* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Do Now - Montville.net
Survey
Document related concepts
Signal transduction wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cell membrane wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Endomembrane system wikipedia , lookup
Programmed cell death wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Transcript
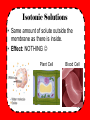
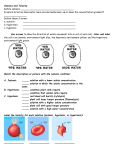
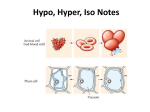
Do Now • What is osmosis? • What happened when we put our gummi bears into water? • WHY did this happen? Objectives • To understand what happens during osmosis in regards to osmotic pressure. • To compare and contrast hypertonic, hypotonic, and isotonic solutions. • To explain different real life applications of these solutions. Osmotic Pressure • What is osmosis? • Whichever has MORE water (either the solution or the solution inside the cell) is going to be the one to have a higher osmotic pressure and the water will flow in the direction of lower osmotic pressure. “Tonicity” • The measure of osmotic pressure • There are 3 types of solutions in regards to tonicity – Isotonic – Hypertonic – Hypotonic Quick Refresher! • What is a solution? • Solute vs. solvent? • Example?- Isotonic Solutions • Same amount of solute outside the membrane as there is inside. • Effect: NOTHING Plant Cell Blood Cell 11,397x Hypertonic Solutions • Solution contains MORE SOLUTE than the cell, water flows out of the cell. • Effect: cell shrinks/shrivels “Plasmolysis” “Crenates” Hypotonic Solutions • Solution contains LESS SOLUTE than the cell, water flows into the cell. • Effect: cell swells or bursts! / • (remember the grocery store misters?) Animations • http://www.biologyjunction.com/tonicity%2 0animations.htm High or low Solute? Cell in ________ Solution H2O H 2O Cell in ________ Solution H 2O H 2O Cell in ________ Solution H 2O H 2O Real Life Application • Turn to the person sitting next to you. In the next few slides you will see real life scenarios using the 3 types of solutions we have discussed. For each scenario you and your partner should determine what type of solution is being described as well as what will happen. Scenario 1 A salt water fish is put into a freshwater aquarium. • What type of solution is the freshwater? • What is going to happen to the cells of the fish? Scenario 2 A patient is given an IV, which contains the perfect balance of glucose • What type of solution is in the IV? • What is going to happen to the cells of the human? Scenario 3 Your garden is infested with slugs so you go around pouring salt on them • What type of solution is this salt? • What is going to happen to the cells of the slug? (don’t do this to the poor slugs ) Practice • By yourself or with a partner (anyone), work on the diffusion problems. • First try to do this by yourself, then verify your answers with a partner. Try to help each other. If you are confused, raise your hand and I will come help you!! • If you finish early, try to answer the “Think about it” questions on my desk! If you don’t finish, please finish it for homework. 3-2-1 Exit Slip • On a SEPARATE sheet of paper… • List 3 things you learned today • List 2 things you are confused about • List 1 thing you found interesting