS suggest that profound deficits in neurotransmission are
likely to occur as a consequence of the mutation. However, the relatively mild phenotype
and results from studies of neurotransmission suggest a more moderate effect of the
tottering mutation on neurotransmitter release. In theory, such moderate effects can be
44
due to a less significant effect of the mutation on release than is expected, or may result
from compensation by other classes of VDCCs. It is possible that the phenotypes of the
tottering mouse are due largely to the moderate functional consequence of the P/Q-type
mutation in regions with little compensation by other channel subtypes. Conversely, the
lack of more widespread and severe consequences of the tottering mutation is likely due
to compensation by other VDCCs in other regions.
45
CHAPTER 3. ROLE OF PURKINJE CELLS IN THE
EXPRESSION OF TOTTERING DYSTONIA
Abstract
The tottering mouse is a neurologic mouse mutant characterized by an ataxic gait,
polyspike EEG recordings, and spontaneous intermittent dystonic episodes.
The
mutation responsible for the tottering mouse has been mapped to a missense mutation in
the ? 1A subunit of P/Q-type voltage dependent calcium channel (Fletcher CF, 1996).
The ? 1A subunit is highly expressed in the granule and Purkinje cells of the cerebellar
cortex. Furthermore, these cell types are rapidly and markedly activated during tottering
dystonic episodes as revealed by proto-oncogene, c-fos, expression (Campbell DB,
1998). It is hypothesized that output from the cerebellar cortex via Purkinje cells is an
absolute and necessary step in the expression of tottering dystonia. Transgenic mice that
express the SV40 T antigen specifically in Purkinje cells via the pcp-2 promoter have
been generated; the transgene causes the selective elimination of Purkinje cells postdevelopmentally over many weeks (Fedderson RM, 1992). The pcp-2-SV40 transgene
was bred onto the tottering mouse strain. The double mutation appears additive with no
gross abnormalities. Prior to Purkinje cell degeneration, double mutant mice exhibit
classical tottering dystonic events; however, these same animals fail to exhibit dystonia
after Purkinje cell loss has occurred in adulthood. In this double mutant experiment,
every tottering mouse acted as its own control, eliminating genetic background as a
behavioral variable. The loss of the dystonic phenotype in double mutant mice indicates
that Purkinje cells and the cerebellar cortex participate in the pathogenesis of dystonia in
the tottering mouse.
46
Introduction
Tottering mice represent a unique opportunity to examine the pathophysiology of
dystonia, particularly paroxysmal dystonia. The genetic mutation responsible for the
tottering syndrome has been defined as a calcium channel mutation and thus places the
tottering mouse in a growing population of disorders due to ion channel mutations termed
channelopathies. Channelopathies are an extremely unique and interesting group of
disorders characterized by marked genetic heterogeneity and episodic manifestation of
phenotype in otherwise normal individuals. The tottering mouse displays a paroxysmal
dystonic phenotype strikingly similar to paroxysmal non-kinesigenic dyskinesia (PNKD)
in onset, movements, duration, frequency, triggers, and pharmacological treatment
profile. While it is likely that PNKD is caused by channelopathies, it is not necessarily
due to a calcium channel mutation per se. Mutations in different ion channels can cause
similar phenotypes and different mutations in the same ion channel can cause different
phenotypes.
Furthermore, it is not suggested that dystonia as a whole is caused by
mutations in calcium channels, however, the tottering mouse may provide a model for
analysis of neuronal circuits involved in at least this form of dystonia. The utility of
dissecting the pathophysiology behind genetic animal models of dystonia is similar to the
usefulness of studying secondary dystonias. Determination of the brain regions and
neuronal circuits involved in dystonic states will identify generic or common pathways
affected and ultimately suggest appropriate steps in intervention of the disease process.
The neuronal circuitry activated during the course of tottering dystonic attacks was
assessed by Campbell et al. using immediate early gene, c-fos, mRNA expression as a
marker for neuronal activity. Their findings indicated that the cerebellum and principal
47
cerebellar relay nuclei are activated during tottering attacks and that the activation
preceded temporally a more modest activation in the cerebral cortex.
Within the
cerebellum, granule cells, PC and DCN are all activated early in the course of an attack.
These data suggest a central role for the cerebellum in the initiation and/or maintenance
of tottering paroxysmal dystonia (Campbell DB, 1998). It is not at all surprising that the
cerebellum is involved in tottering mouse phenotypes due to the abundant expression of
the mutated P/Q-type channel in both cerebellar granule and Purkinje neurons. Together,
the abundant cerebellar expression of P/Q-type channels and the marked activation of the
cerebellum during tottering attacks led Campbell et al. to further investigate the role of
the cerebellar PC in the generation and/or maintenance of tottering dystonia.
To this end, the mutation pcd was used to lesion cerebellar PC. Purkinje cell
degeneration (pcd) is a mouse mutant characterized by the rapid death of PC at postnatal
days 15-29. Genetic lesioning of the cerebellar PC of tottering mice through the pcd
mutation eliminated the tottering dystonic attacks. These data suggest that cerebellar
output is necessary for the induction of tottering dystonic attacks.
Since the time these experiments were undertaken a slightly different approach to
address the same question was developed. Possible confounds of the above experiments
include phenotypic effects of the pcd mutation on the CNS of the double mutants. Until
the gene responsible for the pcd mutant is discovered, it is unlikely that confounding
effects of the mutation on tottering dystonic attacks can be ruled out completely. Further,
the genetics of this experiment prohibited the generation of completely appropriate
control mice. Therefore, a new approach to evaluate the role of PC in tottering dystonia
was devised using a more defined system and internal controls. Here, tottering mice were
48
bred with transgenic mice expressing the SV40 large T antigen (described in detail
below) to produce tottering mice that lose their PC slowly over time after cerebellar
development is complete. The advantage to this model system is that each mouse serves
as its own control; at early time points, tottering dystonia should be unaffected and over
time the effect of graded PC loss can be evaluated. Furthermore, the transgene results in
death of PC only, abrogating any confounding effects of the second 'mutant’. Through
the use of this model, the role of the PC as a necessary and essential component in the
expression of tottering dystonic attacks is evaluated.
Materials and Methods
Transgenic and mutant mice
Mice from the SV4 transgenic mouse line were obtained as a generous gift from Dr.
R.M. Fedderson.
SV4 mice were generated by driving expression of the SV40 T antigen in PCs using
the pcp-2 promoter. The pcp-2 protein is expressed exclusively in PCs and the simian
virus 40 T antigen is known to disrupt cell cycle regulation in post-mitotic cells.
Although cerebellar development proceeds normally in this line, PCs gradually die over
the course of several months beginning at ~P23. Very few PCs remain by 5 months of
age. This loss of PCs occurs relatively slowly over time and is generally reproducible
between mice.
Only occasional PCs remain by P70; at all times, T antigen
immunoreactivity is restricted to PCs (Fedderson RM, 1992).
Transgenic mice received from Dr. Fedderson were on the FVB strain, derived from
the original SV4 line (Fedderson RM, 1992). Because tottering is on the C57Bl/6J
background, FVB transgenic mice were bred onto the C57Bl/6J background for 5
49
generations prior to breeding with tottering animals. F5 generation transgenic females
(SV4 +/-; +/+) were bred with tottering males (SV4 -/-; tg/tg) to produce F6 generation
transgenic animals heterozygous for the tottering mutation (SV4 +/-; +/tg). F6 female
transgenic animals were bred with tottering males again to produce F7 transgenic
tottering animals (SV4 +/-; tg/tg) and control genotypes (SV4 +/-; +/tg, SV4 -/-; tg/tg,
SV4 -/-; +/tg). While back-crossing the transgenic line onto C57Bl/6J for 5 generations,
a smaller group of animals was bred directly with tottering animals. In this group of
mice, the experimental animals were the F3 generation of transgenic animals onto
C57Bl/6J. These two genetically distinct groups will be referred to as F7 and F3,
respectively. All animals were housed on a 12-hour light/dark cycle in the vivarium at
Penn State University College of Medicine and had access to food and water ad libitum.
Genotyping
Presence of the SV40 transgene was determined by PCR analysis. Ear punch DNA
was prepared by adding 300? l of 0.05 M NaOH to the punch and heating at 95oC for 10
minutes. The punch was then vortexed, neutralized with 50? l of 1 M Tris pH8.0 and
vortexed again. Punch debris was spun down at 14,000rpm for 6 minutes and the
supernatant removed. One microliter of the unpurified supernatant was added to 10? l
PCR reactions containing 400nM each dNTP, 10? M each forward 5' (5'AGTACTGTCCCCCAAGAGATAGTAG-3') and reverse 3' (5'-CCATTCATCAGTTC
CATAGGTTGG-3') primers, 1X Vent (exo-) DNA polymerase buffer (10mM KCL,
10mM (NH4)2SO4, 20mM Tris-HCl pH8.8, 2mM MgSO4, 0.1% Triton X-100), and 1 to
1.5 Units Vent (exo-) DNA polymerase (New England Biolabs). The reactions were
denatured at 95oC for 5 min prior to undergoing 30 cycles consisting of 1 min at 95oC, 2
50
minutes at an annealing temperature of 57oC, and 2 min of extension at 72oC. A 7 min
extension at 72oC was added after the cycles completed.
Reaction products were
separated on a 1.2% agarose gel by electrophoresis and viewed after ethidium bromide
staining under UV light. The amplified transgene segment migrates at 1076 bp size.
Dystonia assessment
Animals were brought to the laboratory every three weeks for dystonia assessment
two hours prior to testing. To induce dystonia, animals were injected subcutaneously
with 25mg/kg caffeine at 4 weeks of age and 15mg/kg caffeine at all other timepoints
prepared in 0.9% saline. Animals were assessed every three weeks and sacrificed either
after the initial 4 week assessment or at the age when signs of dystonia were lost. Fourweek-old animals appear slightly more resistant to caffeine induction of dystonia than
older mice and it was imperative that they be correctly phenotyped so the higher dose
was used initially. Animals were observed for one hour and scored every 10 minutes on
a general dystonia scale and according to body regions involved.
The generalized
dystonia rating scale used is as follows (modified from Jinnah HA, 2000):
Score
D0
D1
D2
D3
D4
D5
Behavioral Phenotype
no motor abnormalities
slightly slowed or abnormal motor behavior
rare, isolated dystonic postures seen
mild motor impairment due to dystonic postures
moderate impairment due to dystonic postures
severe immobility due to prolonged dystonic postures
Dystonic severity was also evaluated by noting the presence or absence of hindlimb,
forelimb, trunk, neck, and head involvement. Limb involvement was categorized as
isolated, tonic, or paddling. Trunk dystonia was described as flattened, twisted, hunched,
or rearing. Cranial dystonia was separated into neck flexion or extension and by ear, eye,
or jaw involvement.
51
Histologic analysis
After final behavioral testing, mice were sacrificed by carbon dioxide asphyxiation.
The brains were rapidly removed and frozen in isopentane chilled to -40oC and stored at 70oC until processing. Brains were cut into 20? m coronal sections using a cryostat and
thaw-mounted onto Superfrost plus slides (Fisher, Pittsburgh, PA).
Purkinje cell analysis of F3 generation SV4+/-;tg/tg mice was completed using in situ
hybridization for calbindin mRNA. Calbindin is abundantly and specifically expressed in
Purkinje cells within the cerebellum. Age-matched +/+;tg/tg mice were used as controls
to determine basal Purkinje cell calbindin mRNA levels. The in situ hybridization
protocol is described in detail in the methods section of Chapter 4. cDNA template for
calbindin was obtained as a generous gift from Dr. T.L. Wood and subcloned into
pBluescript SK+. Plasmid was linearized and used to generate antisense single-stranded
radiolabeled RNA probes of 1.2kb in length.
Stereologic cell counting of PC in all folia of the cerebellum in the F7 generation
progeny will be completed in the future.
This technique should allow for precise
correlation between numbers and regions of Purkinje cells necessary for the dystonic
attacks of tottering mice to occur.
Results
Generation of tottering mice carrying the SV40 transgene
All progeny were genotyped for presence of the SV40 T antigen transgene using ear
punch DNA (Figure 3.1). Tottering mice were phenotyped by their characteristic ataxic
gait at the time of weaning and by presence of dystonic attacks. Tottering mice carrying
the SV40 transgene and all control genotypes were generated in ratios expected for
52
autosomal independent segregation of the alleles in both the F3 and F7 groups of mutant
mice (Table 3.1). While tottering mice bearing the transgene were the smallest of the
genotypes, their weights did not differ dramatically from control genotypes (Figure 3.2)
and the animals appeared healthy and behaved normally in general.
53
L
1
2
3
4
5
6
7
8
9
1 kB
Figure 3.1. PCR genotyping for presence of the SV40 transgene. Ear punch DNA was
used as template to amplify the 1076bp fragment of the transgene using primers specific
for the pcp-2 promotor and the SV40 T antigen gene. Lanes 1,2,5,6,7,and 9 contain the
amplified transgene fragment; L denotes 1 kB ladder.
A
Genotype
SV4+/-;tg/tg
SV4-/-;tg/tg
SV4+/-;+/tg
SV4-/-;+/tg
Total
Male
2
3
3
1
9
Female
4
3
2
1
10
Total
6 (31.6)
6 (31.6)
5 (26.3)
2 (10.5)
19
Genotype
SV4+/-;tg/tg
SV4-/-;tg/tg
SV4+/-;+/tg
SV4-/-;+/tg
Total
Male
5
7
3
2
17
Female
5
8
8
3
24
Total
10 (24.4)
15 (36.6)
11 (26.8)
5 (12.2)
41
B
Table 3.1. Genotypes of progeny generated in SV4+/-;+/tg X +/+;tg/tg crosses.
Summary of the genotypes and sexes for F3 (A) and F7 (B) generations determined by
PCR of the SV40 transgene and phenotyping of the tottering homozygous state. Percent
of total mice for each genotype is indicated in parentheses.
54
35
30
Weight (grams)
25
20
15
SV4/tg
SV4
tg
wt
10
5
0
4
7
10
13
16
19
Age (weeks)
Figure 3.2. Body weights of F7 generation progeny. Weights were averaged for all mice
in each genotype. Transgenic tottering mice (SV4/tg) were healthy and viable with body
weights similar to control genotypes: wild type (wt), transgenic (SV4), and tottering (tg).
Single-factor ANOVA analysis revealed no significant difference in weight between
genotypes (p>0.05).
Loss of dystonic phenotype in transgenic tottering mice
In the F3 group of animals, all tottering mice carrying the SV40 T antigen transgene
displayed characteristic tottering dystonic attacks indistinguishable from control tottering
mice at the initial 4 week time point. At the 10 week timepoint, transgenic tottering
animals displayed an extremely mild version of dystonia, marked by involvement of the
limbs only while the head, neck and trunk were largely spared. As the weeks progressed,
individual transgenic tottering animals displayed absolutely no dystonia after caffeine
challenge (Figure 3.3A). In contrast, tottering mice lacking the transgene continued to
respond to caffeine with a dystonic attack. By 18-19 weeks, transgenic tottering animals
55
failed to display any dystonic phenotype. Female animals lost the dystonic phenotype an
average of one month earlier than male animals.
Before loss of dystonic phenotype was confirmed, animals were challenged with
caffeine a second time and by restraint as well. Similar results were obtained in the F7
group of animals (Figure 3.3B). This generation of animals showed somewhat more
delayed loss of the dystonic phenotype however. Head, neck and trunk dystonia were
again lost prior to dystonic limb involvement (Figure 3.4) and female transgenic tottering
animals lost the dystonic phenotype prior to males. Transgenic control animals that were
tottering heterozygotes never displayed any dystonia and control tottering animals
negative for the transgene displayed characteristic dystonic attacks throughout the entire
experiment. In both generations, transgenic tottering mice that had lost the dystonic
phenotype were still distinguishable from their littermates due to a unique ataxic gait,
which was different from that of a transgenic or tottering mouse alone.
56
35
AA
30
Total D Score
25
20
15
10
5
0
3
6
9
12
15
18
21
24
Age (weeks)
35
BB
30
Total D Score
25
20
15
10
5
0
3
6
9
12
15
18
21
24
Age (weeks)
Figure 3.3. Loss of dystonic phenotype in transgenic tottering mice over time. In the F3
generation (A), all transgenic tottering animals (blue circles) were non-dystonic by 19
weeks of age. Similar results were obtained in the F7 generation (B), with a greater
discordance between age of phenotype loss between males (blue circles) and females (red
squares) however. Control tottering mice (pink diamonds) maintained a high level of
dystonia in both experiments (A and B). Linear regression lines are shown for each
grouping.
57
6
6
Head
5
5
4
4
3
3
**
2
2
1
Dystonic Bins
Neck
**
1
**
0
4
7
10
13
16
***
*** ***
19
4
5
4
4
*
2
***
13
16
19
22
Limbs
*
1
***
0
10
13
2
1
7
10
3
**
4
7
6
5
3
***
0
22
Trunk
6
***
16
19
0
22
4
7
10
13
16
19
22
Age (weeks)
Figure 3.4. Regional loss of dystonic phenotype in F7 transgenic tottering mice.
Presence of dystonia was tallied (+ or -) over the one hour observational period (6 total
time bins) and summed by region. Data are presented as number of bins positive for
dystonia by age (weeks) for each region. Transgenic tottering mice (blue bars) lost the
characteristic dystonic phenotype over time while control tottering littermates (pink bars)
sustained dystonic involvement of all regions. Phenotypic loss of head involvement (A)
occurred first, followed by neck (B) and trunk (C) involvement, and lastly by limb (D)
involvement. Error bars represent SEM. Number of dystonic bins were compared
between genotypes using the Student's t-test at each age. Transgenic tottering mice were
sacrificed after disappearance of all dystonia and these animals were given a minimal
score for subsequent timepoints. * indicates p<0.05, ** p<0.005, and *** p<0.0005.
Demonstration of Purkinje cell loss in transgenic tottering animals
Verification of PC ablation after the loss of the dystonic phenotype in all SV4+/-;tg/tg
animals of the F3 generation was performed using in situ hybridization to calbindin
mRNA. Mice were sacrificed shortly after cessation of any dystonic signs or allowed to
58
age for a few more weeks to obtain a range of ages to compare degree of PC loss over
time. One mouse, the 15 week old female (figure 3.5f) still demonstrated some rare
isolated hindlimb dystonia with the rear limb occasionally being held up against the trunk
when sacrificed.
Animals were between 15 and 29 weeks of age when sacrificed.
Results from calbindin mRNA in situ hybridization demonstrate profound loss of
cerebellar Purkinje cells in all transgenic tottering mice compared to age-matched control
tottering mice (Figure 3.5). Degree of PC loss increases substantially with age and with
female sex.
a
c
e
g
b
d
f
h
Figure 3.5. Calbindin mRNA in situ hybridization. Cerebella from F3 transgenic
tottering animals and two age-matched control tottering animals were hybridized with
calbindin antisense mRNA. Transgenic tottering animals (a-f) show marked loss of PC in
comparison to control tottering animals (g-h). Within the transgenic group, increasing
age and female sex are correlated with greater loss of PC: a, 23 week old female, b, 26
week old male, c, 29 week old female, d, 18 week old female, e, 24 week old male, f, 15
week old female. Tottering controls were 19 weeks old.
Discussion
The loss of the characteristic dystonic phenotype in tottering animals after widespread
PC loss demonstrates the necessary role of the cerebellar signaling in the expression of
59
tottering dystonia. Transgenic tottering mice in these experiments served as their own
controls, as initial development and expression of dystonia proceeded normally. Loss of
dystonia corresponded to the death of PC, as confirmed by calbindin in situ hybridization.
These data support those obtained by Campbell et al., in which the tottering dystonic
attacks were eliminated by the pcd mutation (Campbell DB, 1999).
In the latter
experiments however, initial normal development of the attacks could not be
demonstrated due to rapid loss of PC (P15-P29) and unknown widespread effects of the
pcd mutation could not be excluded. Lesion of cerebellar PC specifically with the SV40
T antigen transgene circumvented these issues due to its inherent specificity and slow
progression of cell death.
Generation of healthy transgenic tottering mice proceeded without difficulty. These
animals displayed characteristic tottering dystonic attacks, indicating no gross phenotypic
alterations of the dystonia after cross-breeding. Tottering animals lacking PC were
relatively healthy and viable, suggesting that no genetic interference occurred between
the tottering gene and the SV40 T antigen transgene. In addition to the loss of the
dystonic phenotype however, transgenic tottering mice displayed a unique gait around the
time of dystonia loss that appeared more wobbly and uncoordinated than either transgenic
or tottering gaits alone.
In the initial F3 generation experiment, all transgenic tottering animals displayed
stereotypical generalized dystonia initially. By 8-10 weeks of age, the dystonia was
restricted to the limbs only. As the weeks progressed, the severity continued to lessen
until absolutely no dystonia was observed. Calbindin mRNA in situ hybridization
confirmed the loss of nearly all PC in transgenic tottering mice no longer expressing the
60
dystonic phenotype. Behavioral data from the F7 generation animals was similar to that
from the F3 generation, indicating no gross effect of the heterogenous genetic
background in the original experiments. Characteristic tottering attacks were observed by
four weeks of age in the F7 generation and again at seven weeks of age. A greater time
range for the restriction of the dystonia to the limbs (10-16weeks) and for complete loss
of the phenotype (13-25weeks) was seen in the F7 generation however. This increased
time range is in direct contrast to what would be expected from the increased genetic
homogeneity in the F7 generation animals in comparison to those in the F3 generation.
Therefore, the time of phenotypic onset is more likely due to factors inherent to repetitive
breeding of the transgene as opposed to the genetic background of the mice per se.
Phenotypic differences were noted between male and female mice, with loss of the
dystonic phenotype occurring in female animals by age 14 weeks on average (range 1116) and in males by 19 weeks in the F3 generation. Females in the F7 generation also
exhibited a graded loss of the dystonic phenotype on average of 6 weeks prior to males.
Female transgenic mice appear to develop the characteristic ataxia before males,
indicating earlier PC loss in female transgenic animals in general.
Although not
quantitative, the calbindin in situ hybridization data support this finding. The reasons for
this sex discrepancy are unknown with no sex differences described in the original report
(Fedderson RM, 1992).
PC loss in transgenic animals is reported to occur gradually during the timeframe of
these experiments (Fedderson RM, 1992). Therefore, the graded loss of the dystonic
phenotype in transgenic tottering animals is likely due to decreasing cerebellar cortical
output through remaining PC. The early restriction of the dystonia to include limbs-only
61
rather than simple overall reduction in severity is interesting and suggests regional
control of dystonia through subpopulations of PC. Presumably, the cells lost early were
responsible for more proximal dystonia of the trunk, head, and neck while those PC more
resistant to death caused limb-only dystonia for a number of weeks longer. Future
analysis may indeed demonstrate a somatotopic map of PC loss corresponding to the
regions of the body no longer involved in the dystonic phenotype. Tissue from all F7
transgenic tottering and control genotypes has been collected and will be processed for
stereologic PC counting at timepoints throughout the graded range of dystonia to test this
theory. Initial PC counts in transgenic tottering animals exhibiting characteristic full
body dystonia are expected to be similar to control genotypes. The restricted focus of the
dystonia is expected to occur only after loss of consistent sets of PC corresponding to the
rough cerebellar somatotopic regions for the trunk and head. Further loss of PC in areas
associated with limb motor control are expected to be lost in transgenic tottering animals
exhibiting no dystonia.
Thus, overall severity and regional expression of tottering
dystonia will likely correspond to somatotopically organized PC activity.
While the correspondence between regions of PC loss and body parts involved in
transgenic tottering dystonia remain to be established, the data presented here indicate a
role for cerebellar cortical output in the expression of tottering dystonia. Although the
cerebellum has traditionally been viewed as a secondary processor in motor coordination,
recent evidence has implicated more significant and widespread functions of this region.
Roles in learning, memory, and other cognitive functions have been described for the
cerebellum. In the case of dystonia, aberrant DCN activity has been implicated in
producing the dystonic phenotype of the genetically dystonic rat (LeDoux, 1993) and
62
hypermetabolism in the cerebella of human patients with dystonia syndromes is
frequently noted (Eidelberg D, 1998; Odergren T, 1998; Preibisch C, 2001; Hutchinson
M, 2000). In the tottering mouse, specific activation of the cerebellum and cerebellar
relay nuclei after induction of dystonia implicates this region in the production of
dystonic attacks (Campbell DB, 1998). These data and those presented here suggest an
integral role for the cerebellum in the pathophysiology of dystonia. Evidence supporting
an active role for the cerebellum in a process such as dystonia is intriguing as it upsets
traditional views of cerebellar function. However, traditional studies of the cerebellum
investigated disturbances due to decreased normal function and activity as opposed to the
potential effects of abnormal signals and increased activity within the cerebellum, as
implicated in the aforementioned dystonias.
63
Chapter 4. ROLE OF THE CEREBELLUM IN A NOVEL
ANIMAL MODEL OF DYSTONIA IN WILD TYPE MICE
Abstract
Dystonia is a neurological syndrome characterized by twisting movements or
sustained abnormal postures.
Recent evidence suggests that abnormal cerebellar
signaling contributes to the expression of dystonia. To study the role of the cerebellum in
dystonia, we have developed a novel mouse model. Microinjection of low-doses of
kainic acid into the cerebellar vermis of mice elicited reliable and reproducible dystonia
characterized by hindlimb abduction and extension and a severely flattened trunk. The
severity of the dystonia increased linearly with kainate dose from 0 to 235 picomoles.
Co-injection of the glutamatergic antagonist NBQX with kainic acid dramatically
decreased dystonia verifying that AMPA and/or kainate receptors participate in the
expression of dystonia in this model. The abnormal movements were not associated with
kainate-induced seizures, as EEG recordings showed no epileptiform activity during the
dystonic events. Further, neuronal activation, as assessed by in situ hybridization for cfos, revealed c-fos mRNA expression in the cerebellum, locus ceruleus and red nucleus.
In contrast, regions associated with seizures such as the hippocampus did not exhibit
increased c-fos expression after cerebellar kainate injection. Transgenic mice lacking
Purkinje cells were also microinjected with kainate. Transgenic mice show dramatically
decreased dystonia after kainic acid injection suggesting an important role for Purkinje
cells and the cerebellar cortex in this model of dystonia. Together these data suggest that
the cerebellum plays a role in the pathophysiology underlying dystonia.
64
Introduction
Dystonia is a relatively common neurological disorder characterized by cocontraction of antagonistic muscles and spill over to adjacent muscle groups on EMG
recordings. These effects result in sustained abnormal muscle contractions resulting in
twisting and odd postures (Fahn S, 1987). The pathophysiology of dystonia is poorly
understood, but biochemical changes within the basal ganglia are thought to play an
important role. The hyperkinetic movements of dystonia are theorized to result from
decreased pallidal inhibition of the thalamus, which then in turn would send excessive
excitatory input to the premotor and motor cortices (Berardelli A, 1998; Vitek JL, 2000).
The basal ganglia is frequently the site of pathology in secondary dystonias, but no
consistent changes have been defined in cases of primary dystonia.
Alternative theories suggest however, that impaired inhibition of muscles antagonistic
to those activated occurs at the level of the cerebral cortex or that the cerebrocerebellar
circuit can also lead to excessive excitation of the motor cortex (Halett M, 1998; Richter
A, 1998). Over the last few decades, studies have implicated numerous structures in the
pathophysiology of dystonia including the basal ganglia, thalamus, sensorimotor cortex,
and cerebellum. Genetic rodent models of dystonia implicate the cerebellum in the
production of dystonia. The dystonic rat (LeDoux MS, 1993) and hamster (Richter A,
1998), the dystonia musculorum mouse (Sotelo C, 1988) and the wriggle mouse sagami
(Ikeda M, 1989) all display cerebellar defects to some extent (for review, see Richter A,
1998). Another mouse mutant, tottering, has recently been suggested as a model for
paroxysmal dystonia and supports a role for the cerebellum in the generation of dystonia
as well (Campbell DB, 1998; Campbell DB, 1999). While genetic animal models are
65
useful tools in defining the cellular defects and pathways involved in disease, they also
carry inherent limitations. It is difficult to prove that yet undetermined phenotypes of the
mutation are not involved in a particular phenomenon. Thus, genetic animal models are
most useful in suggesting regions involved in the pathophysiology of a disease that can
later be studied in genetically wild type animals.
Marked activation of the cerebellum occurs in tottering mice upon initiation of
dystonic attacks (Campbell DB, 1998) and increased DCN output is likely responsible for
the generalized dystonia in the dystonic rat (LeDoux MS, 1995). Furthermore, numerous
functional imaging studies in human dystonia patients demonstrate increased metabolic
activity within the cerebellum (Eidelberg D, 1998; Odergren T, 1998; Preibisch C, 2001;
Hutchinson M, 2000). To test the theory that overactivity of the cerebellum is sufficient
to produce a profound dystonic phenotype, local excitation of the cerebellum was
achieved in wild type mice through cerebellar microinjection of kainic acid.
Materials and Methods
Mice
Originally obtained from Jackson Laboratories, wild type C57Bl/6J mice were bred at
the Pennsylvania State University College of Medicine vivarium. Male and female mice
used in the experiments weighed 21-26 grams and were between 2 and 4 months of age.
SV4 transgenic mice were originally obtained as a generous gift from Dr. R.M.
Fedderson on an FVB background. Mice used in these experiments were back-crossed
onto a C57Bl/6J background for five generations before beginning experiments providing
an estimated 96.9% genetic identity between animals. Genotyping for the presence of the
SV40 T-antigen transgene proceeded as described above (Chapter 3) and originally in
66
Fedderson et al., 1992. Male and female transgenic mice used in these experiments were
over 6 months of age to ensure maximal Purkinje cell death prior to experimentation. All
animals were housed on a 12-hour light/dark cycle with access to food and water ad
libitum.
Injections
Kainic acid H2O was obtained from Tocris (Ballwin, MO) and suspended in 0.9%
saline. To visualize injection sites, 1:10 v/v of Trypan Blue (0.4%, Sigma, St. Louis,
MO) was also added to the kainic acid solution. NBQX (Sigma, St. Louis, MO) and
domoic acid (Sigma, St. Louis, MO) were also suspended in 0.9% saline. For the doseresponse and transgenic experiments, animals were anesthetized with methoxyflurane
(Metofane; Mallinckrodt Veterinary Inc., Mundelein, IL) inhalational anesthetic in a
small glass chamber. A midline incision was made over the skull and a small hole was
drilled with a 21-gauge needle over the anterior cerebellum in the midline. A hamilton
syringe with a needle cut to 2mm was placed in the hole and 0.5 ? l kainic acid solution
was delivered over 5 seconds (AP -6.5 mm bregma, Lat 0 mm, Vert -2 mm from skull).
The wound was re-approximated and sealed with Nexaband S/C topical skin closure
(Veterinary Products Laboratories, Phoenix, AZ). All other injections were done under
isofluorane (IsoSol; Vedco, Inc., St. Joseph, MO) anesthesia due to discontinued
production of methoxyfluorane. Anesthetization was induced under 3% isofluorane and
maintained at 1% during injection. Lateral cerebellar injections were made 1-2 mm right
or left of midline with a 2mm needle length (AP -6.5 mm bregma, Lat 1-2 mm, Vert -2
mm from skull). Lateral ventricle(AP –0.5 mm bregma, Lat 1.25 mm, Vert -3 mm from
skull) and striatal injections (AP +1 mm bregma, Lat 2 mm, Vert -3 mm from skull)
67
were also completed. Prior to changing anesthetic, behavioral outcomes were compared
under each anesthetic and found to be identical for wild type mice receiving kainate
injections (data not shown).
Because the effects of kainic acid are immediate in this
paradigm, rapid recovery from anesthesia was necessary. Therefore, injections were
made freehand rather than with the use of a stereotaxic apparatus to limit the amount of
time mice remained under and recovered from the effects of anesthesia. Using this
technique, injection site locations were consistent and reproducible (Figure 4.5).
Behavioral Observation
After wound closure, animals were immediately placed in an empty cage and scored
(see below) for dystonia every 10 minutes after 1 minute of observation.
If no
spontaneous dystonia was seen, mice were disturbed by touch. If no escape or dystonia
resulted, mice were lifted by the tail and placed down again to encourage movement,
which most often preceded the dystonia. The behavioral rating scale illustrated below
was used to score the motor behavior of injected mice. Note that scores of D2-D5 only
are within the range of observed dystonia.
Score
D0
D1
D2
D3
D4
D5
D6
Behavioral Phenotype
no motor abnormalities
no impairment; slightly slowed or abnormal (but not
dystonic) motor behavior
mild impairment; sometimes limited ambulation
unless disturbed, dystonic postures when disturbed
moderate impairment; frequent spontaneous dystonic
postures
severe impairment; sustained dystonic postures and
limited ambulation
prolonged immobility in dystonic postures
erratic running, "popping," and seizures
modified from Jinnah HA, 2000
68
In Situ Hybridization
After the two-hour observation period, mice were sacrificed by carbon dioxide
asphyxiation and the brain removed and frozen in isopentane chilled to –40oC. Frozen
tissue for in situ hybridization was prepared by slicing 20? M coronal sections from fresh
frozen brains stored at -70oC using a cryostat and thaw mounted on Superfrost Plus glass
slides (Fisher, Pittsburgh, PA). After drying, the slide-mounted sections were stored at 70oC. cDNA template for murine c-fos was obtained as a generous gift from Dr. M.E.
Greenberg and subcloned into pBluescript II SK+. Plasmid was linearized to generate
either sense or antisense single-stranded radiolabled RNA probes of 2.2kb in size after in
vitro transcription.
Transcription reactions were incubated for 1-1/2 hr at 37oC in a 25 µl volume
containing 40 mM Tris, pH 7.9, 6 mM MgCl2, 2 mM dithiothreitol (DTT), 40 U RNase
inhibitor (Promega, Madison, WI), 400 µM each ATP, GTP and UTP, 10 µM [35S]CTP
(800 Ci/mmol), 1 µg linearized c-fos template plasmid (sense or antisense) and 20 U T3
or T7 RNA polymerase (Promega, Madison, WI). After transcription was completed,
DNA template was removed by RNase-free DNase (Promega, Madison, WI) digestion
for 30 min at 37oC and riboprobes were reduced to 100-200 bp in size with 0.2 M NaOH
for 45 minutes on ice. Probes were extracted with phenol:chloroform:isoamyl alcohol
(25:24:1) and separated on a G50 Sephadex Nick column (Pharmacia, Piscataway, NJ) to
remove unincorporated nucleotides.
Pretreatment of slide-mounted tissue consisted of fixation in buffered 4%
formaldehyde for 5 min at room temperature followed by a 5 min rinse in 0.1 M
phosphate buffered saline (PBS). Slides were treated with 0.25% acetic anhydride in 0.1
69
M triethanolamine-HCl/0.15 M NaCl (pH 8.0) for 10 min and rinsed in 2X standard
sodium citrate (1X SSC; 0.15 M NaCl, 0.015 M sodium citrate). Sections were
dehydrated in graded ethanols for 1 min each followed by 5 min incubation in
chloroform. After final one min incubations in 100% and 95% ethanol slides were air
dried. Slides were hybridized with 100 µl of hybridization buffer containing 7.5 ng
cRNA probe in 50% formamide, 0.75 M NaCl, 20 mM 1,4-piperazine diethane sulfonic
acid, pH 6.8, 10 mM EDTA, 10% dextran sulfate, 5X Denhardt’s solution (0.02% bovine
serum albumin, 0.02% ficoll, 0.02% polyvinylpyrolidone), 50 mM DTT, 0.2% sodium
dodecyl sulfate and 100 µg/ml each salmon sperm DNA and yeast tRNA. Slides were
coverslipped and hybridized for 16 hr at 56oC in a closed humid chamber.
Following hybridization, coverslips were removed in 4X SSC with 300 mM 2mercaptoethanol at room temperature. Slides were incubated in this solution for 15 min
followed by 15 min in 4X SSC alone. The slides were treated with 50 µg/ml pancreatic
RNase A in 0.5 M NaCl, 50 mM Tris, pH 8.0, 5 mM EDTA for 30 min at 37oC, washed
in graded salt solutions (2X, 1X, and 0.5X SSC each for 5 min at 56oC), and in 0.1X SSC
at 65oC for 30 min. Slides were dipped in 60% ethanol with 0.33 M ammonium acetate
and air dried.
Sections were exposed to x-ray film (DuPont Cronex) and analyzed using MCID
M5+ optical software package. Regions analyzed in detail included the cerebellum, red
nucleus, locus ceruleus, striatum, hippocampus, and motor cortex. After correction for
film background, density of c-fos mRNA hybridization signal was quantified for multiple
sections per animal and averaged. The mean density in a given area per animal was then
averaged for animals receiving the same dose of kainic acid and compared.
70
Injection Site Localization
Tissue was collected for needle localization in the same manner as described for in
situ hybridization. Tissue slices analyzed by in situ hybridization were also studied for
needle tract location with tract markings and peak c-fos intensity as indicators of
injection site.
Tissue not processed for in situ hybridization underwent standard
hematoxylin and eosin staining to verify the injection site.
EEG Recordings
Under isofluorane anesthesia, two small machine screws (1/32" diameter by 1/16"
length; Small Parts Inc.) were placed through the skull of C57Bl/6J mice and wrapped
once with 30 gauge silver-plated copper wire attached to a plug or pin connector.
Colloidal silver paint was applied over the screw and wire to ensure good electrical
conductivity and the apparatus was cemented to the cleaned skull surface using loctite
glue and accelerator (Loctite, Rocky Hill, CT). After a minimum of 24 hours postsurgery, electrode pins were connected to an electroencephalagraph (EEG) recording
apparatus and baseline EEG were recorded. The animal was again anesthetized and 0.5? l
kainic acid (235 pmoles) was injected into the midline anterior cerebellum at a depth of
2mm. The mice were immediately re-connected and EEG recordings were collected for
10 seconds at 5 minute intervals for 90 minutes while behavioral data were collected. In
addition, EEGs were recorded when particularly gross dystonic postures were observed.
After the recording period, mice were injected with 60mg/kg pentalinetetrazol i.p. to
induce generalized seizure activity.
71
Results
Behavioral response to cerebellar kainate microinjection
Wild type mice reliably and reproducibly displayed a dystonic phenotype in response
to low dose kainic acid injection into the cerebellum. Mice typically displayed the first
sign of dystonia 10-20 minutes after injection, with a hindlimb being held up tonically
against the trunk as the mouse was exploring. Within a few minutes, the entire trunk and
all four limbs were involved with the mouse flattened against the cage bottom with an
arched back and the perineum pressed down. The hindlimbs were abducted at the hip and
knee and held out above the base of the tail, often paddling in the air. The forelimbs were
typically held tightly against the trunk or exhibited paddling. The neck often flexed or
extended and the ears were held back against the fur and the eyes closed (Figure 4.1).
This was the most common dystonic posture seen and varied in severity and duration.
Mild and moderately affected mice ambulated or rested normally in between dystonic
attacks that lasted 2 to 15 seconds. Severely dystonic animals remained immobilized in a
tensely held dystonic posture for 2 to 20 minutes at a time. Mildly affected animals
showed dystonia only after being disturbed. Other mice were spontaneously dystonic and
this is reflected in the scoring system. In general, dystonic postures occurred during
initiation of movement.
After being disturbed and attempting to escape, or upon
volitional initiation of movement, dystonia was consistently preceded by a change in
movement. A sudden ambient noise that startled the mice would also frequently incite a
dystonic attack. Severely affected mice immobilized in prolonged postures exhibited
exacerbations defined by further tensing of the muscles in the posture; initiation or
change in movement could not be appreciated in dystonia at this stage of severity.
72
Figure 4.1. Typical dystonic postures after cerebellar kainate injection in wild type mice.
This mouse, injected in the cerebellum with 235 picomoles of kainic acid demonstrates a
flattened, arched trunk with hindlimbs abducted and held out from the body. The photo
on the left also shows some mild flexion of the neck while the photo on the right
illustrates outstretched forelimbs and flattened ears.
Severity of dystonia increases linearly with kainate dosage
The association of the dystonic phenotype with kainate injection was investigated
using a dose-response paradigm. Kainic acid concentrations used were 0-, 10-, 25-, 50-,
75-, 100-, and 150-? g/ml. In the 0.5 ? l delivered, this corresponds to 0-, 25-, 60-, 115-,
175-, 235-, and 350 picomoles kainic acid respectively. Within this dosage range, a full
spectrum of behavior was appreciated. Beginning with no motor abnormalities, the
behaviors continued through a range of dystonic severity and ended with seizure activity
at the highest dose. The severity of dystonia increased linearly with dose, providing a
dose-response correlation coefficient of 0.98 (Figure 4.2A). The behavior returned to
normal or near-normal in all dystonic animals within 2 hours of injection (Figure 4.2B).
Mice given a dose of 350 pmoles kainic acid developed wild and erratic running
behaviors, became seizurigenic and were sacrificed before the observational period
ended. These mice were given a maximal score of D6 for the remaining time in the data
presented in Figure 4.2.
73
6
A
Average D score
5
4
3
2
1
2
R = 0.98
0
0
50
100
150
200
250
300
350
Dose KA (pmoles)
6
B
0pmol
25pmol
60pmol
115pmol
175pmol
235pmol
350pmol
5
D score
4
3
2
1
0
0
10
20
30
40
50
60 70
minutes
80
90
100 110 120
Figure 4.2. Dose-response and dose-recovery curves after cerebellar injection of kainic
acid in wild type mice. Figure A shows the increased severity of dystonia elicited by
increasing dosage of kainic acid. The average D-score was taken at 30 minutes after
injection from 5-6 mice per dosage. Error bars represent SEM and R2 value of 0.98 is
shown. Figure B illustrates the recovery of the mice to non-dystonic behavior within 2
hours of injection (5-6 mice per dosage).
74
in situ hybridization of c-fos expression after cerebellar kainate injection
In situ hybridization of the immediate early gene, c-fos, was performed to determine
the neuronal pathways activated during kainate-induced dystonia. At the end of the twohour behavioral observation period, animals were sacrificed and tissue was collected for
in situ hybridization. Regions of c-fos hybridization analyzed were based on their
proposed relation to dystonia or kainate-induced behaviors. Representative sections from
c-fos in situ hybridization are shown for several regions and over a wide dose range in
Figure 4.3. Intense c-fos induction was seen in the cerebellum of animals receiving high
doses of kainic acid. The lower doses produced modest changes as would be expected
from the mechanical stress of injection. c-fos mRNA hybridization occurred in all
regions of the cerebellum with even more intense induction seen at the needle sites of
injection. Other regions demonstrating c-fos induction in a dose-dependent manner
included the red nucleus and the locus ceruleus. Regions believed to be involved in
dystonia, including the striatum and motor cortex failed to demonstrate substantial c-fos
hybridization.
In fact a significant decrease in c-fos hybridization was seen in the
striatum. The hippocampus also failed to demonstrate c-fos induction and rather showed
significantly decreased expression, indicating that the behavior recorded was not a
kainate-induced seizure. In fact, intense c-fos hybridization in the hippocampus and
motor cortex was seen in one animal that did undergo behaviorally recognized seizures
after injection of kainate presumably too near the inferior colliculi (data not shown).
Mean optical density of c-fos signal was quantified in the aforementioned areas and is
presented in Figure 4.4.
75
0pmoles
60pmoles
115pmoles
235pmoles
Striatum and
Cortex
Hippocampus
Red
Nucleus
Locus
Ceruleus
Cerebellum
Figure 4.3. c-fos in situ hybridization in wild type mice after cerebellar kainate
injection. Representative sections from regions of c-fos activation and other areas of
interest are shown from mice receiving 0, 60, 115, or 235 picomoles kainic acid in a
volume of 0.5? l injected into the anterior cerebellar vermis. Mice receiving kainic acid
displayed dystonic behavior in a dose-dependent manner.
76
0.45
0pmol
60pmol
115pmol
235pmol
0.40
0.35
Optical Density
0.30
0.25
0.20
0.15
0.10
0.05
0.00
ctx
str
hip
rn
lc
cb-ant
cb-post
Figure 4.4. Regional expression of c-fos mRNA after cerebellar kainate injection. In
situ hybridization of c-fos mRNA was quantified two hours after injection into the
cerebellum. Data presented are the mean of four animals at each dosage and error bars
represent SEM. cb-ant, anterior cerebellum, cb-post, posterior cerebellum, rn, red
nucleus, lc, locus ceruleus, str, striatum, hip, hippocampus, ctx, primary motor cortex.
77
Cerebellar needle localization
Confirmation of cerebellar needle tract location was performed to verify accuracy of
freehand injections. At the time of brain extraction, injection site was verified in the
anterior vermis of the cerebellum by gross analysis. Tissue processed for c-fos in situ
hybridization was also analyzed for needle tracts and regions of most intense c-fos
induction as markers for injection location within the cerebellum. Numerous sections
were analyzed for each animal and locations approximated by comparison with Franklin
and Paxanos stereotaxic atlas of the mouse brain (Figure 4.5)
Figure 4.5. Cerebellar injection site localization. Injection sites were localized and
represented as symbols on representative sections modified from Franklin and Paxanos,
1997. Millimeters from bregma are shown for each section. Kainate dosages shown in
legend are in picomoles.
Regionalized kainate injections
To verify that the cerebellum is indeed responsible for the dystonic phenotype seen
after kainate injection, several other brain regions were also injected using this protocol.
First, the cerebellum was injected 1-2 mm lateral from the midline, anterior and in the
vermis. Mice were injected either to the left or right of midline with 235 pmoles kainic
acid at a depth of 2 mm. Circling in the direction contralateral to injection was seen
78
shortly after recovery from anesthesia, immediately prior to and at the onset of dystonia.
Initial mild to moderate dystonia (D2-D3 level) was restricted to the ipsilateral side of the
injection site for 30 to 60 minutes. As the dystonic attacks progressed to more severe
stages (D4-D5), the body became bilaterally affected.
If the kainic acid injected into the cerebellum acted by diffusing to other regions,
injections of kainic acid into the lateral ventricle would also likely result in dystonia.
Therefore, the lateral ventricle was injected in 5-6 mice each with 0 pmoles, 60 pmoles,
115 pmoles, and 235 pmoles kainic acid. No behavioral effect was appreciated at the 0
pmoles and 60 pmoles doses. Mice receiving 115 pmoles showed a paucity of movement
with most of their time spent sitting still in the corner of the observational cage with a
few displaying a seizurigenic phenotype. Mice receiving the highest dose, 235 pmoles,
also remained still in the corner but 60% also had numerous (2+) seizures within the
observational period. The seizures would often begin with the mouse extending at the
neck and having gasping jaw movements in synchrony with the tail jerking above the
body toward the head. After a few seconds the forepaws would begin beating downward
and away from the body again in synchrony with the head and tail movements. Saliva
could often be noted coming out of the mouth and onto the fur. Often the body would
jerk severely enough to cause the mouse to fall to one side and lay on the cage bottom
while the seizure continued. One mouse died in a particularly severe seizure 44 minutes
after injection. In general the seizures lasted 5-15 seconds and the mice were extremely
still between seizures. By the end of the observational period, most seizurigenic mice
had improved and would sniff or explore a bit without as frequent seizure occurrence.
Dystonic postures were never observed after ventricular injection of kainic acid.
79
Because the basal ganglia has been implicated in nearly all movement disorders,
including dystonia, kainic acid was injected into the striatum of wild type mice to
determine if this region could elicit dystonia using this protocol. Mice were again
injected with 0-, 60-, 115-, and 235-pmole doses of kainic acid (5-6mice/dose). Mice
receiving saline typically would explore for the first hour then rest in the second hour.
The 60 pmole dose of kainic acid caused hyperactive grooming in the first hour followed
by resting in the second. Mice receiving 115 pmoles kainic acid never rested for long
periods, they actively groomed and explored for the entire observational period. Mice
would occasionally circle in a direction contralateral to the injection site while exploring.
Mice receiving the highest dose of kainic acid, 235 pmoles, were very actively grooming
and exploring with some circling and two mice had brief, isolated seizures at 1 and 2
hours after injection respectively. As in the ventricular injections, striatal injections
never resulted in dystonic posturing.
Standard hematoxylin and eosin stained sections from all mice receiving regional
kainic acid injections confirmed the position of the freehand injection to either the lateral
ventricle or striatum (data not shown).
Kainate injections in SV4 mice lacking Purkinje cells
To determine that cerebellar signaling is necessary for the kainate-induced dystonia,
transgenic mice lacking Purkinje cells were injected with kainic acid in the anterior
cerebellar vermis in the midline at a depth of ~2 mm. If the cerebellar cortex is indeed
necessary for the production and/or maintenance of dystonia in this model, mice lacking
Purkinje cells, the sole output of the cerebellar cortex, should not display a dystonic
phenotype after kainate injection. Conversely, if cerebellar cortical output is not vital to
80
the production of dystonia, transgenic mice lacking Purkinje cells should display a
dystonic phenotype similar to controls. Fifteen aged transgenic mice were injected with
235pmoles kainic acid and most displayed no notable motor behavior in addition to the
characteristic transgenic ataxia (9/15). A few mice displayed brief and relatively mild
dystonic motor behavior (4/15) and the remaining mice developed brief wild
running/seizure behavior at 10-20 minutes post-injection (2/15).
The seizing mice
quickly recovered and proceeded to maintain odd, stiff arching postures, which were not
typical of the transgenic phenotype. While these postures were also not typical to those
seen with kainate-induced dystonia, they caused the mice to be immobilized in odd
postures and were therefore scored as such (D5). It is also important to note however,
that this behavior remitted temporarily after disturbing the mice. This is in direct contrast
to the exacerabatory effect disturbance has on mice immobilized in typical dystonic
postures.
Average D score over the two-hour observation period was significantly
reduced (p<0.0005) in SV4 mice receiving kainic acid compared to control mice
receiving kainic acid (figure 4.6).
81
45
40
35
Average Total D Score
30
25
***
20
15
10
5
0
WT-0
WT-235
SV4-0
SV4-235
Figure 4.6. Dystonic severity in transgenic mice lacking Purkinje cells. Transgenic mice
(n=15) were injected with 235 pmoles kainic acid in the midline anterior cerebellar
vermis and observed for two hours for dystonic postures (SV4-235). Transgenic animals
were injected with saline (n=6) to demonstrate the basal level of motor disturbance in
these animals (SV4-0). D score was summed over the two-hour observational period and
averaged per group. Wild type data is illustrated for comparison and is the same as
presented above in figure 4.2 (saline n=5, WT-0; kainate n=6, WT-235). *** indicates
significantly reduced dystonia in SV4 mice compared to wild type animals, p<0.0005
using the Student's t-test.
82
EEG recordings of dystonic mice after kainate injection into the cerebellum
Kainic acid is frequently used to induce seizures after i.p. or central injection in
experimental animals. For this reason and because dystonia is sometimes confused with
motor seizures in animal models, EEG recordings were collected during kainate induced
dystonia in mice. EEGs from five mice were recorded during kainate-induced dystonia
(235 pmoles). In order to distinguish the dystonic EEG from that of a seizure, 60 mg/kg
pentylenetetrazole was administered after 90 minutes to induce generalized seizures.
Numerous baseline recordings prior to kainate injection were also recorded and
representative traces of baseline, kainate-induced dystonia, and pentylenetetrazole seizure
are shown in figure 4.7. Eighty EEGs recorded during behavioral dystonia were analyzed
for the five mice and none were associated with epileptiform activity.
83
A. Baseline
B. Kainate-Induced Dystonia
C. PTZ-induced seizure
Figure 4.7. Representative EEG recordings from wild type mice receiving cerebellar
microinjections of kainic acid. EEGs shown were recorded before kainic acid injection
(A), during profound dystonic posturing after kainic acid injection (B), or during seizures
induced after pentylenetetrazole (PTZ) injection (C).
84
NBQX antagonism of kainate-induced dystonia
Kainic acid is known to activate both kainate and AMPA ionotropic glutamate
receptors. In order to verify that kainate is acting locally within the cerebellum at these
receptor sites, NBQX, a kainate and AMPA receptor antagonist, was used to block the
kainate-induced dystonia. Mice were injected in the anterior cerebellar vermis with 0.5? l
of 2.35 nanomoles NBQX alone or 2.35 nmoles NBQX mixed in solution with either
115pmoles or 235pmoles kainic acid. Mice receiving NBQX alone displayed either
normal (2/5), hyperactive/jittery (2/5), or decreased (1/5) locomotor activity.
Combination of NBQX and 115pmoles kainic acid caused 4/5 mice to remain mostly still
during the observational period with slowed ambulation once disturbed. This phenotype
was indistinguishable from the decreased locomotor activity seen in one of the control
NBQX-only injected mice. The remaining NBQX/115 pmoles kainate-injected mouse
showed normal locomotor activity (1/5). Co-injection of NBQX with 115 pmoles kainic
acid never resulted in any dystonic postures. Injection of NBQX together with 235
pmoles kainic acid resulted in 1/5 mice behaving normally, 2/5 mice displaying
decreased activity (as described for both NBQX alone and NBQX/115 pmoles), and 2/5
mice demonstrating a relatively mild dystonic phenotype. Total D score was summed
over two hours of observation and averaged over five mice per group (Figure 4.8).
Statistically significant reduction in total D score was seen in mice injected with 235
pmoles kainic acid in combination with NBQX compared to control kainate-injected
mice (235 pmoles kainate alone).
85
50
Average Total D Score
40
30
**
20
10
0
NBQX
235 KA
Figure 4.8. Dystonic severity
after cerebellar NBQX coinjection with kainic acid.
Mice were injected with NBQX
(2.35 nmoles) alone and in
combination with 235pmoles
kainic acid. Total D score was
summed over two hours of
observation and averaged for 56 mice in each drug paradigm.
** indicates p<0.005 between
kainate with and without
NBQX.
NBQX+235KA
Domoic acid injection induces dystonia in wild type
As a complement to the antagonism provided by the NBQX experiments, domoic
acid was used to duplicate the dystonic phenotype through activation of glutamate
receptors. Similar to kainic acid, domoic acid is an agonist at AMPA and kainate
receptors. Activation of these glutamate receptor subtypes is presumed to underlie the
mechanism of dystonia induction by kainic acid described above. Wild type mice were
injected along the midline in the anterior cerebellar vermis and dystonic behavior
recorded.
Similar to kainate-injected mice, domoate-injected mice displayed a
reproducible phenotype of generalized and intermittent dystonia within 10 to 20 minutes
after injection. Domoate-injected mice were observed to have flattened trunks with the
hindlimbs abducted and externally rotated, suspended in the air. Forelimb paddling and
facial movements were also consistently noted. These dystonic postures were identical to
those observed in mice after kainate injection into the cerebellum. The quality of the
86
dystonia seen after 7.5 pmoles domoate injection was different from kainate-induced
dystonia in one regard however. Domoate mice more frequently remained in odd, semiflattened postures in between dystonic exacerbations than did kainate-injected mice. It is
important to note however, that not all domoate mice displayed this behavior and some
kainate mice also displayed this behavior. Domoate-injected mice displayed a level of
dystonia analogous to that of 235 pmole injected kainate mice (Figure 4.9) and also
recovered to near-normal behavior within two hours after injection (data not shown). As
with kainate, domoic acid induced seizures were seen infrequently when needle
localization was such that the inferior colliculi were likely exposed to concentrated drug.
Average Total D Score
50
40
30
20
10
0
saline
kainate
domoate
Figure 4.9. Dystonic severity
after cerebellar injection of
domoic acid in wild type mice.
Domoate (7.5 pmoles) was
injected
into
the
anterior
cerebellar
vermis
of
five
C57Bl/6J mice and dystonic
behavior recorded. D score was
summed
over
two
hours
immediately following injection
and averaged for 5-6 mice pre
group. Saline and kainate (235
pmoles) data is the same as that
presented above (Figure 4.5).
Error bars represent SEM.
Discussion
Movement disorders are by nature difficult to describe in rodent models. Limited
understanding of the clinical terminology by researchers further compounds this
difficulty making it exceedingly arduous to determine the exact behavior described in the
literature and comparing it to other behaviors seen or described. This is particularly true
87
in the case of dystonia, which historically is misjudged as seizures, myoclonus, ballism,
and chorea. The use of kainic acid in this model of dystonia therefore had to be done
with special attention to the possibility of seizure induction. We believe that the motor
behavior described here represents dystonia and not seizures because of the quality of the
movements, the absence of seizure activity on EEG recordings, and the lack of c-fos
induction in the hippocampus or motor cortex. Furthermore, a few mice did respond to
kainic acid injection with generalized seizures and this was readily discernable from the
dystonic phenotype. In situ hybridization of a seizurigenic mouse showed marked c-fos
induction in the hippocampus and cerebral cortex. This confirmed molecularly what was
very apparent behaviorally and further distinguished the seizure-inducing effects of
kainic acid from the dystonia-producing effects described in this model.
Microinjection of 60 to 250 picomoles of kainic acid (in 0.5? l) into the anterior
cerebellar vermis of C57Bl/6J mice resulted in an acute and reproducible dystonic
phenotype.
Similar to the neurotoxic and seizure-producing effects of kainic acid
(Lothman EW, 1981; Sperk G, 1985), kainate-induced dystonia was positively associated
with increasing kainic acid dosage. All body regions were involved in dystonic postures.
Paddling or tonic extension of the hindlimbs along with a severely flattened and extended
trunk hallmarked the most common dystonic posture. Face, head and neck were also
involved in dystonic postures, categorizing these attacks as a form of generalized
dystonia. Dystonic attacks began 10-20 minutes after kainate injection and lasted for 1-2
hours.
While the most severely affected mice would remain immobilized in tense
dystonic postures for the much of the time, the majority of mice displayed short-lived
intermittent dystonic attacks initiated by movement or startle.
88
The immediate early gene, c-fos, expression was studied to determine the neuronal
pathways activated during kainate-induced dystonia. Two hours after cerebellar kainic
acid injection, nearly the entire cerebellar cortex and the DCN show markedly increased
c-fos induction. Of the cerebellar nuclei targets, only the red nucleus demonstrated c-fos
activation in dystonic mice. The locus ceruleus also demonstrated c-fos induction in a
dose-dependent manner to kainate injection. These changes in c-fos expression
theoretically may have resulted from activation of pathways independent from that
involved in the production of the dystonia. However, the general paucity of neuronal
activation seen after cerebellar kainic acid injection increases the likelihood that these
regions were involved in the pathway responsible for the dystonic phenotype as well.
Cerebellar c-fos induction increased with kainate dosage. Dramatic activation in the
posterior and anterior cerebellum, both medially and laterally, was seen after mid- to
high- dose kainate injections. These dosages corresponded to animals demonstrating
profound dystonic behavior. Low doses of kainic acid resulted in relatively mild and
localized c-fos induction within the cerebellum, while saline injection produced very
slight induction around the needle tract, consistent with mechanical stress.
The
difference between the intense broad activation seen at higher kainate doses compared to
the local mild activation at lower doses is quite remarkable and unlikely to result from
diffusion of kainic acid alone. Broad activation throughout all cerebellar folia despite the
small injection volume (0.5? l) suggests neuron to neuron activation played a role in
spreading as opposed to simple diffusion. It is more reasonable that neuron to neuron
activation played a role because of the distance and number of folia the kainate would
have traveled to activate the distal tissue directly. Furthermore, if diffusion to the CSF
89
between folia occurred, neighboring regions such as the colliculi and brainstem would
show more background activation in animals receiving high kainate doses or generalized
seizure activity would result from high concentrations of kainate circulating in the CSF.
Therefore, these data suggest that neuronal activation of the cerebellum by local kainic
acid injection results in amplification of the signal to result in broad activation of most of
the cerebellum. Whether this phenomenon participates in the production of dystonia or
results from the dystonia-producing signal remains to be seen. c-fos in situ hybridization
studies in transgenic mice lacking Purkinje cells and failing to demonstrate dystonia after
cerebellar kainate injection would address both of these questions. c-fos studies in
transgenic mice would also determine whether induction in the red nucleus and locus
ceruleus is part of the pathway causing dystonia or if these events are independent of one
another.
The red nucleus was the only target of DCN efferents clearly demonstrating c-fos
induction after cerebellar kainate injection. Because efferents of the DCN to the red
nucleus likely represent collaterals of axons destined for the motor thalamus, it is
surprising that these nuclei failed to demonstrate marked activation as well.
The
theorized importance of the motor thalamus in the pathophysiology of dystonia makes
this finding particularly puzzling. The red nucleus is one of the major efferent targets of
the DCN, receiving excitatory input from the contralateral interpositus and dentate nuclei.
The red nucleus also receives afferents from other major motor centers in the brain,
including the motor and premotor cortices, the posterior thalamic nucleus, the basal
ganglia, and the spinal cord. This structure lies in the midbrain of limbed vertebrates and
gives rise to the fibers of the rubrospinal tract. The descending rubrospinal tract
90
originates in somatotopically organized neurons of the red nucleus and terminates on
interneurons in the ventral gray column of the spinal cord. The other major efferents of
the red nucleus form a recurrent loop to the cerebellum. Direct contralateral fibers
synapse directly on the DCN while ipsilateral efferents of the red nucleus indirectly reach
the cerebellar cortex through the precerebellar nuclei. As with the cerebellum, a large
body of evidence is accumulating that suggests a role for the red nucleus in dystonia as
well. In a review of 7 patients with midbrain lesions resulting in dystonia, lesions in 7/7
patients involved the red nucleus, rubro-thalamic, and/or dentate-rubral fibers (Vidailhet
M, 1999). Induction of neck dystonia in rats after intrarubral injection of compounds that
bind opiate sigma receptors implicate involvement of the red nucleus in dystonia (Walker
JM, 1988; Matsumoto RR, 1990). These effects have been determined to act through the
sigma2 receptor subtype (Nakazawa M, 1999). Furthermore, the use of antipsychotics
with sigma receptor activity may induce motor side effects through these receptors rather
than presumed dopaminergic activity (Walker JM, 1988). Numerous abnormal findings
in genetic rodent models also support a role for the red nucleus in the pathophysiology of
dystonia. Magnocellular neurons in the red nucleus of the dystonia musculorum mouse
show pathological features consistently (Messer A, 1980; Stanley E, 1983). The dystonic
rat shows decreased metabolic activity within the red nucleus during dystonic posturing
only (Brown LL, 1989). Alternatively, greatly increased activity was seen in the red
nucleus of dystonic hamsters, again only in the presence of dystonia. Depth electrode
recordings from the red nucleus in these animals also suggests abnormalities in the red
nucleus (reviewed in Richter A, 1998). If the cerebrocerebellar circuit is hyperactive or
otherwise deranged in dystonia, cerebellar efferents to the red nucleus may play a part in
91
expression of the dystonic program. Activation of muscles via the rubrospinal tract may
occur in discordance with activation/inhibition of muscles via the corticospinal tract
resulting in disordered movement as a direct consequence of cerebellar signaling.
Similar to the red nucleus, the locus ceruleus (LC) demonstrated marked activation in
a dose-dependent manner after cerebellar kainate injection. The locus ceruleus is a
relatively small pair of brainstem nuclei that project noradrenergic (NE) terminals widely
throughout the brain. As with the cerebellum and the red nucleus, substantial evidence
supporting a role for the locus ceruleus in the pathophysiology of dystonia continues to
accumulate (reviewed in Adams LM, 1988). Regions involved in motor control receive
substantial LC innervation including the motor cortex, thalamus, and the cerebellum.
Within the cerebellum and elsewhere, norepinephrine released from LC terminals enables
more effective transmission of both excitatory and inhibitory systems converging on the
same target neurons during periods of simultaneous activity (Bloom FE, 1979).
Facilitation of afferent excitatory and inhibitory activity while reducing endogenous
background activity allows LC-NE enhancement of target area function (Woodward DJ,
1979). For example, mossy fiber afferent stimulation of cerebellar granule cells results in
parallel fiber excitation of Purkinje cells lying along the length of the parallel fiber. The
same parallel fiber activates stellate and basket cells to inhibit adjacent Purkinje cells that
lie off that parallel fiber. In this situation, LC-NE input potentiates these effects and
therefore increases the precision of this inhibitory surround mechanism for incoming
excitatory signals (Eccles J, 1967; Woodward DJ, 1979; Moises HC, 1983).
It is
interesting that failure of such center-surround inhibition at the level of the motor cortex
is theorized to result in dystonia (Halett M, 1998). While evidence implicating these
92
functions of the LC in dystonia has not been defined, numerous lines of research have
demonstrated more general involvement of the LC in dystonia.
Biochemical analysis of two human dystonia musculorum deformans samples
revealed marked changes in NE levels in numerous LC targets (Hornykiewicz O, 1986).
Animal models of dystonia also support a role for the LC in the pathophysiology of
dystonia. In an experimental model of dystonia, microinjection of adrenocorticotropin
hormone (ACTH) fragments into the LC of rats caused ipsilateral leaning and postures
reminiscent of human dystonia for a number days depending on dosage. These results
were determined to result from the direct action of NE at ? -adrenergic receptors on
cerebellar PC (Jacquet YF, 1982; Jacquet YF, 1988). Further experimentation revealed
that the action of ACTH likely occurs through modulation of endogenous opiate peptides
in the LC (Bertolini A, 1986). The dystonic rat, dystonia musculorum mouse, tottering
mouse, and dystonic hamster all demonstrate altered NE concentrations, particularly in
the cerebellum (Riker DK, 1981; Richter 1998; Levitt P, 1981). As with the red nucleus,
it seems unlikely that the locus ceruleus alone is responsible for the production of
dystonia. However, LC activity may modulate motor center functions as a primary
component of the dystonia program or a secondary reaction to dystonic activity.
Despite the localization of the cerebellar injection sites, the broad and intense
cerebellar c-fos induction, and the activation of the red nucleus, it is still arguable that the
kainic acid acted to induce dystonia at another site. Therefore, microinjection of kainic
acid in other regions was attempted to verify the inciting role of the cerebellum in this
model of dystonia. First, we lateralized the cerebellar injections from the midline of the
anterior vermis to ~2mm lateral, in the paravermis. Dystonia in these mice remained
93
ipsilateral to the injection site initially during moderate dystonic impairment, eventually
generalizing to both sides of the body as the severity of dystonia increased.
Displacement of the previously bilateral expression of dystonia through lateralization of
the cerebellar injection site strongly supports the importance of cerebellar activation in
the production of dystonia in this model. Injections of 0, 60, 115, or 235 picomoles
kainic acid into the striatum and the lateral ventricles never resulted in dystonia,
emphasizing the specificity of local excitation of the cerebellum in the induction of
dystonia with kainic acid microinjection.
To further illustrate the function of the cerebellum in the production of dystonia,
cerebellar kainic acid microinjection was done in transgenic mice lacking PCs. SV4
transgenic mice lose PCs over a period of weeks post-developmentally with no other
abnormalities reported, cerebellar or otherwise.
Kainate injection in these animals
resulted in a highly significant decrease in average total dystonia. The appearance of
relatively mild dystonic postures in a few of the mice was likely due to the remaining PC
activity at the time of the experiments. The F5 generation of the transgene bred onto
C57Bl/6J background appears phenotypically delayed in comparison to the original
reports (Fedderson R, 1992) and to prior generations. Thus, despite the advanced age of
the transgenic mice used in these experiments (six months old), sparse PCs may have
remained in some animals, resulting in dystonic posturing after kainic acid injection. In
addition to cementing the necessary and inciting role the cerebellum plays in the
induction of dystonia, failure of mice lacking PCs to demonstrate dystonia directly
implies a role for cerebellar cortical output in the production of dystonia. These results
are in agreement with those obtained by Campbell et al., and data presented in this work
94
(Chapter 3) using the tottering mouse as a model of dystonia.
In both of these
experiments, selective destruction of PCs in the dystonic tottering mutant mouse resulted
in loss of the dystonic phenotype (Campbell DB, 1999; Chapter 3). Together these data
suggest that dystonia of cerebellar origin occurs through activation of a circuit involving
the cerebellar PC. Furthermore, the PC is a necessary component of the pathways
involved in the pathophysiology of dystonia in these two very different mouse models.
Data presented here indicate that kainic acid injection into the cerebellar vermis of
wild type mice produces a dystonic phenotype and c-fos induction throughout the
cerebellum, red nucleus, and locus ceruleus in a dose-dependent manner. The cellular
mechanism of kainate-induced dystonia remains to be uncovered. Kainic acid is a
glutamate analogue and an agonist at both kainate and AMPA receptors. AMPA (along
with NMDA) receptors are thought to mediate the majority of fast excitatory
neurotransmission in the CNS while kainate receptors contribute a relatively minor
component. A second presynaptic function of kainate receptors has been suggested with
modulation (depression) of GABAergic transmission being demonstrated (RodriguezMoreno A, 1997). Antagonism of kainate-induced dystonia with co-injection of NBQX
and replication of the behavior with cerebellar domoic acid injections verify the notion
that kainic acid induces dystonia through activity at these receptors.
In summary, microinjection of kainic acid into the cerebellar vermis is sufficient to
produce profound dystonia in wild type mice.
These data illustrate the potentially
powerful role the cerebellum may play in the production of dystonia because no
abnormalities in any other brain region existed at the time of injection.
While a
contributory role for the red nucleus and the locus ceruleus remain to be established,
95
these brainstem nuclei showed marked activation after cerebellar kainate injection in
dystonic animals. The well-defined and genetically normal background of these mice
eliminates confounding factors in the examination of dystonia pathophysiology in this
animal model. Surgical and pharmacological manipulations of this model may provide
profound insight not only to the pathophysiology of dystonia, but to possible
interventions as well. The production of profound and reproducible dystonia in wild type
animals after localized excitation of the cerebellum suggests that this region no longer be
overlooked in the pathophysiology of this devastating neurological disease.
97
Chapter 5. Discussion
5.1.
MOUSE MODELS OF DYSTONIA
Animal models of human disease represent powerful tools to examine
pathophysiology of disease and to identify possible interventions in the disease process.
Limitations of models arise from the innate differences between the physiologies of
humans versus laboratory animals; however, tremendous amounts of information
pertaining to the human disease state may be discovered through animal research.
Demonstration of similar findings in multiple models, each with different strengths and
limitations, lends support to the validity of the results, reducing the likelihood that they
represent an idiosyncrasy of an individual model.
Primary dystonia represents a common neurological disease of unknown etiology.
Numerous neuroanatomical regions have been implicated in the pathophysiology of this
disorder, however none have been definitively identified as responsible for this disease
process. Therefore, studies in current animal models and development of novel models
of dystonia can provide crucial insight to the pathophysiology and potential interventions
of this devastating disorder.
The tottering mouse
Current rodent models of dystonia are limited in usefulness because the primary gene
defect resulting in the phenotype is unknown. The tottering mouse, therefore has a
unique advantage as a rodent model of dystonia because of the well-defined nature of the
genetic mutation and a growing understanding of the resultant behavioral and cellular
phenotypes. Discovery of the neuronal basis of tottering mouse dystonia can further
define the neuronal networks capable of producing dystonia of other etiologies.
98
Description
Tottering mice display striking episodic attacks of severe motor dysfunction
remarkably similar to human generalized dystonia. Described in detail in Chapter 1.2,
the attacks typically last 30-60 minutes and occur spontaneously or in response to several
known stressors and pharmacological agents with no discernable refractory period.
Characterization of attack induction after exposure to various environmental stimuli and
compounds has greatly increased the utility of this model in the study of dystonia.
Caffeine, alcohol, and stress (e.g., restraint, environmental novelty) reliably trigger
attacks in ~60-100% of tottering mice (Campbell DB, 1998; Fureman BE, 2001).
Furthermore, administration of common anticonvulsants not only fails to block attacks,
but some actually induce attacks in tottering mice (Syapin PJ, 1983).
Conversely,
administration of L-type calcium channel antagonists (Campbell DB, 1999; Fureman BE,
2001) and benzodiazepines (Syapin PJ, 1983) reduces tottering dystonic attack frequency.
The phenotypic characteristics and pharmacology of tottering dystonia strikingly
resemble those of human PNKD (Chapter 1.1) and are highlighted in Table 5.1.
Characteristic
Attack Duration
Attack Frequency
Tottering Mouse
30-60 minutes
Unknown
Occurrence
Common Triggers
Other
Spontaneous
Stress, caffeine, alcohol
Severity may wax and wane during
attacks
No discernable refractory period
+ L-type Ca2+ channel antagonists
+ benzodiazepines
- anticonvulsants
? utosomal recessive inheritance
? 1A VDCC subunit mutation
Drug Response
Genetics
Pathophysiology
Strong evidence for role of aberrant
cerebellar signaling
PNKD
30-60 minutes
2-3/month (range from 2-3/day to 23/year)
Spontaneous
Stress, caffeine, alcohol, fatigue
Severity may wax and wane during
attacks
No discernable refractory period
+ benzodiazepines (moderate effect)
- anticonvulsants
Autosomal dominant inheritance
Linkage to 2q33-35, in area of suspected
ion channel
Unknown
Table 5.1. Comparison of salient features of tottering dystonia and PNKD.
99
Role of the cerebellum in tottering dystonia
The tottering mouse has been studied as a model of dystonia with particular relevance
to PNKD (Fureman BE, 2001). Previous reports have implicated the cerebellum in the
production of tottering dystonia. Increased cerebellar L-type VDCC expression and
suppression of attacks with L-type calcium channel antagonists (Campbell DB, 1999)
lend circumstantial evidence for a role of the cerebellum in tottering dystonia. Intense cfos induction within the cerebellum during attacks also supports a role for this region in
the pathophysiology of tottering dystonia (Campbell DB, 1998). Elimination of the
phenotype on the pcd background provides even stronger evidence that the cerebellum,
and the cerebellar PC in particular, induce and/or maintain the dystonic phenotype of the
tottering mouse (Campbell DB, 1999). In addition, surgical removal of small portions of
the anterior cerebellar vermis reduce the duration and number of attacks while lesions of
the posterior cerebellar vermis show no differences. Similar results are obtained after
excitotoxic lesions of the same areas. (Abbott LC, 2000).
Data presented in this work
further define an important role for the cerebellum in the generation of tottering dystonia
through the elimination of the dystonic phenotype after transgene-induced PC death
(Chapter 3). Future experiments in these mice will define subsets of PC responsible for
the regional expression of tottering dystonia.
Other data presented here demonstrate decreased excitatory neurotransmission in the
tottering cerebellum with no concomitant change in inhibitory transmission (Chapter 2).
These findings suggest an imbalance in the overall signaling in the tottering cerebellum
and provide evidence of a potential link between the genetic mutation and the behavioral
phenotype, as they represent a molecular consequence in a region defined in the
100
pathophysiology
of
the
disease.
Future
experiments
designed
to
analyze
neurotransmission of single cell types in the tottering cerebellum would further define the
transmitter imbalance and dissect out cell types involved in the production of dystonia in
this mutant.
Future Studies
The PC has already been identified as an absolute and necessary component of the
dystonia-producing substrate in tottering mice (Campbell DB, 1999; Chapter 3). The
function of the PC as the sole output of the cerebellar cortex therefore indicates this
region in the pathophysiology of dystonia. Components of the circuitry upstream of PC
function need to be evaluated to determine whether the dystonia-producing signal
originates in the PC or if this cell type simply represents a crucial link between the origin
and expression of dystonia. Purkinje cells receive excitatory input from climbing fibers
and parallel fibers while inhibitory input is derived from stellate and basket cells.
Because PCs are of central importance to the generation of tottering dystonic attacks,
disruption of the PC spike rate through climbing fiber denervation was attempted in the
tottering mouse. Lesioning of climbing fibers through ablation of the inferior olive (IO)
with 3-acetylpyridine (3-AP) injection was not achieved however, even after
administration of high doses of 3-AP (300mg/kg; data not shown). Therefore, other
methods or further modifications of 3-AP intoxication should be attempted to evaluate
the role of climbing fiber excitation of PC in the expression of tottering dystonia.
Mossy fibers represent a second extracerebellar input to the cerebellar cortex and act
to excite PC through relay with granule cells. Lesioning of mossy fibers or granule cells
would therefore elucidate the role these inputs have in tottering dystonia. Breeding
101
genetic mouse mutants that develop lesions of mossy fibers, granule cells, and other
combinations of cell types involved in the cerebellar circuitry onto the tottering mouse
would determine the effects these inputs have on the expression of tottering dystonia.
However, epigenetic effects and the contribution of extraneous phenotypes of the second
mutant may be difficult to evaluate.
The effect of GABAergic innervation on relatively small areas of PCs can be
evaluated through local application of GABA agonists/antagonists followed by dystonia
induction in tottering mice.
Analysis of tritiated drug diffusion could verify the
distribution in the cerebellar cortex, eliminating the concern that the drugs affected the
DCN directly. Spatial restriction of locally applied drugs to relatively few PCs may limit
their potential response.
However, small areas of the anterior cerebellar cortex
profoundly affect expression of dystonia in both ablative studies (Abbott LC, 2000) and
local excitation (Chapter 4) using glutamate agonists, suggesting that this region may be
sufficient to elicit a response.
Microinjection of GABA agonists/antagonists in the
anterior cerebellar vermis in tottering mice can be done rapidly under isofluorane
anesthesia and the effect on dystonia evaluated almost immediately.
In fact,
administration of GABA agonists or antagonists through an indwelling catheter to the
cerebellar cortex during a tottering dystonic attack can directly assess the effect inhibition
or release of PC activity has on the expression of dystonia. Resultant effects on dystonia
after PC GABA-receptor activation/inhibition would implicate a role for basket and/or
stellate cells in the modulation of dystonia-producing PC activity. These data would
furthermore reveal whether PC firing patterns are likely increased or decreased in
102
tottering dystonia. This is an intriguing and complicated question and is discussed further
below in section 5.2.
In summary, the tottering mouse represents a unique animal model of dystonia that
closely resembles PNKD. Analysis of the neuroanatomical and functional consequences
of the P/Q-type VDCC mutation has determined that the cerebellar cortex is vital in the
pathophysiology of dystonia in this mutant. Further delineation of the dystonic origin
and the circuits involved in the expression of dystonia in tottering mice will greatly
expand current understanding of the pathomechanisms capable of producing dystonia.
Kainate-induced dystonia
Limitations of the tottering mouse as an animal model of dystonia arise from wide
spread neurological effects of the mutation. Despite limitations, consistent findings of
abnormal cerebellar structure and function in genetic rodent models of dystonia lend
strong support to a role of the cerebellum in dystonia. The next step in the use of animal
models of dystonia would then be to use genetically normal animals to replicate the
dystonia-producing signals implicated in the more limited genetic models of dystonia.
We present here, a novel animal model suited to the study of neuronal networks capable
of causing dystonia in wild type mice using a well-defined excitatory amino acid
microinjection protocol.
Description
The kainate-induced dystonia described here represents a novel drug-induced animal
model of human dystonia (Chapter 4). Microinjection of low-doses of kainic acid into
the cerebellar vermis of mice elicited reliable and reproducible generalized dystonia that
began 10-20 minutes after kainate injection and lasted for less than two hours. While the
103
most severely affected mice remained immobilized in tense dystonic postures for the
much of the time, the majority of mice displayed short-lived intermittent dystonic attacks
initiated by movement or startle. The clinical spectrum of kainate-induced dystonia was
noted to somewhat resemble that of human PKD specifically (see Chapter 1.1).
Characteristics of these two forms of dystonia are compared in Table 5.2.
Characteristic
Attack Duration
Attack Frequency
Occurrence
Drug Response
Pathophysiology
Kainate Model
Seconds to minutes
~10-100 attacks in 2 hours after injection
Initiation of movement, startle
Not tested
Aberrant cerebellar excitation
PKD
Seconds to minutes
1-10/day (up to 100/day reported)
Initiation of movement, startle
+ anticonvulsants
Unknown
Table 5.2. Comparison of salient features of the kainate model of dystonia and PKD.
Role of the cerebellum in kainate-induced dystonia
Kainic acid is a glutamate agonist at both kainate and AMPA ionotropic glutamate
receptors. Microinjection of kainic acid into the cerebellum of mice elicited dystonia
through actions at these receptor subtypes, as co-injection with NBQX antagonized these
effects and administration of domoic acid replicated the results. Furthermore, neuronal
activation, as assessed by in situ hybridization for c-fos mRNA, revealed specific patterns
of activity in the cerebellum, locus ceruleus and red nucleus. The specificity of the
neuronal induction occurring with kainate-induced dystonia suggests that localized
cerebellar effects were indeed responsible for the phenotype. Failure to induce dystonia
after microinjection of the striatum or lateral ventricle and lateralization of the phenotype
after paravermal cerebellar injection also suggest that kainate is acting locally within the
cerebellum to induce dystonia. Transgenic mice lacking Purkinje cells show dramatically
decreased dystonia after kainic acid injection suggesting an important role for Purkinje
cells and the cerebellar cortex in this model of dystonia as well. Together, these data
104
suggest that aberrant cerebellar excitation is sufficient to produce dystonia and the
cerebellar cortex plays an essential role in generation of the dystonic signal or circuitry.
Future Studies
Further development of this model of dystonia using wild type and various mutant
mice can dissect out specific cellular components of the dystonia-producing pathway and
also determine possible interventions to the process. Mice lacking specific subsets of
neurons involved in cerebellar circuitry can more fully evaluate the neuroanatomical
substrate of kainate-induced dystonia. Lesioning of other brain regions implicated in the
pathophysiology of dystonia can determine whether these regions are essential in the
expression of dystonia using this model. For example, dopamine-depleted or dopamine
receptor knockout mice can be used in this microinjection protocol to determine whether
this dystonia of cerebellar origin acts in isolation from motor pattern modulation by the
basal ganglia. Lack of basal ganglia c-fos activation already suggests independence of
these two pathways in kainite-induced dystonia.
In summary, kainate-induced dystonia in wild type mice implicates the cerebellum as
an important component in the pathophysiology of dystonia. The cerebellar cortex plays
an essential role in the production of dystonia in this model. Traditional views of
cerebellar function would not predict such a phenotype being of cerebellar origin.
However, traditional studies of the cerebellum investigated disturbances due to decreased
normal function and activity as opposed to the potential effects of abnormal signals and
increased activity, as implicated in the rodent models of dystonia. As animal models and
human imaging studies continue to demonstrate abnormal cerebellar activity, the
perception of the cerebellum in health and disease may necessitate reevaluation.
105
5.2. THE CEREBELLUM AND DYSTONIA
In addition to the data discussed in detail throughout this work, other aspects of
cerebellar anatomy and function raise provocative questions concerning the role of this
region in dystonia. Therefore, discussion of cerebellar circuitry and aspects of cerebellar
function pertinent to dystonia follow.
Cerebellar Circuitry
Intrinsic Cerebellar Circuitry
The intrinsic circuitry of the cerebellum is relatively simple and illustrated in Figure
2.1. Cerebellar PCs supply the sole output from the cerebellar cortex and form inhibitory
synapses directly on the DCN. This corticonuclear projection is the principal input to the
DCN and remains strictly ipsilateral. PCs maintain a roughly radial arrangement in their
projections to DCN with vermal PCs (medial) projecting to the fastigial nuclei while
paravermal (intermediate) and hemispheral PCs (lateral) project mainly to the interpositus
and dentate nuclei respectively (Fundamental Neuroscience, Development of the
Cerebellar System, and Principles of Neural Science).
Afferent Cerebellar Circuitry
Afferents into the cerebellum consist of two main types, climbing fibers from the
inferior olive and mossy fibers from various other precerebellar nuclei. Climbing fibers
exert a powerful excitatory influence on PCs, which then act to inhibit the DCN. Mossy
fibers arising from precerebellar nuclei such as spinocerebellar nuclei, lateral reticular
nucleus, prepositus nucleus, vestibular nuclei, pontine gray nucleus, and the pontine
reticulo-tegmental nucleus all form excitatory synapses with granule cells which in turn
excite PCs. A third, less well defined type of input to the cerebellum, consists of
106
nonlaminar afferents from the locus ceruleus, raphe nuclei, and other undetermined
brainstem structures. These afferents synapse on PCs and throughout the cerebellum,
releasing NE, serotonin, and acetylcholine respectively (Fundamental Neuroscience,
Development of the Cerebellar System, and Principles of Neural Science).
Efferent Cerebellar Circuitry
The cerebellar DCN represent the output of the cerebellum and send efferents to
every major region involved in motor control except for the basal ganglia. DCN efferents
are excitatory with the exception of those reaching the inferior olive. Fibers originating
from the interpositus and dentate synapse in the contralateral red nucleus, superior
colliculus, and other visually related structures in the mesencephalon. Fibers terminating
in the diencephalon do so largely in the ventrolateral complex of the motor thalamus.
The interpositus and dentate nuclei also send contralateral fibers to the pontine grey
nuclei, the reticulotegmental nucleus, and the inferior olive, while the ipsilateral fibers
terminate in the reticular formation. All of these structures reached by the descending
branch of cerebellar efferents are involved in motor behaviors and are also precerebellar
nuclei that send mossy fibers into the cerebellum. Efferent fibers from the fastigial
nucleus synapse on the vestibular nuclei and numerous other precerebellar nuclei with a
few fibers reaching the spinal cord. Fibers originating in the fastigial nucleus also
terminate in the superior colliculus, visual nuclei, and the ventrolateral complex of the
motor thalamus. All projections to the red nucleus and to the motor thalamus maintain a
rough somatopographic organization, not as clearly defined as that of the cerebral motor
and sensory cortices.
107
Cerebellar efferents affect the production and control of movement largely through
input to the cortex via the thalamus and to the red nucleus. Each of these areas in turn
send afferents back to the cerebellum to create upper and lower transcerebellar loops
respectively (Figure 5.1). The motor thalamus receives the largest component of
cerebellar efferents originating in the interpositus and dentate nuclei. The fastigial nuclei
also send a smaller bundle of fibers to this area of thalamus. The premotor and motor
areas of the cortex are the ultimate targets of the cerebellothalamic pathway. In addition
to the corticospinal tract, the cortex in turn sends descending fibers to the pontine gray
nucleus and other precerebellar nuclei that project mossy fibers back to the cerebellar
cortex, finishing this upper transcerebellar loop.
The red nucleus is the second major efferent target of the DCN that substantially
affects motor control.
Contralateral fibers originating from the interpositus nucleus
synapse in the phylogenetically older magnocellular portion of the red nucleus, while
those from the dentate nucleus synapse with the parvicellular portion. It is believed that
these axons are collaterals of those ascending to the motor thalamus and are excitatory in
nature.
The red nucleus also receives inputs from other structures, including large
somatotopically organized input from the premotor and motor cortex to the parvicellular
portion. Efferents of the red nucleus include the rubrospinal tract, arising largely from
the magnocellular neurons in a somatotopically-organized fashion. This tract is a motor
output pathway that terminates on interneurons in the ventral grey column of the spinal
cord. The parvicellular portion of the red nucleus sends efferents back to the cerebellum
via a direct contralateral pathway and an indirect ipsilateral pathway via precerebellar
nuclei. This circuit represents the lower transcerebellar loop (Fundamental Neuroscience,
108
Development of the Cerebellar System, and Principles of Neural Science).
Altered
cerebellar output may act through the thalmo-cortical or rubrospinal paths to affect
muscle function. Each of the cerebellar loops may also serve to augment or spread a
locally aberrant signal within the cerebellum to include larger somatotopically organized
cerebellar regions.
DYSTONIA
MOTOR CORTEX
3
2
Somatosensory
Cortex
Thalamus
1
A
Precerebellar
nuclei
B
Basal
Ganglia
4
RED
NUCLEUS
CEREBELLUM
Inferior
Olive
Figure 5.1. Schematic diagram of pathways theorized to malfunction in cerebellarinduced dystonia. As depicted previously, there are four main regions currently
implicated in the pathophysiology of dystonia: (1) the basal ganglia, (2) the thalamus, (3)
the sensorimotor cortex, and (4) the cerebellum. Additional regions involved in
cerebellar circuitry are included as work presented here implicates disordered cerebellar
activity may induce dystonia through connections with these regions. The upper
cerebellar loop (A) is depicted in red and the lower cerebellar loop (B) in blue.
109
Cerebellar Function
Traditional views implicate the cerebellum in the timing and coordination of
movements. Studies in people and animal models after cerebellar lesioning typically
demonstrate relatively mild motor dysfunctions hallmarked by symptoms such as ataxia,
tremor, dysmetria, and other signs of general incoordination. A role in motor learning is
also attributed to the cerebellum and more recently, even cognitive functions have been
attributed to the cerebellum. The overall function of the cerebellum is excitation of
motor pathways after integration of tremendous amounts of afferent information derived
from motor, perceptual, cognitive, and sensory input from the length of the neuraxis.
Cerebellar output through the DCN is tonically active with a resting frequency of
firing at 40-50Hz. This frequency can either increase or decrease during initiation and
execution of movements, modulating the activity of motor pattern generators. Each of
the three DCN divisions acts in different aspects of motor function with slight overlap
occurring. The medial fastigial nuclei are largely involved with postural control and gait.
The interpositus nuclei act in stretch, contact, and other reflexes suggesting functional
involvement in compensatory or corrective movements. The dentate nuclei participate in
initiation of voluntary movements (Fundamental Neuroscience).
Interpositus nuclei
The interpositus nuclei seem most likely to be involved in dystonia as they function in
co-contraction of antagonistic muscles, reciprocal inhibition in reflexes, and motor
response to sensory input. It is important to note however that the interpositus is one of
many regions involved in each of these functions, as would be expected from the
extensive circuitry involved in generation and execution of motor programs. One
110
connection between the interpositus nuclei and dystonia is the involvement of the
interpositus in somesthetic behaviors and the abnormal sensory findings in patients with
dystonia. Interpositus neurons modulate their activity in response to sensory feedback.
Studies of tremor accompanying movement demonstrate efferent control of the antagonist
muscle by the interpositus to dampen the tremor. This finding is consistent with the idea
that the interpositus is involved in somesthetic behaviors (Fundamental Neuroscience).
In dystonia, common sensory prodromata may trigger abnormal interpositus activity thus
inducing a dystonia-producing activity. Sensory tricks used to arrest development of
dystonia may also act at the interpositus, which receives prominent somatosensory and
proprioceptive input before sending efferent signals. Alterations of dystonic postures by
various sensory stimuli led the renowned neurologist, Denny-Brown to theorize that
dystonia resulted from an imbalance of reflex responses to natural stimulation, both
tonically and phasically (Denny-Brown D, 1965).
Other work suggests that the interpositus contributes to stretch reflex excitability by
controlling the discharge of gamma motor neurons. Gamma motor neurons modulate
overall sensitivity to stretch through activation of intrafusal muscle fibers, which in turn
affect the tension on the muscle spindle (Fundamental Neuroscience).
Impaired
reciprocal inhibition during such reflexes is a common and possibly pathoneumonic sign
in dystonia (Rothwell JC, 1988). While these reflexes are controlled at the spinal,
brainstem, and central levels, it is believed that central disturbance is responsible for the
decreased inhibition of antagonistic muscles after reflex-inducing activation of the
agonistic muscles.
Interpositus control of gamma motor neurons therefore may
contribute to the weakened reciprocal inhibition seen in dystonic patients.
111
Control of agonist and antagonist muscle contraction around joints is another role of
the interpositus potentially deranged in dystonia. Interpositous ablation causes tremor
supporting the idea that the interpositus is most concerned with balancing the agonistantagonist muscle activity of a limb as it moves. Lesioning of the interpositus nucleus
also causes a deficit in the ability to bring a limb back to its hold position after being
disturbed. Interpositus neurons fire when the holding position of a limb is perturbed,
activating antagonist muscles that prevent overshooting the position by contraction of the
agonistic muscle.
Thus, the interpositus neurons are involved in the timing of
antagonistic muscle contractions around joints (Fundamental Neuroscience).
The
interpositus also acts to determine whether the muscles around a joint will undergo
reciprocal activation or co-contraction. During fine motor behaviors that involve cocontraction, interpositus neurons fire as if activating both agonist and antagonist muscles,
and PC are silent (Wetts R, 1985). Conversely, the interpositus and PCs fire in similar
patterns during behaviors where agonists and antagonists are sequentially active.
Patterned PC inhibition of interpositus neuron firing results in a similar pattern of activity
in the interpositus neurons, which in turn produces the alternation between contraction of
agonist and antagonist muscles.
These data suggest that the cerebellum plays an
important role in switching excitation and inhibition between antagonist muscles (Wetts
R, 1985). The mechanism of such control was presented in review by Richter and
Loscher:
"Parts of the excitatory efferences from the DCN (particularly the
interpositus nucleus) projecting to the ventrolateral thalamus form the
interposito-thalamiccortical path (Mori et al., 1995). There seems to exist
a reciprocal relationship between the activities of interpositus neurons and
corticospinal neurons. Thus, the activity of interpositus neurons, which
discharge tonically in the absence of movements and rhythmically during
112
stepping movements, has been found to be precisely in opposite to that of
corticospinal neurons. It has been concluded that the interpositus nucleus
efferents which activate a particular muscle via the rubrospinal pathway
simultaneously inhibit via the interposito-thalamocortical path
corticospinal neurons that control the antagonistic muscle (Mori et al.,
1995)." (Richter A, 1998)
While these three aspects of interpositus function potentially relate to the
pathophysiology of dystonia, other regions, namely the basal ganglia and motor cortex
have also been implicated in these functions and in dystonia as well. It is unclear under
what circumstances one of these regions dominates the others or how conflicting motor
programs are resolved, if at all. Therefore, the ability of each of these regions to induce
co-contraction, perhaps through the ultimate loss of center-surround inhibition at each
level, truly augments the difficulty of determining the pathophysiology underlying
dystonia.
Pathophysiology of Dystonia
In contrast to the basal ganglia, the cerebellum produces tonic excitatory rather than
inhibitory output. This explains why lesions of the basal ganglia frequently produce
positive motor signs while cerebellar lesions produce negative motor signs. As ablation
of normal activity represents the most common form of neuropathology, these regions are
largely associated positive and negative motor signs respectively. While deficits in
neuronal function are more common, increased activity in a region of the brain can be
produced by selective destruction of inhibitory interneurons or an ectopic focus of
possibly synchronized neuronal activity such as in epilepsy. The work presented here
suggests that an abnormal focus of activity involving the cerebellar cortex produces the
positive motor signs characteristic of dystonia.
Increased or decreased PC activity?
113
I believe that within the tottering mouse cerebellum periods of disturbed
neurotransmitter release result in abnormal cerebellar cortical activity that in turn changes
DCN firing patterns to those that produce dystonia. Similarly, profound excitation of the
anterior cerebellar vermis though kainate microinjection also changes cerebellar cortical
activity such that DCN firing patterns elicit dystonia.
This theory becomes more complicated when the activity of the PC is theorized. As
described above, interpositus neurons fire as if activating both agonist and antagonist
muscles and PC are silent during fine motor behaviors that involve co-contraction (Wetts
R, 1985). Therefore, theoretical strong net inhibition of PCs would silence their activity,
allowing interpositus neurons to activate both agonist and antagonist muscles
simultaneously rather than sequentially. Decreased excitatory versus inhibitory input to
PCs in tottering mice may cause sufficient net inhibition and is suggested by data
presented in Chapter 2. In line with this theory, ablation of PCs would also release DCN
from inhibition and cause co-contraction. However, the exact opposite occurs and these
animals fail to demonstrate co-contraction. Failure to induce co-contraction after PC
ablation in the kainate model is also observed.
Together these data suggest that either
silencing of PCs is not central to the induction of interpositus neuron firing patterns
causing co-contraction or deafferentation of interpositus neurons in transgenic animals
lacking PCs changes the inherent activity of these cells. PC death may also result in
pathological changes in presynaptic climbing fibers, which send excitatory collaterals to
DCN as they coarse through the granule cell layer.
The balance between afferent
collateral excitation and PC inhibition is thought to set the basal rate of DCN discharge.
Therefore, deafferentation effects on the DCN directly or through potential alterations in
114
climbing fibers may reset the basal activity of the DCN. It seems more likely however,
that silencing of PCs is not complete and/or sufficient to induce co-contraction. A
particular motor program may need to be initiated through PC activity initially or for the
duration of DCN output. Thus, PC activity early in the dystonic program or specific
patterns of PC activity throughout may be necessary in the induction of DCN based cocontraction.
Determining the activity of PCs during tottering and kainate-induced dystonia would
address this theory. While direct measure of PC activity would be ideal, recording such
activity in live mice is difficult. Prominent activation of both PCs and DCN in both
tottering mice and kainate-injected mice during dystonia supports the presence of
patterned activity between PCs and DCN rather than PC silencing. It also seems unlikely
that basket and stellate cell activation after kainate injection could fully overcome direct
excitation of PCs to cause PC silencing in the kainate model of dystonia. An initial
approach in determining whether PC firing patterns are likely increased or decreased in
tottering dystonia was discussed above under "future studies" (Chapter 5.1). Effects on
tottering dystonia after administration of GABA agonists or antagonists through an
indwelling catheter to the cerebellar cortex would reveal whether PC firing patterns are
likely increased or decreased during attacks.
Cessation of an ongoing attack after
application of a GABA agonist would suggest the necessity of PC activity during the
expression of dystonia. Conversely, termination of an attack due to GABA antagonist
application would imply a need for silencing PC activity during dystonia.
The confusion over why inhibitory cerebellar cortical output through the PC is
necessary for the production of dystonia when disinhibition of the target DCN is likely
115
involved in the expression of the dystonia is paralleled within the basal ganglia circuitry.
The Gpi/SNr output of the basal ganglia is one of tonic inhibition on the thalamus,
analogous to the effect PC output has on DCN.
Disinhibition of the thalamus by
pathologically decreased output of the basal ganglia is traditionally thought to cause
dystonia. However, surgically-induced GPi lesions can improve dystonia symptoms.
Improvement of dystonia after removal of the inhibitory structure (PC or Gpi) when the
activity level of that structure is theorized to be reduced by the disease process itself is
perplexing.
A more complex theory was presented in the introduction with the conclusion that
temporal and/or spatial fluctuations in Gpi/SNr activity are responsible for dystonia
(Crossman AR, 1998). Similarly, the effect of the PC derangement on DCN output
resulting in dystonia likely represents disordered activity rather than simply decreased
activity as well. This theoretical analogy is depicted in Figure 5.2. In the cerebellarinduced dystonia systems presented in this work, removal of the cerebellar PC output
through transgenic loss of PC in both tottering and kainate models restores the normal
state. This is similar to the effect surgical Gpi ablation has on the analogous basal
ganglia system.
116
Basal Ganglia
Gpi
Gpi
Cerebellum
Gpi
PC
Thalamus
Thalamus
DCN
?
Normal
Dystonic
PC
?
?
Thalamus
PC
DCN
DCN
?
Treated
Normal
Dystonic
Treated
Figure 5.2. Comparison of release of inhibition treating dystonia in the basal ganglia and
the cerebellum. Depiction of globus pallidus (Gpi) inhibition of tonically active thalamus
in dystonia and Purkinje cell (PC) inhibition of tonically active deep cerebellar nuclei
(DCN) in dystonia. Destruction of somehow aberrant inhibitory signals returns each
system to its normal state.
Summary
The work presented here suggests that an abnormal focus of activity involving the
cerebellar cortex produces the positive motor signs characteristic of dystonia..
Furthermore, it is theorized that the resultant DCN activity, particularly that of the
interpositus, acts to induce involuntary co-contraction of antagonistic muscles by
disordered activation of opposing muscles through the red nucleus and cerebral cortex.
The DCN may fail to coordinate activity of agonistic and antagonistic muscles controlled
by the rubrospinal and corticospinal tracts respectively.
The mechanism within the
cerebellar cortex resulting in pathologic DCN firing probably involves failure of centersurround inhibition of PCs. The locus ceruleus, in turn, may attempt to correct ongoing
117
co-contraction through NE- mediated focusing of the center-surround mechanism, but to
no avail. Similar derangements occurring in the basal ganglia and motor cortex may
result in dystonia. However, the abundance and consistency of cerebellar findings in
studies of dystonia in addition to the role of the cerebellum in normal motor function,
suggests a role for this structure in the expression if not the origin of the dystoniaproducing signal.
118
REFERENCES
Abbott LC, Bump M, Brandl A, and DeLaune S. 2000. Investigation of the myocloniclike movement disorder exhibited by tottering mice. Movement Disorders. 15Suppl.1:5359.
Adler CH, 2000. Stratagies for controlling dystonia. Postgraduate Medicine. 108(5):151160.
Almasy L, Bressman SB, and Raymond D. 1997. Idiopathic torsion dystonia linked to
chromosome 8 in two Mennonite families. Annals of Neurology. 42:670-673.
Development of the Cerebellar System. Eds. Altman J and Bayer SA. CRC Press: Boca
Raton, FL. 1997. pp.26-80.
Auburger G, Ratzlaff T, Lunkes A, Nelles HW, Leube B, Binkofski F, Kugel H, Heindel
W, Seitz R, Benecke R, Witte OW, and Voit T. 1996. A gene for autosomal dominant
paroxysmal choreoathetosis/spasicity (CSE) maps to the vicinity of a potassium channel
gene cluster on chromosome 1p, probably within 2cM between D1S443 and D1S197.
Genomics. 31:90-94.
Ayata C., Shimizu-Sasamata M., Lo E.H., Noebels J.L., and Moskowitz M.A. 2000.
Impaired neurotransmitter release and elevated threshold for cortical spreading
depression in mice with mutations in the a1A subunit of P/Q type calcium channels.
Neuroscience. 95(3):639-645.
Ben-Ari Y. 1985. Limbic seizures and brain damage produced by kainic acid:
mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 14:375-403.
Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, and Marsden CD.
1998. The pathophysiology of primary dystonia. Brain. 121:1195-1212.
Bertolini A, Vergoni AV, Poggioli R, and Gessa GL. 1986. Morphine and endorphine
antagonize posture and locomotor disorders induced by the injection of ACTH 1-24 in
the rat locus ceruleus. Life Science. 38:373-377.
Bhatia KP. 1999. The paroxysmal dyskinesias. Journal of Neurology. 246:149-155.
Bressman SB, de Leon D, Raymond D, Ozelius LJ, Breakefield XO, Nygaard TG,
Almasy L, Risch NJ, and Kramer PL. 1998. Clinical-genetic spectrum of primary
dystonia. Dystonia 3: Advances in Neurology, 78:79-91.
Browne DL, Gancher ST, Nutt JG, Brunt ERP, Smith EA, KramerP, and Litt M. 1994.
Episodic ataxia/myokymia syndrome is associated with point mutations in the human
potassium channel gene, KCNA1. Nature Genetics. 8:136-140.
119
Burguera JA, Catala J, Casanova B. 1991. Thalamic demylination and paroxysmal
dystonia in multiple sclerosis. Movement Disorders. 6:379-381.
Caddick SJ, Wang C, Fletcher CF, Jenkins NA, Copeland NG, and Hosford DA. 1999.
Excitatory but not inhibitory synaptic transmission is reduced in lethargic (Cacnb4lh) and
tottering (Cacna1atg) mouse thalami. Journal of Neurophysiology. 81:2066-2074.
Camac A, Greene P, and Khandji A. 1990. Paroxysmal kinesigenic dystonic
choreoathetosis associated with a thalamic infarct. Movement Disorders. 5:235-238.
Campbell DB and Hess EJ. 1998. Cerebellar circuitry is activated during convulsive
episodes in the tottering tg/tg mutant mouse. Neuroscience. 85:773-783.
Campbell DB and Hess EJ. 1999. L-type calcium channels contribute to the tottering
mouse dystonic episodes. Molecular Pharmacology. 55:23-31.
Campbell DB, North JB, and Hess EJ, 1999. Tottering mouse motor dysfunction is
abolished on the Purkinje cell degeneration (pcd) mutant background. Experimental
Neurology. 160:268-278.
Catterall WA. 1988. Structure and function of voltage-sensitive ion channels. Science.
242:50-60.
Crossman AR and Brotchie JM. 1998. Pathophysiology of dystonia. Dystonia 3:
Advances in Neurology, 78:19-25.
Demirkiran M and Jankovic J, 1995. Paroxysmal dyskinesias: clinical features and
classification. Annals of Neurology. 38:571-579.
Denny-Brown, D. 1965. The nature of dystonia. Bulletins of the New York Academy of
Medicine. 41:858-869.
Dunlap K, Luebke JI, and Turner TJ. 1995. Exocytotic Ca2+ channels in mammalian
central neurons. Trends in Neurological Sciences. 18(2):89-98.
Eidelberg D, Takikawa S, Wilhemsen K, Dhawan V, Chaly T, Robeson W, Dahl R,
Margouleff D, Greene P, Hunt A, Przedborski S, and Fahn S. 1993. Positron emmision
tomographic findings in Filipino X-linked dystonia-parkonsinism. Annals of Neurology.
34:185-191.
Eidelberg D, Moeller JR, Antonin A, Kazumata K, Nakamura T, Dhawan V, Spetsieris P,
deLeon D, Bressman SB, Fahn S. 1998. Functional brain networks in DYT1 dystonia.
Annals of Neurology. 44(3):299-300.
Fahn S, Marsden CD, and Calne DB. 1987. Classification and Investigation of Dystonia.
In: Marsden CD, Fahn S, eds. Movement Disorders 2. London: Butterworths,:332-358.
120
Fahn S. 1988. Concept and classification of dystonia. Dystonia 2: Advances in
Neurology, 50:1-7.
Fahn S, Bressman SB, and Brin MF. 1995. Chapter 107: Dystonia. In Merritt’s Textbook
of Neurology, 9th edition. Ed. Rowland LP. Williams and Wilkins. Media, PA. Pp.705712.
Fahn, S, Bressman SB, and Marsden, CD. 1998. Classification of dystonia. Dystonia 3:
Advances in Neurology, 78:1-10.
Feddersen RM, Ehlenfeldt R, Yunis WS, Clark HB, and Orr HT. 1992. Disrupted
cerebellar cortical development and progressive degeneration of Purkinje cells in SV40 T
antigen transgenic mice. Neuron. 9:955-966.
Fink JK, Rainier S, Wilkowski J, Jones SM, Kume A, Hedera P, Albin R, Mathay J,
Girbach L, Varvil T, Otterund B, and Leppert M. 1996. Paroxysmal dystonic
choreoathetosis: tight linkage to chromosome 2q. American Journal of Human Genetics.
59:140-145.
Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy Jr. JD, Hawkes R, Frankel WN,
Copeland NG, and Jenkins NA. 1996. Absence epilepsy in tottering mutant mice is
associated with calcium channel defects. Cell. 87:607-617.
Fouad GT, Servidei S, Durcan D, Bertini E, Ptacek LJ. 1996. A gene for paroxysmal
dyskinesia (FPD1) maps to chromosome 2q. American Journal of Human Genetics.
59:135-139.
Franklin KBJ and Paxanos G. 1997. The Mouse Brain In Stereotaxic Coordinates.
Academic Press. San Diego, CA.
Fundamental Neuroscience. 1999. Eds. Zigmond MJ, Bloom FE, Landis SC, Roberts JL,
and Squire LR. Academic Press. San Diego, CA. Chapter 35.
Fureman BE, 2001. L-type calcium channel regulation in the P/Q-type calcium channel
mutant mouse, tottering, a model for episodic neurological disorders. Ph.D. thesis, The
Pennsylvania State University.
Goodenough DJ, Fariello RG, Annis BL, and Chun WM. 1978. Familial and aquired
paroxysmal dyskinesias: A proposed classification with deliniation of clinical features.
Archives of Neurology. 35:827-831.
Green MC and Sidman RL. 1962. Tottering - a neuromuscular mutation in the mouse.
Journal of Heredity. 53:233-237.
Herndon RM and Coyle JT. 1977. Selective destruction of neurons by a transmitter
agonist. Science. 198:71-72.
121
Herndon RM, Coyle JT and Addicks E. 1980. Ultrastructural analysis of kainic acid
lesion to cerebellar cortex. Neuroscience. 5:1015-1026.
Hess EJ and Wilson MC, 1991. Tottering and leaner mutations perturb transient
developmental expression of tyrosine hydroxylase in embryologically distinct Purkinje
cells. Neuron. 6:123-132.
Horne AL, and Kemp JA. 1991. The effect of ? -conotoxin GIVA on synaptic
transmission within the nucleus accumbens and hippocampus of the rat in vitro. British
Journal of Pharmacology. 103:1733-1739.
Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, Nomura Y, Endo K,
Tanaka H, Tsuji S, Fujita K, and Nagatsu T. 1994. Hereditary progressive dystonia with
marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene.
Nature Genetics. 8:236-242.
Hallett, M. 1998. The neurophysiology of dystonia. Arch Neurology. 55:601-603.
Hofele K, Benecke R, and Auburger G. 1997. Gene locus FPD1 of the dystonic MountReback type of autosomal-dominant paroxysmal choreoathetosis. Neurology. 49:12521256.
Houser MK, Soland VL, Bhatia KP, Quinn NP, and Marsden CD. 1999. Paroxysmal
kinesigenic choreoathetosis: a report of 26 patients. Journal of Neurology. 246:120-126.
Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, and
Eidelberg D. 2000. The metabolic topography of essential blepharospasm: a focal
dystonia with general implications. Neurology. 55:673-677.
Jarman PR, Bhatia KP, Davie C, Heales SJR, Turjanski N, Taylor-Robinson SD,
Marsden CD, Wood NW. 2000. Paroxysmal dystonic choreoathetosis: Clinical features
and investigation of pathophysiology in a large family. Movement Disorders. 15(4):648657.
Jinnah HA, Sepkuty JP, Ho T, Yitta S, Drew T, Rothstein JD, and Hess EJ. 2000.
Calcium channel agonists and dystonia in the mouse. Movement Disorders. 15(3):542551.
Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams
ME, Scheller RH, Tsien RW, and Shin HS. 1999. Ablation of P/Q-type Ca2+ channel
currents, altered synaptic transmission, and progressive ataxia in mice lacking the a1Asubunit. Proceedings of the National Acadamy of Sciences. 96(26):15245-15250.
122
Kaplan BJ, Seyfried TN, and Glaser GH, 1979. Spontaneous polyspike discharges in an
epileptic mutant mouse (tottering). Experimental Neurology. 66:577-586.
Kluge A, Kettner B, Zschenderlein R, Sandrock D, Munz DL, Hesse S, and Meierkord H.
1998. Changes in perfusion pattern using ECD_SPECT indicate frontal lobe and
cerebellar involvement in exercise-induced paroxysmal dystonia. Movement disorders.
13(1):125-134.
Knappskog PM, Flatmark T, Mallet J, Ludecke B, Bartholome K. 1995. Recessively
inherited L-dopa-responsive dystonia caused by a point mutation (Q381K) in the tyrosine
hydroxylase gene. Human Molecular Genetics. 4:1209-1212.
Kostic VS, Stojanovic-Svetel, and Kacar A. 1996. Symptomatic dystonias associated
with structural brain lesions: report of 16 cases. Canadian Journal of Neurological
Science. 23:53-56.
Kramer LP, Ozelius L, de Leon D, Risch N, Bressmen SB, Brin MF, Schuback DE,
Burke RE, Kwiatkowski OJ, Shale H, Gusella JF, Breakfield XO, and Fahn S. 1990.
Dystonia gene in Ashkenazi Jewish population located on chromosome 9q32-34. Annals
of Neurology. 27:114-120.
Kwiatkowski OJ, Ozelius L, Kramer PL, Perman S,Schuback DE, Gusella JF, Fahn S ,
and Breakfield XO. 1991. Torsion dystonia genes in two populations confined to a small
region on chromosome 9q32-34. American Journal of Human Genetics. 49:366-371.
Lance JW. 1977. Familial paroxysmal dystonic choreoathetosis and its differentiation
from related syndromes. Annals of Neurology. 2:285-293.
LeDoux MS, Rutledge SL, Mountz JM, and Darji JT. 1995. SPECT abnormalities in
generalized dystonia. Pediatric neurology. 13(1):5-10.
LeDoux MS, Lorden JF, and Meinzen-Derr J. 1995. Selective elimination of cerebellar
output in the genetically dystonic rat. Brain Research. 697:91-103.
Lee MS and Marsden CD. 1994. Movement disorders following lesions of the thalamus
or subthalamic region. Movement Disorders. 9:493-507.
Lehericy S, Vidailhet M, Dormont D, Pierot L, Chiras J, Mazetti P, Marsault C, and Agid
Y. 1996. Striatopallidal and thalamic dystonia. Archives of Neurology. 53:241-250.
Leube B, Doda R, Ratzlaff T, Kessler K, Benecke R, and Auberger G. 1996. Idiopathic
torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult
onset, autosomal inheritance and purely focal distribution. Human Molecular Genetics.
5:1673-1677.
123
Levitt P and Noebels JL. 1981. Mutant mouse tottering: selective increase of locus
ceruleus axons in a defined single-locus mutation. Proceedings of the National Academy
of the Sciences. 78(7):4630-4634.
Liu H, Cao Y, Basbaum AI, Mazarati AM, Sankar R, and Wasterlain CG. 1999.
Resistance to excitotoxin-induced seizures and neuronal death in mice lacking the
preprotachykinin A gene. Proceedings of the National Academy of Sciences.
96(21):12096-12101.
Lothman EW and Collins RC. 1981. Kainic acid induced limbic seizures: metabolic,
behavioral, electroencephalographic and neuropathological correlates. Brain Research.
218:299-318.
Luebke JI., Dunlap K, and Turner TJ. 1993. Multiple calcium channel types control
glutamatergic synaptic transmission in the hippocampus. Neuron. 11:895-902.
Matsumoto RR, Hemstreet MK, Lai NL, Thurkauf A, De Costa BR, Rice KC, Hellewell
SB, Bowen WD, and Walker JM. 1990. Drug specificity of pharmacological dystonia.
Pharmacology and Biochemistry of Behavior. 36(1):151-155.
Mayeux R, and Fahn S. 1982. Paroxysmal dystonic choreoathetosis in a patient with
familial ataxia. Neurology. 32:1184-1186.
Momiyama A and Takahashi T. 1994. Calcium channels responsible for potassiuminduced transmitter release at rat cerebellar synapses. Journal of Physiology. 476:197202.
Munchau A, Mathen D, Cox T, Quinn NP, Marsden CD, and Bhatia KP. 2000. Unilateral
lesions of the globus pallidus: report of four patients presenting with focal or segmantal
dystonia. Journal of Neurology Neurosurgery Psychiatry. 69:494-498.
Nakazawa M, Kobayashi T, Matsuno K, and Mita S. Possible involvement of a sigma
receptor subtype in the neck dystonia in rats. Pharmacology and Biochemistry of
Behavior. 62(1):123-126.
Nardocci N, Lamperti E, Rumi V, and Angelini L. 1989. Typical and atypical forms of
paroxysmal choreoathetosis. Developmental Medicine and Child Neurology. 31:670-681.
Nijssen PCG, Tijssen CG. 1992. Stimulus sensitive paroxysmal dyskinesias associated
with a thalamic infarct. Movement Disorders. 7:364-366.
Noebels JL and Sidman RL. 1979. Inherited epilepsy: Spike-wave and focal motor
seizures in the mutant mouse tottering. Science. 204:1334-1336.
Noebels JL. 1984. A single gene error of noradrenergic axon growth synchronizes central
neurones. Nature. 310:409-411.
124
Nutt JG, Muenter MD, Aronson A, Kurland LT, and Melton LJ. 1988. Epidemiology of
focal and generalized dystonia in Rochester, Minnesota. Movement Disorders. 3:188194.
Odergren T, Stone-Elander S, and Ingvar M. 1998. Cerebral and cerebellar activation in
correlation to the action induced dystonia in writer's cramp. Movement Disorders.
13(3):497-508.
Olney JW, Rhee V, and Ho OL. 1974. Kainic acid: a powerful neurotoxic analogue of
glutamate. Brain Research. 77:507-512.
Ozelius L, Kramer PL, Moskowitz CB, Kwiatkowski OJ, Brin MF, Bressmen SB,
Schuback DE, Falk CT, Risch N, de Leon D, Haines J, Gusella JF, Fahn S, and
Breakfield XO. 1989. Human gene for torsion dystonia located on chromosome 9q32-34.
Neuron. 2:1427-1434.
Playford Ed, Fletcher NA, Sawle GV, Marsden CD, and Brooks DJ. 1993. Striatal
[18F]dopa uptake in familial idiopathic dystonia. Brain. 116:1191-1199.
Plomp JJ, Vergouwe MN, Van de Maagdenberg AM, Ferrari MD, Frants RR, and
Molenaar PC. 2000. Abnormal transmitter release at neuromuscular junctions of mice
carrying the tottering ? 1A Ca2+ cannel mutation. Brain. 123:463-471.
Preibisch C, Berg D, Hofmann e, Solymosi L, and Naumann M. 2001. Cerebral
activation patterns in patients with writer's cramp: a functional magnetic resonance
imaging study. Journal of Neurology. 248:10-17.
Principles of Neural Science. Eds. Kandel ER, Schwartz JH, and Jessel TM. 4th edition.
2001. Mc-Graw-Hill: New York, NY. pp.832-851.
Qian J and Noebels JL. 2000. Presynaptic Ca2+ influx at a mouse central synapse with
Ca2+ channel subunit mutations. The Journal of Neuroscience. 20(1):163-170.
Richter A and Loscher W. 1998. Pathophysiology of idiopathic dystonia: findings from
genetic animal models. Progress in Neurobiology. 54:633-677.
Rodriguez-Moreno A, Herreras O, and Lerma J. 1997. Kainate receptors presynaptically
downregulate GABAergic inhibition in the rat hippocamous. Neuron. 19:893-901.
Rothwell JC, Day BL, Obeso JA, Berardelli A, and Marsden CD. 1988. Reciprocal
inhibition between muscles of the human forearm in normal subjects and in patients with
idiopathic torsion dystonia. Dystonia 2: Advances in Neurology, 50:133-141.
.
125
Royle SJ, Collins FC, Rupniak HP, Barnes JC, and Anderson R. 1999. Behavioral
analysis and susceptibility to CNS injury of four inbred strains of mice. Brain Research.
816:337-349.
Schwarcz R, Zaczek R, and Coyle JT. 1978. Microinjection of kainic acid into the rat
hippocampus. European Journal of Pharmacology. 50:209-220.
Snyder, et al. 1992. Journal of Gerontology. 47(6):B190-B-197.
Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, Scheffer
IE, and Berkovic SF. 1995. A missense mutation in the neuronal nicotinic acetylcholine
receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe
epilepsy. Nature Genetics. 11:201-203.
Sunohara N, Mukoyama M, Mano Y, Satoyoshi E. 1984. Action induced rhythmic
dystonia: an autopsy case. Neurology. 34:321-327.
Syapin PJ. 1983. Inhibition of pentylenetetrazol induced genetically-determined
stereotypic convulsions in tottering mutant mice by diazepam. Pharmacology,
Biochemistry, and Behavior. 18:389-394.
Takahashi T and Momiyama A. 1993. Different types of calcium channels mediate
central synaptic transmission. Nature. 366:156-158.
Turner TJ, Adams ME, and Dunlap K. 1993. Multipla Ca2+ channel types coexist to
regulate synaptosomal neurotransmitter release. Proceedings of the National Academy of
Sciences. 90:9518-9522.
Vidailhet M, Dupel C, Lehericy s, Remy P, Dormont D, Serdaru M, Jedynak P, Veber H,
Samson Y, Marsault C, and Agid Y. 1999. Dopaminergic dysfunction in midbrain
dystonia. Archives of Neurology. 56:982-989.
Vitek JL and Giroux M. 2000. Physiology of hypokinetic and hyperkinetic movement
disorders: model for dyskinesia. Annals of Neurology. 47(supp.1):S131-S140.
Wakamori M, Yamazaki K, Matsunodaira H, Teramoto T, Tanaka I, Niidome T, Sawada
K, Nishizawa Y, Sekiguchi N, Mori E, Mori Y, and Imoto K. 1998. Single tottering
mutations responsible for the neuropathic phenotype of the P-type calcium channel. The
Journal of Biological Chemistry. 273(52):34857-34867.
Walker D, and DeWaard M. 1998. Subunit interaction sites in voltage-dependent Ca2+
channels: role in channel function. Trends in Neurological Sciences. 21(4):148-154.
Wetts R, Kalaska JF, and Smith AM. 1985. Cerebellar nuclear cell activity during
antagonistic cocontraction and reciprocal inhibition of forearm muscles. Journal of
Neurophysiology. 54(2):231-244.
Carolyn E. Pizoli
EDUCATION
Pennsylvania State University College of Medicine, Hershey, Pennsylvania
M.D./Ph.D. degree candidate, anticipated graduation date: May 2003
Goucher College, Towson, Maryland
B.A. in Biological Sciences and Chemistry earned in 1996
Degree with Distinction and Honors in Biological Sciences and Chemistry
1996-2003
1992-1996
RESEARCH
Penn State University College of Medicine
1998-2001
Graduate Thesis Research. Conducted research in the neuroscience laboratory of Dr. Ellen Hess
to determine the pathophysiology of dystonia. The role of the cerebellum in a genetic mouse
model of dystonia, tottering, and in a novel model using wild type mice was analyzed using
biochemical, cellular, pharmacological and behavioral approaches.
Goucher College
1995-1996
Independent Research. Conducted senior independent research project to determine effects of
DNA conformation on RNA polymerase kinetics.
Johns Hopkins University
Summer 1995
Research Fellow. Planned and conducted experiments analyzing RNA polymerase kinetics under
a Howard Hughes research fellowship.
University of Maryland School of Medicine
Summer 1994
Research Fellow. Conducted experiments to clone the sodium/calcium exchanger gene in squid
under a Summer Undergraduate Research Fellowship.
HONORS/AWARDS
The Stimson-Duvall Fellowship
The Gairdner Moment Prize in Biology
The Louise Kelly Prize in Chemistry
Grace T. Lewis Merit Scholarship
1996
1996
1996
1992-1996
PUBLICATIONS / ABSTRACTS
Carolyn Pizoli, Hyder Jinnah, Melvin Billingsley, and Ellen Hess. Abnormal Cerebellar Signaling
Induces Dystonia in Mice. Journal of Neuroscience. Sept. 1, 2002, 22(17): 7825-7833.
Carolyn Pizoli, Corey Hart, Steven Dear, Melvin Billingsley, and Ellen Hess. Cerebellar
Activation Induces Dystonia in Mice. Society for Neuroscience Abstracts. 2001, 27: #294.12
Carolyn Pizoli and Ellen Hess. Selective Elimination of Purkinje Cells in the Tottering Mouse.
Society for Neuroscience Abstracts. 2000, 26:#135.6
Carolyn Pizoli and Ellen Hess. Potassium Stimulated Release of 3H-Glutamate from Tottering
Mouse Cerebellar Slices. Society for Neuroscience Abstracts. 1999, 25:#286.6
ACTIVITIES
Physician Scientist Student Association, co-president (1997-1999)
Childlife volunteer, Hershey Medical Center
Shock Trauma Unit volunteer, University of Maryland Hospital
Varsity Swim Team, Goucher College
Special Olympics Swim Coach, Pittsburgh, PA
1
1996-present
Summer 1997
Summer 1995
1992-1993
1990-1991