* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Binding of a Growth Hormone- Inducible Nuclear Factor Is Mediated
Metalloprotein wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene regulatory network wikipedia , lookup
Drug design wikipedia , lookup
Expression vector wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Gene expression wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Proteolysis wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein purification wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Biochemical cascade wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Western blot wikipedia , lookup
Ligand binding assay wikipedia , lookup
Paracrine signalling wikipedia , lookup
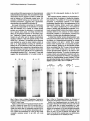
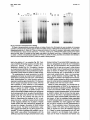
Binding of a Growth HormoneInducible Nuclear Factor Is Mediated by Tyrosine Phosphorylation Susan A. Berry, Pearl Howard C. Towle L. Bergad, Carmella DeRose Whaley, and Departments of Pediatrics (S.A.B., P.L.B., C.D.W.) and Biochemistry (H.C.T.) Institute of Human Genetics (S.A.B., H.C.T.) University of Minnesota Minneapolis, Minnesota 55455 achieving normalsomaticgrowth and fuel homeostasis. GH action is initiated by bindingto a specific GH receptor on the cell surface, followed by activation of the receptor-associated tyrosine kinase JAK2 (1). The mode of signaltransduction that is elaborated by this kinaseactivation in responseto GH is unknown. One of the principaleffects of GH binding is to alter specific gene expression in target cells. A number of messengerRNA specieshave beenfound to be induced by GH treatment. Two hepatic genesbelongingto this class are the serine protease inhibitor genes Spi 2.1 and 2.3 (2). We have previously characterized an element in the 5’-flanking region of Spi 2.1 that is capable of conferring GH responsivenessto a heterologous promotor (GHRE). This elementbindsa hepatic nuclear protein in a GH state-specific manner and does not requirede nova protein synthesisfor activation of binding (3). This observation suggestedthat GH-responsive bindingto the element requiresa reversibleposttranslationalmodification.Consideringthe activation of JAK2 tyrosine kinase activity upon GH binding, it seemed logical to consider phosphorylation as a candidate mechanismfor this modification.We examinedthe possible role of phosphorylationin the binding of the GHinducible nuclear factor (GHINF) and investigated whether p91, a protein recently implicated in JAKB mediatedsignaltransduction(4, 5) might participate in GHINF bindingto the Spi 2.1 promotor elementin viva. The nuclear mechanism by which GH acts to induce gene expression after binding to its receptor on the cell surface is not defined. We have characterized an element in the 5’-flanking region of the rat GHresponsive serine protease inhibitor @pi) 2.1 gene responsible for its induction by GH. This element binds a hepatic nuclear protein(s) in a GH statespecific manner. Activation of binding by GH does not require de nova protein synthesis, suggesting that a reversible posttranslational process is required for binding to the element. To define the mechanism of this process, hepatic nuclear extracts were analyzed by electrophoretic mobility shift assays using a DNA fragment (-147 to -103) of the Spi 2.1 gene. Treatment of extracts with phosphatases resulted in a marked reduction of GH statespecific binding. Addition of phosphatase inhibitors antagonized the reduction in binding after phosphatase treatment. The specific nature of the phosphorylation event involved in binding was explored using phosphotyrosine antibodies and a protein tyrosine phosphatase. Treatment of nuclear extracts with either of these reagents ablated binding to the response element. Because the tyrosine-phosphorylated transcription factor protein p91 has recently been implicated in cytokine signal transduction mediated by JAKS, we sought evidence that p91 was part of the GH-responsive binding complex. Analysis of an enriched preparation of GH-inducible binding complexes by Western blots using anti-p91 demonstrated no immunoreactivity. We conclude that tyrosine phosphorylation of a nuclear factor is required for GH state-specific binding to this GH response element in wivo, but that p91 is not present in the binding complex. (Molecular Endocrinology 8:1714-1719,1994) RESULTS INTRODUCTION GH is one of the principal developmental hormones elaborated by the pituitary. Its action is critical for o&la8E10/94$03.00/0 Mdecular Endocrirology Copyright CD 1994 by The Endoaine Society 1714 AND DISCUSSION We have previously demonstrated GH state-specific binding of a hepatic nuclear factor to a DNA fragment (-147 to -103) of the Spi 2.1 gene in electrophoretic mobility shift assays.This site correlated with GHstatespecific deoxyribonuclease-l-hypersensitive sites located in proximity to this fragment of Spi 2.1 flanking DNA. A concatamer of this fragment coupled to the thymidine kinasepromotor and bacterial chloramphenicol acetyltransferasetransfected into isolated hepato- GHINF Binding Is Mediated by Y Phosphorylation 1715 cytes conferred GH responsivenessto chloramphenicol acetyltransferaseexpression.We concludedfrom these observationsthat this fragment contains a GHRE that acts by binding to a GH-inducible nuclear factor. GH activation of GHINF bindingwas not blocked by cycloheximide pretreatment, suggesting that a posttranslational process was required for activation (3). We sought information concerning the modification that might be responsiblefor the alteration in binding after GH treatment. As a number of nuclear binding proteins are modulated by alterations in phosphorylation of specific amino acids, we initially examined the effects of phosphatasetreatment on the binding reaction. Treatment of hepatic nuclear extracts from GHtreated rats with either acid or alkaline phosphatases resulted in ablation of GH state-specific binding to the GHRE(Fig. 1, left panel). The specificity of the response was assessed by using the same treated extracts to bind to an oligonucleotide including the USF/MLTFbinding site of the adenovirus major late promotor, a bindingreaction that is insensitiveto phosphatasetreatment (6). Binding of upstream stimulatory factor/major late transcriptionfactor (USF/MLTF) was unaffected by phosphatasetreatment (Fig. 1, right panel). These data suggest that phosphorylation of a nuclear protein is GH alk ac alk ac GH critical for GH state-specific binding to the Spi 2.1 GHRE. We examined whether inhibitors of phosphataseactivity would with 1 mM sodium of binding by phospha- vanadate (Fig. 2). The marked de- crease in binding after phosphatasetreatment of extracts containing GHINF without the inhibitors was sub- stantially altered by the presence of these inhibitors. Nonspecific inhibition of phosphatase prevented the reduction of bindingafter phosphatasetreatment. To determine whether tyrosine phosphorylationwas critical to GH-induciblenuclearfactor binding,we tested the effects of a phosphotyrosineantibody on nuclear protein binding to the Spi 2.1 GHRE. When nuclear extracts were incubated with a monoclonalphosphotyrosine antibody, binding by the GH-induciblenuclear factor was ablated(Fig. 3A). The useof other antibodies failed to alter GHINF binding (data not shown). Thus, tyrosine phosphorylationappears to be critical to GHmediatedfactor activation. To confirm this observation, nuclearextracts were subjectedto treatment with protein tyrosine phosphatase-1B (PTPase),a phosphatase specific for phosphorylated tyrosine residues.PTPase alsoeliminatedbinding,and this ablationcould be inhibMEND-12 Fig. 1. Effect of Acid or Alkaline Phosphatase Treatment ot Nuclear Extracts on Binding to Labeled Spi 2.1 GHRE (lefi) or USF/MLTF (fight) Probes Hepatic nuclear extracts from hypophysectomized rats without (Hx) or treated with human GH (150 pg/lOO g BW) 1 h before liver harvest (all others) were used in electrophoretic mobility shii assays after no phosphatase treatment (GH) or after exposure to 0.3 U calf intestinal phosphatase (alk) or 0.01 U potato acid phosphatase (ac). There are also smaller nonspecific complexes present in gel shifts with crude extracts. block the ablation tase treatment. The addition of 10 mMpara-nitrophenol phosphate, a phosphatase substrate, to the alkaline phosphatasetreatment led to the reappearanceof GH state-specific binding. Similar results were obtained 231141 FIG 2 18 X 17.9 (3614) Fig. 2. Effect of Phosphatase Inhibitors on Diminution of Binding after Phosphatase Treatment of Nuclear Extracts Extracts from hypophysectomized rats treated with GH were used in electrophoretic mobility shift assays with the Spi 2.1 GHRE probe after no alkaline phosphatase (0) or after treatment with 0.1 or 0.3 U alkaline phosphatase (Alk Phos). Inhibitors of phosphatase [lo mM para-nitrophenol phosphate (pNPP) or 1 mu sodium vanadate] were added as indicated above the lanes, as noted in Materials and Methods. Also shown to the right of the vanadate lanes is an additional lane with no phosphatase and no vanadate (No inhib). MOL ENDO. 1994 1716 Vol8No.12 Fig. 3. Involvement of Phosphotyrosine in Binding A, Effects of phosphotyrosine monoclonal antibody on binding. Extracts from GH-treated rats were incubated with increasing quantities of phosphotyrosine antibody (anti-pY) before the addition of Spi 2.1 GHRE probe and electrophoresis. Hx, Extract from hypophysectomized rats. B, Effects of PTPase on binding and inhibition of this effect by vanadate. Using hepatic nuclear extracts from GH-treated rats, reaction mixtures had N&VO,, 1 mM (Van), or 500 ng (10 pl) PTPase (PTP) added according to the scheme above the lanes, where (+) indicates that that reagent was added to the reaction mixture and (-) indicates that the reagent was not added. C, Effects of phosphotyrosine antibody and PTPase on USF/MLTF binding. Quantities and conditions were identical to those in A and B, except that the USF/MLTF probe was added to binding reactions. ited by the addition of 1 mM vanadate (Fig. 38). Treatment of USF/MLTF-binding reactions with either phosphotyrosine antibody or PTPase resulted in no alterationsin binding(Fig. 3C). The ablation of binding by phosphotyrosineantibody and a phosphatasespecific for phosphotyrosineis strong evidencethat a phosphorylatedtyrosine residueis critical for GHINFbinding. The understandingof signaltransduction by certain polypeptide hormoneshas recently been facilitated by observationsconcerningthe role of p91, a DNA-binding factor phosphorylated at a critical tyrosine residue in responseto cytokine signaltransduction(7,8). Because the GH receptor-associatedtyrosine kinaseJAK2 has been implicated in some instances of p91-mediated geneactivation (4,5), and becausetyrosine phosphorylation is required for GHINF binding, we examined whether p91 was present in the GHINF complex. We useda commerciallyavailableantibody that recognizes the N-terminalportion of p91 as well as the alternatively splicedprotein ~84, which derives from the N-terminal portion of p91 (9). This antibody has been used by others to demonstrate the presence of p91 in complexes formed by y-interferon-treated extracts binding to their regulatory element (10). Incubation of hepatic nuclear extracts with this N-terminal p91 antibody did not result in a change in the pattern of GHINF binding (data not shown). The failure to demonstrate a supershift suggeststhat neither p91 nor p84 is present in the complex. To further investigate this possibility, we purified GHINF using affinity chromatography with the GHRE and examinedthe highly enriched fraction using Western blotting. The enrichedGHINF preparationcontains two polypeptides of about 93 and 70 kilodaltons (kDa) that are immunoreactive with phosphotyrosine antibodies(Fig. 4A) and are not seen in crude extracts from the hepatic nuclei of hypophysectomized animals (data not shown). A Western blot using p91 antibody shows that although p91 is demonstrablein GH-activated crude nuclear extracts, there is no immunoreactivity in purified fractions containing GHINF (Fig. 48). Although p91 is presentin GH-treated rat liver extracts, the GHINF complex isolatedby affinity chromatography doesnot contain proteinsrecognizableby an N-terminal p91 antibody even when not bound to DNA. Based on these observations, we conclude that tyrosine phosphorylationof GHINF is the mechanismby which GH activates the bindingof GHINF.Alternatively, a factor that activates GHINF binding could itself be activated by tyrosine phosphorylation,so that the effect on GHINF is indirect. We do not favor the latter possibility because the effect of phosphatasetreatment is preserved in highly enrichedGHINF preparations(data not shown). As yet, the candidate kinase responsible for GHINF phosphotylationin responseto GHtreatment remains unidentified. Our work suggests that neither p91 nor p84 is involved in the GH responsivenessof Spi 2.1. This is surprisingin view of the known participation of JAK2 in GH action and the observation that JAK2 is required for some p91-mediated responses (11). Clearly, a protein immunoreactivewith p91 antibody is present in our GH-treated crude nuclear extracts and in those of Gronowski and Rotwein (12). 1717 GHINF Binding Is Mediated by Y Phosphorylation A B anti -pY antLp9m4 217 32.5 Fig. 4. Antibodies to p91/84 Do not Recognize the Tyrosine Phosphorylated Proteins Obtained after GHRE-Specific Affinity Chromatography Purification of GHINF A, lmmunoblot of crudeGH-treatedrat livernuclearextract (cr) and affinity-purifiednuclearextracts (aff) incubatedwith monoclonal phosphotyrosine antibody (anti-pY). B, Immunoblot of crude GH-treated rat liver nuclear extract (cr) and affinity-purified nuclear extracts (aff) incubated with N-terminal ISGFB (p91/84) antibody (anti-p91 /84). Mol wt markers (xl 03) are shown to the left. This protein is activated in responseto GH treatment, as p91 immunoreactivityis absent in our crude extracts from hypophysectomized liver (data not shown) (12). Further, in GH-activated cell lines,a protein comigrating with p91 was induced in responseto GH (13, 14). However, activation of gene expression by other polypeptide hormones via tyrosine phosphorylation in the absenceof immunoreactivep91 has bean demonstrated (1516) suggestingthat the GH responsecould have features in common with activation of gene expressionby these hormones.Thus, the GH-mediated pathway of signal transduction may diverge into p91dependentand -independentpathways. There are other proteinsimmunologicallyrelated to p91 that serve similar roles in signaling pathways for cytokines; for example,the acute phaseresponsefactor APRF(Stat3) in the interleukin-6 pathway (17-19). This protein has 52.5% amino acid homology with p91 (18). Additional proteins in this gene family have also been described (20). There is a family of p91-like genes, presumably with different binding and transcriptional activation activities. Our studies cannot determine whether GHINF is a memberof such a p91 gene family, but immunologically distinct from it. Finbloomet al. (21) demonstrated the presenceof a 93-kDa tyrosine-phosphorylatedprotein in GH-activated IM-9 cell nuclear extracts, which was present in complexes bound to an oligonucleotideof the r-response region of the FCy-receptor factor. A p91 antiserumfailed to produce a supershift, but when the extracts were purified by affinity chromatography, both an internal and C-terminal p91 antibody recog- nized a 93-kDa protein on Western blots of sodium dodecyl sulfate-polyacrylamidegels of the purified extracts (21). The 93-kDa tyrosine-phosphorylatedprotein seenin our affinity-purified fraction could be this protein despite the absence of immunoreactivity to N-terminal p91 antibodies. The signalingpathways of growth factor activation of Ras proteins, followed by activation of a kinase cascade, may also be activated by GH. It has been demonstrated that mitogen-activatedprotein (MAP) kinases are part of the phosphorylationcascadein the response after GH binding to GH receptor in 3T3-F442A preadipocytes (22, 23). This also was noted in Chinesehamster ovary cells transfected with GH receptor (24) and in GH-treated liver nuclear extracts (12). Ultimately, many aspectsof MAP kinase-activatedsignaltransduction are mediatedby Jun/Fos binding to Hela cell activator protein-l (AP-1) elements. As Fos and Jun both demonstrate GH responsiveness(25, 26) this may be an alternative pathway for GH-mediatedgene activation. The Spi 2.1 gene does not contain a sequence related to the consensusAP-l-binding site. However, Fos expressionitself is may be mediatedby a p91-like protein in a complex with other phosphoproteins.Thus, GH may activate both p91-type and MAP kinasepathways in affecting different metabolicresponses. Our observations indicate that tyrosine phosphorylation of a nuclear protein is critical in the in vivo responseof rat liver to GH treatment. This is madeevident by the observation of GH state-specific binding of GHINF to the GHRE of Spi 2.1, which is altered by antagonism of tyrosine phosphotylation. The exact pathway(s) by which GH-responsive Spi 2.1 gene expression could be modulated by reversibletyrosine phosphorylation of a nuclear binding protein remains undefined.Emergingevidence about GH responsecurrently supports the relatively simple mechanismof direct activation of a latent cytoplasmic DNA-binding protein, but if GHINF is a p91-like protein, it is immunologicallydistinct from it, suggestingthat it is neither p91 nor the immunologicallyrelated Stat3 protein. Provocative evidence supporting polypeptide activation of the complex MAP kinase pathway in GH action also exists, and we cannot rule out activation of Spi 2.1 gene expression by this signal transduction cascade. The resolution of this uncertainty will be provided ultimately by analysis of GHINF and its phosphorylation site(s). MATERIALS AND METHODS Nuclear Extract Preparation Male rats (Fischer strain) were hypophysectomized at lOO125 g by the supplier (Taconic Farms, Germantown, NY) and observed for at least 3 weeks to confirm growth failure. All animals were maintained in accordance with the NIH Guide for the Care andUseof LaboratoryAnimalsunderthe supervision of Research Animal Resources of the University of Minnesota. They were given 150 pg human GH (Genentech, South San MOL 1718 ENDO. 1994 Vol8 Francisco, CA)/1 00 g BW, iv, and their livers were removed 1 h later. All subsequent work was performed at 4 C unless otherwise specified. Nuclei were isolated according to the method of Gorski et al. (27). Extracts of nuclei were prepared by resuspension in nuclear lysis buffer [lo mM HEPES (pH 7.6). 0.1 M KCI, 3 mM MgCI2.0.1 mrv EDTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonylfluoride, and 10% glycerol] in a Dounce homogenizer (4 ml/O.5 ml nuclear vol). While stirring gently, nuclear lysis buffer containing 1.2 M KCI was added dropwise to a final concentration of 0.4 M. After 30 min, the mixture was centrifuged at 35,000 rpm in a Beckman Ti-75 rotor (Beckman, Palo Alto, CA) for 60 min. The resultant supematant was either frozen in aliquots immediately or dialyzed against 25 mM HEPES (pH 7.6) 0.1 M KCI, 0.1 mM EDTA, 1 mM dithiothreitol, and 10% glycerol twice for 2 h each, then frozen in aliquots. All aliquots were stored at -80 C. Protein concentrations were determined using the Bio-Rad Protein Assay Kit (Richmond, CA). Probe Preparation An oligonucleotide corresponding to the coding strand of the GHRE located from -147 to -103 of the rat Spi 2.1 gene promoter sequence (3) was synthesized, including Taql recognition sites at both ends for cloning purposes. A short oligonucleotide complementary to the 3’end of the oligonucleotide (13 basepairs) was also synthesized. The annealed duplex was extended using Escherichia co/i polymerase-I (Klenow) with deoxy-ATP as the radioactive nucleotide. It was purified through a B&Spin 6 column (BioRad Laboratories). Its specific activity was 1 x 1 Og cpm/pg. A synthetic duplex corresponding to the adenovirus USF/ MLTF-binding site (6) was labeled by fill-in using E. co/i polymerase-l (Klenow) with deoxy-CTP as the radioactive nucleotide. It was also purified through a Bio-Spin 6 column and had a specific activity of 1.5 X 1 O8 cpm/pg. Electrophoretic Mobility Shift Assay All electrophoretic mobility shift assays were performed in a buffer containing 20 mM HEPES (pH 7.6) 10% glycerol, 2 mM MgC12, 5 rnhf &Cl,, 0.1 mg/ml .BSA, 4% Ficoli 400, 1 mM spermidine, 1 mM DlT, and 1 mM phenylmethylsulfonylfluoride. Poly(dldC~poly(dldC) (1.5 pg) and poly(dAdT)-poly(dAdT) (0.5 pg; Pharmacia-LKB, Piscataway, NJ) were added to each reaction. For assays with the USF/MLTF probe, all conditions were identical, except that MgC12 and CaC12 were omitted from the binding buffer. For every 19 ~1 reaction mixture, 6 fig nuclear orotein extract were added. and the final KCI concentration was adjusted to 50 mM if necessary. Where indicated, para-nitrophenol phosphate at a final concentration of 10 mM or sodium vanadate (N&VO,) at a final concentration of 1 rnt+t was also added. In examination of the effects of phosphatase on binding to the GHRE. alkaline phosphatase or potato acid phosphatase (both from Boehringer Mannheim Biochemicals, Indianapolis, IN) was appropriately diluted in 0.1 mg/ml BSA and added to the binding mixture. After a brief mixing, the mixture was incubated at 30 C for 20 min. For PTPase (Upstate Biotechnology, Lake Placid, NY) studies, 500 ng PTPase on agarose beads were added, and the mixture was incubated at 37 C for 30 min. After phosphatase treatments of the extracts, 1 ~1 containing 20 fmol labeled GHRE was added to the treated extracts, and the mixture was further incubated at 30 C for an additional 20 min. For examination of alterations of binding by antibodies, the monoclonal phosphotyrosine antibody 4GlO (Upstate Biotechnology) or anti-ISGF3 (p91/84) polyclonal antibody (Transduction Laboratories, Lexington, KY) was added to the reaction mixture and incubated at 4 C for 1 h. One microliter containing 20 fmol labeled GHRE was added, and the mixture was further incubated at 30 C for an additional 20 min. The reaction mixtures were loaded onto a nondenaturing, No. 12 3.2% glycerol, 4% polyacrylamide gel (39.51 acrylamide-bisacrylamide) in a Bio-Rad Protean II system in 0.5 x TBE (45 mM Tris, 45 mM boric acid, and 1 mM EDTA, pH 8.3) and electrophoresed at 150 V for 2.5 h. The gels were dried and exposed to film. Affinity Purification and lmmunoblotting of GHINF GHINF was purified from crude nuclear extracts by chromatography on separation, using heparin agarose followed by calf thymus-DNA Sepharose. The active fractions were affinity purified by chromatography, using an octomer of the Spi 2.1 GHRE fragment from -147 to -102 (3) coupled to cyanogen bromide-activated Sepharose4B, as previously described (28). All elutions were monitored by electrophoretic mobility shift assay with the GHRE probe. Details of the purification will be published elsewhere (Bergad, P. L. , H. C. Towle, S. J. Schwarzenberg, H.-M. Shih, and S. A. Berry, manuscript in preparation). Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 7.5% gel, followed by transfer to BA-S nitrocellulose. The blots were incubated with either phosphotyrosine antibody or anti-ISGFB and developed with alkaline phosphatase-conjugated secondary antibodies according to manufacturers’ protocols. Acknowledgments Received March 3, 1994. Revision received July 28, 1994. Accepted August 23, 1994. Address requests for reprints to: Susan A. Berry, M.D., Department of Pediatrics, University of Minnesota, 420 Delaware Street SE, Box 75 UMHC, Minneapolis. Minnesota 55455. This work was performed with the support of the Vikings Children’s Fund. and the Deoartment of Pediatrics and the Institute for Disabilities Studies, University of Minnesota. Portions of the work were supported by NIH Grant DK-32817. REFERENCES 1. Argetsinger LS, Campbell GS, Yang XN, Witthuhn BA, Silvennoinen 0, lhle J, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237-244 2. Yoon J-B, Towle HC, Seelig S 1987 Growth hormone induces two mRNA species of the serine protease inhibitor gene family in rat liver. J Biol Chem 262:4284-4289 3. Yoon J-B, Berry SA, Seelig S, Towle HC 1990 An inducible nuclear factor binds to a growth hormone-regulated gene. J Biol Chem 265: 19947-l 9954 4. Sadowski HB, Shuai K, Damell JEJ, Gilman MZ 1993 A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science 261:1739-1744 5. Silvennoinen 0, Schindler C, Schlessinger J, Levy DE 1993 &s-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science 261:1736-1739 6. Kugler W, Kaling M, Ross K, Wagner U, Ryffel GU 1990 BAP, a rat liver protein that activates transcription through a promoter element with similarity to the USF/MLTF binding site. Nucleic Acids Res 18:6943-6951 7. Schindler C, Shuai K, Prezioso VR, Damell JE 1992 Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science 257:809-813 8. Shuai K, Stark GR, Kerr IM, Damell JE 1993 A single GHINF 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. Binding Is Mediated by Y Phosphorylation phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science 261 :1744-l 746 Schindler C, Fu X-Y, lmprota T, Aebersold R, Damell JE 1992 Proteins of transcription factor ISGF-3: one gene encodes the 91- and 84 kDa ISGF-3 proteins that are activated by interferon a. Proc Natl Acad Sci USA 89:7836-7839 RuffJamison S, Chen K, Cohen S 1993 Induction by EGF and interferon-r of tyrosine phosphorylated DNA-binding proteins in mouse liver nuclei. Science 261:1733-1736 Watling D, Guschin D, Muller M, Silvennoinen 0, Witthuhn BA, Quelle FW, Rogers NC, Schindler C, Stark GR, lhle JN, Kerr M 1993 Complementation by the protein tyrosine kinase JAKP of a mutant cell line defective in the interferon? signal transduction pathway. Nature 366:166170 Gronowski AM, Rotwein P 1994 Rapid changes in nuclear protein tyrosine phosphorylation after growth hormone treatment in viva: identification of phosphorylated mitogen-activated protein kinase and STAT91. J Biol Chem 269:7874-7878 Meyer DJ, Campbell GS, Cochran BH, Argetsinger LS, Lamer AC, Finbloom DS, Carter-Su C, Schwartz J 1994 Growth hormone induces a DNA binding factor related to the interferon-stimulated 91 -kDa transcription factor. J Biol Chem 269:4701-4704 Kilgour E, Anderson NG 1994 Growth hormone induces the tyrosine phosphorylation and nuclear accumulation of components of the ISGFB transcription factor complex. FEBS Lett 343:205-207 Lamer AC, David M, Feldman GM, lgarashi K, Hackett RH. Webb DSA. Sweitzer SM. Petricoin EF. Finbloom DS 1993 Tvrosine phosphorvlation of DNA binding proteins by multiple cytokines. S&nce 261:1730-l 733Kotanides H. Reich NC 1993 Reouirement of tvrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science 262:1265-l 267 Lutticken C, Wegenka UM, Yuan JP, Buschmann J, Schindler C, Ziemiecki A, Harpur AG, Wilks AF, Yasukawa K, Taga T, Kishimoto T, Babieri G, Pellegrini S, Sendtner M, Heintich PC, Horn F 1994 Association of transcription factor APRF and protein kinase Jakl with the interleukin6 signal transducer gpl30. Science 263:89-92 Akira S, Nishio Y, lnoue M, Wang XJ, Wei S, Matsusaka 1719 T, Yoshida K, Sudo T, Naruto M, Kishimoto T 1994 Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the o~130-mediated sianalina Dathwav. Cell 77:63-71 19. I’hong Z, Wen ZL,bam&‘JE 1994 Stat3: a STAT family member activated by tyrosine phosphorylation in re sponse to epidermal growth factor and interleukin-6. Science 264:95-98 20. Zhong Z, Wen Z. Damell JE 1994 Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci USA 91:4806-4810 21. Finbloom DS, Petricoin EF, Hackett RH, David M, Feldman GM, lgarashi K, Fibach E, Weber MJ, Thorner MO, Silva CM, Lamer AC 1994 Growth hormone and erythropoietin differentially activate DNA-binding proteins by tyrosine phosphorylation. Mol Cell Biol 14:2113-2118 22. Winston LA, Bertics P 1992 Growth hormone stimulates the tyrosine phosphorylation of 42- and 45-kDa ERKrelated proteins. J Biol Chem 267:4747-4751 23. Anderson NG 1992 Growth hormone activates mitogenactivated protein kinase and S6 kinase and promotes intracellular tyrosine phosphorvlation in 3T3-F442A preadipocytes. Biochem J 284649-652 24. Moller C. Hansson A. Enbera B. Lobie PE. Norstedt G 1992 Growth hormone (GH) induction of tyrosine phosphorylation and activation of mitogen-activated protein kinases in cells transfected with rat GH receptor cDNA. J Biol Chem 267:23403-23408 25. Slootweg MD, de Groot RP, Hernnann-Erlee MPM, Koomneef I, Kruiker W, Kramer YM 1991 Growth hormone induces expression of c-jun and jun B oncogenes and employs a protein kinase C signal transduction pathway for the induction of c-fos oncogene expression. J Mol Endocrinol6:179-188 26. Meyer DJ, Stephenson EW, Johnson L, Cochran BH, Schwartz J 1993 The serum response element can me diate induction of c-fos by growth hormone. Proc Natl Acad Sci USA 90:6721-6725 27. Gorski K, Cameiro M. Schibler U 1986 Tissue smfic in vitro transcription from the mouse albumin prom&or. Cell 471767-776 28. Kadonaga JT 1991 Purification of sequence-specific binding proteins by DNA affinity chromatography. Methods Enzymol208:l O-23