* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Describing Chemical Reactions
Relativistic quantum mechanics wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Double layer forces wikipedia , lookup
American Chemical Society wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemical warfare wikipedia , lookup
Destruction of Syria's chemical weapons wikipedia , lookup
Atomic theory wikipedia , lookup
Fine chemical wikipedia , lookup
History of molecular theory wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Drug discovery wikipedia , lookup
Chemical imaging wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Physical organic chemistry wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Process chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Chemical reaction wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical potential wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical Corps wikipedia , lookup
Transition state theory wikipedia , lookup
Stoichiometry wikipedia , lookup
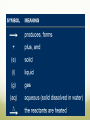
Describing Chemical Reactions Objectives: List three observations that suggest that a chemical reaction has taken place List three requirements for a correctly written chemical equation Write a word equation and a formula equation for a given chemical reaction Balance a formula equation by inspection What Is a chemical reaction? Chemical reaction: the process by which one or more substances are changed into one or more new substances Represented by chemical equations Chemical equation: a shorthand expression that represents a chemical reaction Shows the relative amount of each substance taking place in a chemical reaction Signs of a Chemical Reaction To know for certain that a chemical reaction has taken place requires evidence that one or more substances have undergone a change in identity Certain easily observed changes usually indicate that a chemical reaction has occurred: Evolution of heat and light Production of a gas Formation of a precipitate Precipitate: a solid that is produced as a result of a chemical reaction in solution and that separates from the solution Color change Chemical Equations A+B C+D REACTANTS PRODUCTS Reactants: starting substances in a chemical reaction Products: the substances formed in a chemical reaction Characteristics of a Chemical Equation The equation must represent known facts. All reactants and products must be identified. The equation must contain the correct formulas for the reactants and products. The law of conservation of mass must be satisfied Law of Conservation of Mass Mass is neither created nor destroyed in a chemical reaction Total mass stays the same Atoms can only rearrange Writing Equations Identify the substances involved Use symbols to show: How many? – coefficient Coefficient: a small whole number that appears in front of a chemical formula in a chemical equation Of what? – chemical formulas In what state? – physical state Letters in parentheses indicate the physical state of each substance involved in the reaction (g) gas ; (l) liquid ; (s) solid ; (aq) aqueous solution Balancing Chemical Equations 1. Write the unbalanced chemical equation. 2. Count the atoms of each type on each side of the equation. 3. Add coefficients to make the number of atoms equal. (Remember: the law of conservation of mass must be satisfied!) Coefficient subscript = # of atoms 4. Reduce coefficients to the lowest possible ratio, if necessary. 5. Double check atom balance! Sample Problem Aluminum, Al, and copper (II) chloride, CuCl2, react to form copper, Cu, and aluminum chloride, AlCl. CuCl2 Al + 2 Cu + 3 AlCl3 3 2 2 2 1 Al 1 3 1 Cu 1 3 6 2 Cl 3 6 Helpful Tips for Balancing Equations Balance one element at a time Update ALL atoms counts after adding a coefficient If an element appears more than once per side, balance it last Balance polyatomic ions as single units Ex: “1 SO4” instead of “1 S” and “4 O” If you find an element difficult to balance, save it for last If you use a fraction during the balancing process, you can eliminate it later by multiplying each coefficient by 2 In general, it may be helpful to balance H and O last Chagi Fan! Balance each of the following chemical reactions: 1. Mg(s) + O2(g) MgO(s) 2Mg + O2 2MgO 2. Fe(s) + O2(g) Fe2O3(s) 4Fe + 3O2 2Fe2O3 Practice Balance the following equations: 1. 2. 3. 4. 5. 6. __Mg + __HCl __MgCl2 + __H2 __H2 + __Cl2 __HCl __ Ga + __ O2 __ Ga2O3 __Al + __Fe2O3 __Al2O3 + __Fe __GeCl4 + __ H2O __ GeO2 + __ HCl __Fe + __H2O __Fe3O4 + __H2