* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Essential Cell Biology Chapter 4 excerpt
Endomembrane system wikipedia , lookup
Phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Signal transduction wikipedia , lookup
Magnesium transporter wikipedia , lookup
Homology modeling wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein domain wikipedia , lookup
Protein folding wikipedia , lookup
Protein moonlighting wikipedia , lookup
List of types of proteins wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein purification wikipedia , lookup
Western blot wikipedia , lookup
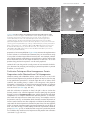
124 Chapter 4 protein Structure and Function figure 4–5 Hydrophobic forces help proteins fold into compact conformations. the polar amino acid side chains tend to fall on the outside of the folded protein, where they can interact with water; the nonpolar amino acid side chains are buried on the inside to form a highly packed hydrophobic core of atoms that are hidden from water. In this very schematic drawing, the protein contains only about 30 amino acids. polar side chains nonpolar side chains hydrophobic core region contains nonpolar side chains unfolded polypeptide hydrogen bonds can be formed to the polar side chains on the outside of the molecule folded conformation in aqueous environment proteins fold into a conformation of lowest energy Each type of protein has a particular three-dimensional structure, which is determined by the order of the amino acids in its chain. The final folded structure, or conformation, adopted by any polypeptide chain is determined by energetic considerations: a protein generally folds into the shape in which the free energy (G) is minimized (see p. 91). Protein folding has been studied in the laboratory using highly purified proteins. A ECB3 m3.05/4.05 protein can be unfolded, or denatured, by treatment with solvents that disrupt the noncovalent interactions holding the folded chain together. This treatment converts the protein into a flexible polypeptide chain that has lost its natural shape. When the denaturing solvent is removed, the protein often refolds spontaneously, or renatures, into its original conformation (figure 4–7). The fact that a renatured protein can, on its own, regain the correct conformation indicates that all the information necessary to specify the three-dimensional shape of a protein is contained in its amino acid sequence. Each protein normally folds into a single stable conformation. This conformation, however, often changes slightly when the protein interacts with other molecules in the cell. This change in shape is crucial to the function of the protein, as we shall see later in this chapter. figure 4–6 Hydrogen bonds within a protein molecule help stabilize its folded shape. large numbers of hydrogen bonds form between adjacent regions of the folded polypeptide chain. the structure depicted is a portion of the enzyme lysozyme. the polypeptide backbone is colored green. hydrogen bonds between backbone atoms are shown in red; those between atoms of a peptide bond and a side chain in yellow; and those between atoms of two side chains in blue. note that the same amino acid side chain can make several hydrogen bonds. (after C.K. Mathews, K.e. van holde, and K.G. ahern, Biochemistry, 3rd ed. San Francisco: Benjamin Cummings, 2000.) hydrogen bond between atoms of two peptide bonds hydrogen bond between atoms of a peptide bond and an amino acid side chain hydrogen bond between two amino acid side chains backbone to backbone backbone to side chain side chain to side chain the Shape and Structure of proteins EXPOSE TO A HIGH CONCENTRATION OF UREA purified protein isolated from cells figure 4–7 Denatured proteins can recover their natural shapes. this type of experiment demonstrates that the conformation of a protein is determined solely by its amino acid sequence. renaturation works best for small proteins. REMOVE UREA denatured protein 125 original conformation of protein re-forms When proteins fold incorrectly, they sometimes form aggregates that can damage cells and even whole tissues. Aggregated proteins underlie a number of neurodegenerative disorders, including Alzheimer’s disease and Huntington’s disease. Prion diseases—such as scrapie in sheep, bovine spongiform encephalopathy (BSE, or “mad cow” disease) in cattle, and Creutzfeldt–Jacob disease (CJD) in humans—are also caused by protein aggregates. The prion protein, PrP, can adopt a misfolded form that is considered “infectious” because can convert properly folded PrP proECB3 ite4.07/4.07 teins in the infected brain into the abnormal conformation (figure 4–8). This allows the misfolded form of PrP to spread rapidly from cell to cell, causing the death of the infected animal or human. Although a protein chain can fold into its correct conformation without outside help, protein folding in a living cell is generally assisted by special proteins called molecular chaperones. These proteins bind to partly folded chains and help them to fold along the most energetically favorable pathway, as we shall discuss in Chapter 7. Chaperones are vital in the crowded conditions of the cytoplasm, because they prevent newly synthesized protein chains from associating with the wrong partners. Nevertheless, the final three-dimensional shape of the protein is still specified by its amino acid sequence; chaperones merely make the folding process more efficient and reliable. proteins come in a wide Variety of complicated Shapes Proteins are the most structurally diverse macromolecules in the cell. Although they range in size from about 30 amino acids to more than 10,000, the vast majority of proteins are between 50 and 2000 amino acids long. Proteins can be globular or fibrous; they can form filaments, sheets, rings, or spheres. figure 4–9 presents a sampling of proteins whose exact structures are known. We will encounter many of these proteins later in this chapter and throughout the book. Resolving a protein’s structure often begins with determining its amino acid sequence, a task that can be accomplished in several ways. For many years, protein sequencing was accomplished by directly analyzing the amino acids in the purified protein; the first protein sequenced was the hormone insulin, in 1955. Today we can determine the order of amino acids in a protein much more easily by sequencing the gene that encodes it (discussed in Chapter 10). Once the order of the nucleotides in the DNA that encodes a protein is known, this information can be converted into an amino acid sequence by applying the genetic code (discussed in Chapter 7). The amino acid sequences of millions of proteins have already been determined in this way, and they have been collected into vast electronic databases that allow users to obtain the amino acid sequence of any protein almost instantaneously. figure 4–8 Prion diseases are caused by rare proteins whose misfolding is infectious. the mammalian protein prp is the best known of these proteins, but other examples are known. (a) the protein undergoes a rare conformational change to give an abnormally folded prion form. (B) the abnormal form causes the conversion of normal prp proteins in the host’s brain into the misfolded form, which forms protein aggregates that disrupt brain function and cause disease. Question 4–1 urea used in the experiment shown in figure 4–7 is a molecule that disrupts the hydrogen-bonded network of water molecules. why might high concentrations of urea unfold proteins? The structure of urea is shown here. O C H2N NH2 ? (A) prion protein can adopt an abnormal, misfolded form very rare conformational change normal Prp protein abnormal prion form of PrP protein (B) misfolded protein can induce formation of protein aggregates heterodimer misfolded protein converts normal PrP into abnormal conformation homodimer converting more PrP to misfolded form creates an aggregate protein aggregate 126 Chapter 4 protein Structure and Function SH2 domain lysozyme catalase myoglobin hemoglobin DNA deoxyribonuclease collagen porin cytochrome c chymotrypsin calmodulin aspartate transcarbamoylase insulin alcohol dehydrogenase 5 nm figure 4–9 Proteins come in a variety of shapes and sizes. each folded polypeptide is shown as a space-filling model, represented at the same scale. In the top left corner is the Sh2 domain, which is featured in greater detail in panel 4–2 (pp. 128–129). For comparison, part of a Dna molecule (gray) bound to a protein is illustrated. (after David S. Goodsell, Our Molecular nature. new York: SpringerVerlag, 1996. With permission from Springer Science and Business Media.) ECB3 m3.23/4.09 the Shape and Structure of proteins Although all the information required for a polypeptide chain to fold is contained in its amino acid sequence, we have not yet learned how to reliably predict a protein’s detailed three-dimensional conformation—the spatial arrangement of its atoms—from its sequence alone. At present, the only way to discover the precise folding pattern of any protein is by experiment, using either X-ray crystallography or nuclear magnetic resonance (NMR) spectroscopy, as we discuss later in the chapter. So far, the structures of about 20,000 different proteins have been completely analyzed by these techniques. Most have a three-dimensional conformation so intricate and irregular that their structure would require an entire chapter to describe in detail. Because the structure of a large protein can be overwhelming to look at, we will illustrate the intricacies of protein conformation by examining the structure of a smaller protein domain. As we discuss shortly, most proteins are formed from multiple domains, each folding into a compact three-dimensional structure. In panel 4–2 (pp. 128–129), we present four different depictions of SH2, a protein domain that—as a part of proteins involved in cell signaling—has important functions in eucaryotic cells. Built from a string of 100 amino acids, the structure is displayed as (A) a polypeptide backbone model, (B) a ribbon model, (C) a wire model that includes the amino acid side chains, and (D) a space-filling model. As indicated in the panel, each model emphasizes different features of the polypeptide. The three horizontal rows show the SH2 domain in different orientations, and the images are colored to distinguish the path of the polypeptide chain, from its N-terminus (purple) to its C-terminus (red). We will describe the different structural elements in this protein domain shortly. From Panel 4–2, we can clearly see how amazingly complex protein conformation is, even for a small domain like SH2. To visualize such complicated structures, scientists have developed various graphical and computer-based tools that generate a variety of images of a protein, some of which are depicted in Panel 4–2. These images can be displayed on a screen and rotated to view all aspects of the structure (Movie 4.1). In addition, describing and presenting such complex protein structures is made easier by recognizing that several common folding patterns underlie these conformations, as we discuss next. The a helix and the b Sheet are common folding patterns When the three-dimensional structures of many different protein molecules are compared, it becomes clear that, although the overall conformation of each protein is unique, two regular folding patterns are often present. Both were discovered more than 50 years ago from studies of hair and silk. The first folding pattern to be discovered, called the a helix, was found in the protein a-keratin, which is abundant in skin and its derivatives—such as hair, nails, and horns. Within a year of the discovery of the a helix, a second folded structure, called a b sheet, was found in the protein fibroin, the major constituent of silk. (Biologists often use Greek letters to name their discoveries, with the first example receiving the designation a, the second b, and so on.) These two folding patterns are particularly common because they result from hydrogen bonds that form between the N–H and C=O groups in the polypeptide backbone. Because the amino acid side chains are not involved in forming these hydrogen bonds, a helices and b sheets can be generated by many different amino acid sequences. In each case, the protein chain adopts a regular, repeating form or motif. These structural features, and the shorthand cartoon symbols that are often used to represent them in models of protein structures, are presented in figure 4–10. 127 how proteins are Studied tions of these 20 modifications is possible, the protein’s behavior can in principle be altered in a huge number of ways. The set of covalent modifications that a protein contains at any moment constitutes an important combinatorial regulatory protein code. The attachment or removal of these modifying groups controls the behavior of a protein, changing its activity or stability, its binding partners, or its location inside the cell (see Figure 4–44). This regulatory code enables the cell to make optimal use of its proteins, and it allows the cell to respond rapidly to changes in its condition or environment. how proTeinS are STudied Understanding how a particular protein functions requires detailed structural and biochemical analyses—both of which require large amounts of pure protein. But isolating a single type of protein from the thousands of other proteins present in a cell is a formidable task. For many years, proteins had to be purified directly from the source: the tissues in which they are most plentiful. That approach was inconvenient, entailing, for example, early-morning trips to the slaughterhouse. More importantly, the complexity of intact tissues and organs is a major disadvantage when trying to purify particular molecules, because a long series of chromatography steps is generally required. These procedures not only take weeks to perform but they also yield only a few milligrams of pure protein. Nowadays, proteins are more often isolated from cells that are grown in a laboratory. Often these cells have even been “tricked” into making large quantities of a given protein using the genetic engineering techniques that we will describe in Chapter 10. Such cells generally allow large amounts of pure protein to be obtained in only a few days. In this section—and in Panels 4–4 to 4–6 (pp. 164–167)—we outline how cells can be grown in culture and how proteins are purified from these and other cells. In the how we know section (pp. 158–160), we describe how these proteins are analyzed to determine their amino acid sequence and their three-dimensional structure. Finally, we shall discuss how efforts to probe protein structure and function are being conducted on a large scale, with the hope of obtaining a deeper understanding of how sets of proteins cooperate to make life possible. cells can Be Grown in a culture dish Given the appropriate surroundings, most plant and animal cells will live, proliferate, and even express specialized properties in a tissue-culture dish. Experiments performed using cultured cells are sometimes said to be carried out in vitro (literally, “in glass”) to contrast them with experiments on intact organisms, which are said to be carried out in vivo (literally, “in the living”). These terms can be confusing, however, because they are often used in a very different sense by biochemists. In the biochemistry laboratory, in vitro refers to reactions carried out in a test tube in the absence of cells, whereas in vivo refers to any reaction taking place inside a living cell, even cells that are growing in culture. Although not true for all types of cells, most cells grown in culture display the differentiated properties appropriate to their origin: fibroblasts, the precursor cells that give rise to connective tissue, continue to secrete collagen; cells derived from embryonic skeletal muscle fuse to form muscle fibers that contract spontaneously in the culture dish; nerve cells extend axons that are electrically excitable and make synapses with other nerve cells; and epithelial cells form extensive sheets with many of the 157 how proteins are Studied (A) (C) (B) 20 mm 100 mm 50 mm figure 4–48 Cells in culture often display properties that reflect their origin. (a) phase-contrast micrograph of fibroblasts in culture. (B) Micrograph of cultured myoblasts, the precursor cells that give rise to muscle, shows cells fusing to form multinucleate muscle cells. (C) Cultured precursor cells that give rise to oligodendrocytes, the glial cells that support and nurture neurons in the brain. (D) Cultured epithelial cells can form cell sheets. (e) tobacco cells, from an immortal cell line, grown in liquid culture. (a, courtesy of Daniel Zicha; B, courtesy of rosalind Zalin; C, from tang et al., J. Cell Biol. 148:971–984, 2000, with permission from the rockefeller University press; D, from K.B. Chua et al., Proc. Natl. Acad. Sci. USA 104:11424–11429, 2007, with permission from the national academy of Sciences; e, courtesy of Gethin roberts.) properties of an intact epithelium (figure 4–48). Because these phenomena occur in culture, in a controlled environment, they are accessible to study in ways that are often not possible in intact tissues. For example, cultured cells can be exposed to hormones or growth factors, and the effects that these molecules have on the shape or behavior of the cells—or on the proteins they produce in response—can be easily explored. (D) 100 mm Cultured cells can also provide a ready source of raw materials for biologists interested in purifying and studying a particular protein or protein machine, as we see next. purification Techniques allow homogeneous protein preparations to Be obtained from cell homogenates Whether starting with a fibroblast culture, a piece of liver, or a vat of cells that have been engineered to produce the protein of interest, the first step in any purification procedure is to break open the cells to release their contents; the resulting slurry is called a cell homogenate. This physical disruption is followed by an initial fractionation procedure to separate out the class of molecules of interest—for example, all the soluble proteins in the cell (panel 4–4, pp. 164–165). With this collection of proteins in hand, the job is then to isolate the desired protein. The standard approach involves purifying the protein through a series of chromatography steps, which separate the individual components of a complex mixture into different portions, or fractions. After each such step, one uses some sort of assay—for example, a test for the protein’s activity—to determine which fractions contain the protein of ECB3 M10.03/4.45 interest. Such fractions are then subjected to additional chromatography steps until the desired protein is obtained in pure form. The most popular forms of protein chromatography separate polypeptides on the basis of their size, their charge, or their ability to bind to a particular chemical group (panel 4–5, p. 166). If antibodies that recognize a particular protein are available, they can be used to help extract that protein from a mixture (see Panel 4–3, pp. 144–145). (E) 50 mm 161 162 Chapter 4 protein Structure and Function protein X covalently attached to column matrix matrix of affinity column MIXTURE OF PROTEINS APPLIED TO COLUMN proteins that bind to protein X adhere to column figure 4–49 Affinity chromatography can be used to isolate the binding partners of a protein of interest. the purified protein of interest, here protein X, is covalently attached to the matrix of a chromatography column. an extract containing a mixture of proteins is then loaded onto the column. those proteins that associate with protein X inside the cell will bind to it on the column. proteins not bound to the column pass right through, and the proteins that are bound tightly to protein X can then be released by changing the ph or ionic composition of the washing solution. A similar approach can be used to isolate those proteins that interact physically with the protein being studied. In this case, the purified protein of interest is attached to the matrix of the chromatography column; the proteins that bind to this protein will collect in the column and can then be eluted by changing the composition of the washing solution (figure 4–49). most proteins pass through the column ELUTION WITH HIGH SALT purified proteins Proteins can also be separated by electrophoresis. In this technique, a mixture of proteins is loaded onto a polymer gel and subjected to an electric field; the polypeptides will then migrate through the gel at different speeds depending on their size and net charge (panel 4–6, p. 167). If too many proteins are present in the sample, or if the proteins are very similar in their migration rate, they can be resolved further using twodimensional gel electrophoresis (see Panel 4–6). These electrophoretic approaches yield a number of bands or spots that can be visualized by staining, each one containing a different protein. Electrophoresis and chromatography—each developed more than 50 years ago—have been instrumental in building an understanding of what proteins look like and how they behave (Table 4–2). Both techniques are still used very frequently in laboratories. Once a protein has been obtained in pure form, it can be used in biochemical assays to study the details of its activity, and it can be subjected to techniques that reveal its amino acid sequence and precise three-dimensional structure (see How We Know, pp. 158–160). TABle 4–2 HISTORICAl lAnDMARkS In OUR UnDeRSTAnDInG OF PROTeInS 1838 the name “protein” (from the Greek proteios, “primary”) was suggested by Berzelius for the complex nitrogen-rich substance found in the cells of all animals and plants. 1819–1904 Most of the 20 common amino acids found in proteins were discovered. 1864 hoppe-Seyler crystallized, and named, the protein hemoglobin. 1894 Fischer proposed a lock-and-key analogy for enzyme–substrate interactions. 1897 Buchner and Buchner showed that cell-free extracts of yeast can ferment sucrose to form carbon dioxide and ethanol, thereby laying the foundations of enzymology. 1926 ECB3 N4.300/4.46 Sumner crystallized urease in pure form, demonstrating that proteins could possess the catalytic activity of enzymes; Svedberg developed the first analytical ultracentrifuge and used it to estimate the correct molecular weight of hemoglobin. 1933 tiselius introduced electrophoresis for separating proteins in solution. 1934 Bernal and Crowfoot presented the first detailed X-ray diffraction patterns of a protein, obtained from crystals of the enzyme pepsin. 1942 Martin and Synge developed chromatography, a technique now widely used to separate proteins. 1951 pauling and Corey proposed the structure of a helical conformation of a chain of amino acids—the a helix—and the structure of the b sheet, both of which were later found in many proteins. 1955 Sanger obtained the amino acid sequence of insulin, the first protein whose amino acid sequence was determined. 1956 Ingram produced the first protein fingerprints, showing that the difference between sickle-cell hemoglobin and normal hemoglobin is due to a change in a single amino acid (Movie 4.12). 1960 Kendrew described the first detailed three-dimensional structure of a protein (sperm whale myoglobin) to a resolution of 0.2 nm, and perutz proposed a lower-resolution structure for hemoglobin. 1963 Monod, Jacob, and Changeux recognized that many enzymes are regulated through allosteric changes in their conformation. how proteins are Studied large amounts of almost any protein can be produced by Genetic engineering Techniques Advances in genetic engineering techniques now permit the production of large quantities of almost any desired protein. In addition to making life much easier for biochemists interested in purifying specific proteins, this ability to churn out huge quantities of protein has given rise to an entire biotechnology industry (figure 4–50). Companies now use bacteria, yeast, or cultured mammalian cells to mass produce all sorts of proteins that are used therapeutically, such as insulin, human growth hormone, and even the fertility-enhancing drugs used to boost egg production in women undergoing in vitro fertilization. Preparing these proteins previously required the collection and processing of vast amounts of tissue and other biological products—including, in the case of the fertility drugs, the urine of postmenopausal nuns. Using the same sorts of genetic engineering techniques, scientists can also design proteins that perform novel tasks: metabolizing toxic wastes, synthesizing life-saving drugs, or operating under conditions that would destroy most biological catalysts. We shall discuss these methods in great detail in Chapter 10. automated Studies of protein Structure and function are increasing the pace of discovery Biochemists have made enormous progress in understanding the structure and function of proteins over the past 150 years (see Table 4–2, p. 162). These advances are the fruits of decades of painstaking research on isolated proteins, performed by individual scientists working tirelessly on single proteins or protein families, one by one, sometimes for their entire careers. But many future advances may come from proteomics, the largescale study of cellular proteins in which the activities or structures of hundreds—even thousands—of proteins are analyzed by highly sensitive, automated techniques. If scientists can perfect such methods, they might some day be able to monitor all of the proteins that are present in a cell: assessing whether they are switched on (or off) and seeing which proteins they are partnered with—all in a single experiment. Large-scale analyses of protein structures are already under way. Techniques that have been miniaturized and automated allow researchers to rapidly clone genes, produce proteins, grow crystals, and collect X-ray diffraction data for hundreds of proteins at a time. Through X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy (see How We Know, p. 160), we now know the three-dimensional shapes of more than 20,000 proteins. These structures are archived in large, publicly available databases (Movie 4.13). By analyzing the conformations of these proteins, biologists have come to the conclusion that the vast majority of protein domains fold up into a limited number of patterns—perhaps as few as 2000. The structures of about 800 of these protein folds have been determined so far. By studying how these patterns form, scientists hope to develop computational methods that will be able to take any amino acid sequence and predict both the structure and the function of the protein. Even with such information, it will still be an enormous challenge to decipher exactly how all these proteins—about 400 in the smallest bacterium and 10,000 in a typical human cell—work together to form a living cell. Figuring out exactly how proteins collaborate to create and maintain order in a universe that is always tending toward disorder will require both the continual development of new techniques and a great deal of human ingenuity. But the closer we get to answering this question, the closer we will be to understanding the fundamental basis for life. figure 4–50 Biotechnology companies produce mass quantities of useful proteins. Shown is a photograph of the fermenters used to grow the cells needed for such large-scale protein production. (Courtesy of Bioengineering aG, ECB3 n4.400/4.46.5 Switzerland.) 163 panel 4–5 166 Protein separation by chromatography PROTEIN SEPARATION + _ + + + _ + _ + COLUMN CHROMATOGRAPHY _ _ _ _ Proteins are often fractionated by column chromatography. A mixture of proteins in solution is applied to the top of a cylindrical column filled with a permeable solid matrix immersed in solvent. A large amount of solvent is then pumped through the column. Because different proteins are retarded to different extents by their interaction with the matrix, they can be collected separately as they flow out from the bottom. According to the choice of matrix, proteins can be separated according to their charge, hydrophobicity, size, or ability to bind to particular chemical groups (see below ). sample applied + solvent continuously applied to the top of column from a large reservoir of solvent Proteins are very diverse. They differ in size, shape, charge, hydrophobicity, and their affinity for other molecules. All of these properties can be exploited to separate them from one another so that they can be studied individually. THREE KINDS OF CHROMATOGRAPHY Although the material used to form the matrix for column chromatography varies, it is usually packed in the column in the form of small beads. A typical protein purification strategy might employ in turn each of the three kinds of matrix described below, with a final protein purification of up to 10,000-fold. Purity can easily be assessed by gel electrophoresis (Panel 4–6). solvent flow + + + + porous plug test tube time solvent flow + + + + + + + + + + ++ + + + + + + + + + + + + + + + + + + + + + + + + + + + solid matrix positively charged bead + + + bound negatively charged molecule free positively charged molecule (A) ION-EXCHANGE CHROMATOGRAPHY Ion-exchange columns are packed with small beads carrying either positive or negative charges that retard proteins of the opposite charge. The association between a protein and the matrix depends on the pH and ionic strength of the solution passing down the column. These can be varied in a controlled way to achieve an effective separation. fractionated molecules eluted and collected solvent flow porous beads small molecules retarded large molecules unretarded bead with covalently attached substrate bound enzyme molecule other proteins pass through (B) GEL-FILTRATION CHROMATOGRAPHY (C) AFFINITY CHROMATOGRAPHY Gel-filtration columns separate proteins according to their size. The matrix consists of tiny porous beads. Protein molecules that are small enough to enter the holes in the beads are delayed and travel more slowly through the column. Proteins that cannot enter the beads are washed out of the column first. Such columns also allow an estimate of protein size. Affinity columns contain a matrix covalently coupled to a molecule that interacts specifically with the protein of interest (e.g., an antibody, or an enzyme substrate). Proteins that bind specifically to such a column can subsequently be released by a pH change or by concentrated salt solutions, and they emerge highly purified (see also Figure 4–49). panel 4–6 167 Protein separation by electrophoresis GEL ELECTROPHORESIS sample loaded onto gel by pipette cathode plastic casing The detergent sodium dodecyl sulfate (SDS) is used to solubilize proteins for SDS polyacrylamidegel electrophoresis. protein with two subunits, A and B, joined by a disulfide bridge CH3 CH2 CH2 A CH2 single subunit protein B C S-S CH2 CH2 CH2 HEATED WITH SDS AND MERCAPTOETHANOL CH2 _ __ __ _ __ __ ___ ___ ___ __ __ ___ ___ _ _ _ __ _____ ___ __ _ _ _ __ _ _ __ _ _ _ _ _ __ __ _SH__ ____ __ __ _ _ ___ _ _ _ _ _ ___ __ _ __ _ __ __ __ _____ __ _ __ _ _ __ __ __ __ _ __ __ _ __ _ ___ _HS _ __ _ _ _ ___ __ _ _ __ _ _ _ _____ __ _ _ ___ ___ ___ negatively __ _ _ _ _ ___ _ _ _ ___ __ _ C _ charged SDS _ _ __ _ _ __ molecules A B CH2 buffer CH2 + anode gel CH2 CH2 O O buffer O When an electric field is applied to a solution containing protein molecules, the molecules will migrate in a direction and at a speed that reflects their size and net charge. This forms the basis of the technique called electrophoresis. ISOELECTRIC FOCUSING For any protein there is a characteristic pH, called the isoelectric point, at which the protein has no net charge and therefore will not move in an electric field. In isoelectric focusing, proteins are electrophoresed in a narrow tube of polyacrylamide gel in which a pH gradient is established by a mixture of special buffers. Each protein moves to a point in the gradient that corresponds to its isoelectric point and stays there. stable pH gradient 9 8 7 6 5 4 At low pH, the protein is positively charged. At high pH, the protein is negatively charged. + _+_ +_ _+ + _+_ +_ _+ + + __ _+ _ __+ ++ _ +_ _+ + POLYACRYLAMIDE-GEL ELECTROPHORESIS Na + B SDS polyacrylamide-gel electrophoresis (SDS-PAGE) Individual polypeptide chains form a C complex with negatively charged molecules of sodium dodecyl sulfate (SDS) and therefore migrate as a negatively charged SDS–protein complex through a slab of porous polyacrylamide gel. The A apparatus used for this electrophoresis technique is shown above (left ). A reducing agent (mercaptoethanol) is usually added to break any –S–S– linkages in or between proteins. Under these conditions, proteins migrate at a rate that reflects their molecular weight. + slab of polyacrylamide gel TWO-DIMENSIONAL POLYACRYLAMIDE-GEL ELECTROPHORESIS Complex mixtures of proteins cannot be resolved well on one-dimensional gels, but two-dimensional gel electrophoresis, combining two different separation methods, can be used to resolve more than 1000 proteins in a two-dimensional protein map. In the first step, native proteins are separated in a narrow gel on the basis of their intrinsic charge using isoelectric focusing (see left ). In the second step, this gel is placed on top of a gel slab, and the proteins are subjected to SDS-PAGE (see above ) in a direction perpendicular to that used in the first step. Each protein migrates to form a discrete spot. + +++ + + +++ ___ _ _ ___ O SDS The protein shown here has an isoelectric pH of 6.5. All the proteins in an E. coli bacterial cell are separated in this 2-D gel, in which each spot corresponds to a different polypeptide chain. They are separated according to their isoelectric point from left to right and to their molecular weight from top to bottom. (Courtesy of Patrick O'Farrell.) basic SDS migration (mol. wt. x 10–3) 10 S 100 50 25 stable pH gradient acidic