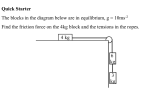
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1. The Model: implementation of the numerical responses of
Scientific opinion on climate change wikipedia , lookup
Early 2014 North American cold wave wikipedia , lookup
Public opinion on global warming wikipedia , lookup
Climate change and poverty wikipedia , lookup
Effects of global warming on humans wikipedia , lookup
General circulation model wikipedia , lookup
Effects of global warming on Australia wikipedia , lookup
Surveys of scientists' views on climate change wikipedia , lookup
Climate change and cyclic predator-prey population
dynamics in the high-Arctic
Olivier Gilg, Benoît Sittler & Ilkka Hanski
Supplementary Material
The six sections below present additional material that is not mandatory for understanding the
results in the main paper but will provide to the interested reader with further details on
material, methods, simulation results based on alternative model assumptions, and
interpretation of the results. We also summarize here data and results that are described at
length in other published papers.
1. The Model: implementation of the numerical responses of the predators (Methods)
The numerical responses of the snowy owl, the long-tailed skua and the Arctic fox were
implemented as follows (parameter names and values as in Table 1 in the main text). N is the
current lemming density for any given date except for snow melt. Lemming density at snow
melt is denoted by N’, which is used in the calculation of the numerical responses of the
predators, which are determined for the rest of the summer by lemming densities at snow
melt, the time of the year when the predators decide to settle or not in the area. The dates are
the values given for the baseline model by Gilg et al. (2003), but they are varied in the present
simulations according to the different climate change scenarios as described below. For more
details on the functional and numerical responses see Gilg et al. (2006).
The numerical response for the adult owls with density denoted by P1 is given by
Adult owls:
P1
b1 ( N ' 2)
Y1 ( N ' 4)
for the period from May 1st (“arrival of owls”) to September 30 (“departure of owls”) given
that N (before snow melt; June 15) or N’ (after snow melt) > 2. In words, the snowy owls stay
in the area until the end of the summer if lemming density at snowmelt exceeds 2 per ha. b1
and Y1 are two parameters (see Table 1 in the main text).
1
The numerical response for the young owls with density denoted by P1 young is given
by
Young owls:
P1 young
b1 young ( N ' 2)
1
young
0.36("age in days"9)
Y1
( N ' 4) 1 e
for the period from June 25 (“hatching of owls” ) until September 30 (“departure of owls”),
assuming that adult owl density (P1) is greater than zero in that year. Note that the second part
of the function, adapted from Ricklefs’s logistic growth curve (Ricklefs 1967), allows us to
count the impact of hatchlings and fledging in terms of “adult equivalents” (the constant
values are from published growth curves as in Gilg et al.(2003). b1 young and Y1 young are two
parameters (see Table 1 in the main text).
The numerical response for the adult skuas with density denoted by P2 is given by
Adult skua:
P2 0.02
from June 5 (“arrival of skuas”) until August 15 (“departure of skuas”). Adult skuas are
strongly territorial in Northeast Greenland, their density is hence assumed to be constant for a
given study area.
The numerical response for the young skuas with density denoted by P2 young is given by
Young skua:
young
2
P
b2young N '2
young 2
Y2
N '2
1
0.464("age in days" 4.55)
1 e
from July 14 (“hatching of skuas”) until August 15 (“departure of skuas”). This is an
analogous equation to that for young owls.
The numerical response for the adult foxes with density denoted by P3 is given by
Adult fox:
P3 a3
(b3 a3 ) N '2
Y32 N '2
from May 1st (“arrival of foxes”) until October 22 (“departure of foxes”), with N’ replaced by
N before snow melt (June 15).
Finally, the numerical response for the young foxes with density denoted by P3 young is
given by
Young fox:
young
3
P
b3young N '2
1
young 2
2
0.36("age in days"9)
Y3
N ' 1 e
2
from June 15 (“birth of young foxes”) until October 22 (“departure of foxes”). This is an
analogous equation to that for young owls.
The model has been constructed and parameter values estimated (Table 1) with
information from the long-term study in the Karupelv Valley. The same model was used to
run simulations for the Zackenberg study site, except for a few parameters that have been
recently estimated specifically for this site (Schmidt et al. 2008). These parameters include
the densities of the skua and the fox (see Table 1). The adult fox does not respond numerically
at Zackenberg (hence b3 = a3). We used our own estimates for the maximum density of snowy
owls for the Zackenberg simulations, since only one data point was available from
Zackenberg. We used a slope of 2.5 for the numerical response of young foxes at Zackenberg,
to take into account that foxes breed at lower lemming densities at Zackenberg than at
Karupelv. Indeed, and as also suggested by the constant density of adult foxes, the fox
population is not so greatly influenced by lemming dynamics at Zackenberg as in Karupelv,
probably because at Zackenberg muskox carcasses are more abundant and more regularly
available in winter, improving the survival and breeding quality of adult foxes in the
following spring (see below and Schmidt et al., 2008).
2. Differences in the responses of the four predators at Karupelv and Zackenberg
Though the lemming dynamics have been largely synchronous between the two sites in 19952007, Karupelv has had a strongly cyclic lemming population and predators with pronounced
numerical responses, while at Zackenberg the dynamics of both the prey and the predators
have been more stable (Gilg et al. 2006; Schmidt et al. 2008).
For example, the long-tailed skua, which breeds successfully at relatively low
lemming density (around 1 per ha) in northeast Greenland (Gilg et al. 2006), breeds twice as
often at Zackenberg (75% of years) than at Karupelv (37% of years). The variance in its
annual breeding success is also only half at Zackenberg of that at Karupelv (0.09 vs. 0.18
fledglings per km2 in 1995-2006), but because the lemming peaks reach higher values at
Karupelv, the average annual breeding success remains similar at both sites (0.21 and 0.20 at
Karupelv and at Zackenberg, respectively).
The snowy owl requires higher lemming densities to breed (≥2 lemmings per ha at
Karupelv; Gilg et al. 2006) and is therefore not expected to occur regularly at Zackenberg.
The species has been found breeding only once (one pair) at Zackenberg in 1995-2006,
though former successful breeding events have been reported (Pantenburg 1952). This
discrepancy may also be due to the relatively small size and narrowness of the inner valley in
Zackenberg (compared to the valley at Karupelv); the snowy owl avoids steep rocky slopes
and requires at least 5 km² per pair on average in most high-Arctic regions (Gilg,
3
unpublished). At Karupelv, the snowy owl has bred in every summer when the lemming
density was above the threshold of 2 lemming per ha.
The densities of the Arctic fox vary 5-fold at Karupelv, which is due to a few
additional breeding pairs during high peak years. In most years of the lemming cycle, there is
no numerical response of the fox to lemming density at either site, though the minimum
constant fox density is reported to be twice as high at Zackenberg than at Karupelv (Gilg et al.
2006; Schmidt et al. 2008).
Finally, the stoat, the only true lemming specialist predator in northeast Greenland,
apparently imposes a more stable predation rate at Zackenberg (log-transformed ratio of
lemming winter nests predated by stoat: 0.67 ± 0.27 SE) than at Karupelv (0.28 ± 0.44).
However, counts of depredated winter nest must be interpreted cautiously for Zackenberg,
since the area monitored (2.05 km²) is by far too small to detect stoat presence when its
density is low. For instance, one should not consider the zero counts for 2004-06 as an
indication of local extinction, especially since living stoats were seen elsewhere in the valley
during these years (as reported in Zackenberg annual reports). At Karupelv, we would have
missed all stoat winter nests in most years by reducing our monitoring area to 2 km² (in 63%
of the years there were <0.5 stoat nests per km²), while in practice there has been only one
year with almost no stoat winter nests (one nest found at the border of the study area).
For the Zackenberg simulations and as explained in the Mateerial and methods and
presented in Table 1, the functional response of the fox was implemented with a higher (more
generalist like) half-saturation constant, and their densities were set to a minimum constant
value regardless of lemming density. By doing so, we followed Schmidt et al.’s (2008)
published parameter values and Forchhammer et al. (2008) conclusions. The likely
explanation for such different responses by the same predator species at the two sites is the
presence of muskox carcasses. At Zackenberg, the number of breeding foxes may be more
dependent on the number of muskox carcasses than on lemming density; muskox carcasses
have been much more common at Zackenberg than at Karupelv. In the period 1996-2003, the
two years with the highest carcass densities, 1996 and 2000, were the two best breeding years
for the fox at Zackenberg, even if these years were among the three lowest lemming years.
Similarly, the year with the highest breeding success (young per km² corrected for the number
of dens monitored in each year) was also the one with the highest number of muskox
carcasses, and it was the second lowest year in terms of lemming densities.
The Arctic fox is a typical generalist predator which commonly breeds in the Arctic
regions where there are no rodents (e.g. Svalbard, West Greenland). Hence, it is not surprising
that when alternate food becomes available in significant numbers, which is often the case at
Zackenberg but not at Karupelv, the foxes display only a weak numerical response to
4
lemming densities.
3. Evidence of lemming mortality in winter
In 1989-2007, all lemming carcasses found in lemming winter nest aggregations have been
counted by B.S. The proportion of nest aggregations with juvenile carcasses (lemmings less
than ca. 35g when alive, empirically aged according to the carcass size) was twice as high
after 1998 than before (Fig. S2). For the period 2000-02, when the expected peak phase was
aborted, this ratio was four times higher than the average for the entire period (t=3.37,
P<0.01). Whether lemmings died because of food limitation, poor insulation by snow cover or
because of diseases cannot be determined, but it is unlikely that entire carcasses are left over
by predators (single skulls and other such remains were carefully excluded from this
calculation). On the other hand, the juveniles are more sensitive to unfavorable environmental
conditions than adult lemmings, and it is possible that the exceptionally high death rate of the
juveniles was caused by melt-frost events in the spring. Note that these exceptional deaths
occurred during the period when the regular cycles disappeared. It is also worth mentioning
that the years 2000-2004 had by far the longest ice-free period recorded between 1951-2006
in the region (see Fig. 3 in Christiansen et al. 2008). Lack of sea-ice induces a shift from
continental to oceanic climate in northeast Greenland, with higher temperatures and
precipitations than usual (Hansen et al. 2008; Hinkler et al. 2008; Stendel et al. 2008).
Finally, though elevated lemming mortality in the spring may be linked to the climate change
induced high frequency of frost-melt events, high lemming mortality in the spring has been
reported also previously. Exactly one century ago, Manniche (1910) wrote: “In March and
April I frequently found on the snow lemmings which had been starved or perhaps more
likely frozen to death. It was evident that in several places the animals had tried in vain to
break through the glazing of the snow crust”.
4. Alternative assumption about reduced lemming growth rate in winter
The observed increase in the frequency of frost-melt events (Fig. 2c), which is assumed to
increase stochastic mortality of lemmings at snow melt, would likely reduce the regularity of
population cycles. Though adult lemmings are particularly well adapted to the subnivean
environment and may survive qualitative changes in their habitat, such as the formation of ice
crust within or at the bottom of the snow layer, the growth rate of their populations would be
reduced. We run another set of simulations with alternative assumptions, in which stochastic
mortality at snow melt (Sto) was replaced by reduced growth rate of lemmings in winter (rw;
Figs. S3-S5). For the three probes (cycle length, amplitude and peak density), these
5
simulations produced qualitatively similar results to the ones presented on Figs. 3 and 4, but a
two-fold decline in winter growth rate appeared to have a stronger impact on lemming
dynamics (especially on cycle length) than stochastic spring mortality (in the range from 0 to
90%), the former often leading to non-cyclic dynamics while the latter mostly producing
weakly cyclic dynamics (compare Fig. 3 with Fig. S3). Similar altered dynamics were
obtained for stochastic mortality in the range 0-30% and winter growth rate of 3.8 (compare
Fig. 4ef with Fig. S4ef), or 0-60% and 3.6, respectively (compare Fig. 4gh and Fig. S4gh).
5. Environmental stochasticity in the remaining parameters
As presented in the Material and methods, the six parameters whose values were varied in the
four scenarios in the main text were chosen because they are the ones whose values are most
likely to be affected by climate change. To assess the significance of moderate stochastic
variation (environmental stochasticity) in the remaining parameters, we included normally
distributed random variation in all the constant parameters listed in Table 1. We used random
variation around to mean value up to 10% or 20% of the mean. Ranges of the mean parameter
values and the number of simulations were the same as in Fig. 3 and Table 2.
As expected, adding environmental stochasticity increased variance in the results: see
e.g. increased mixing and wider distribution of all the scatter plots in Fig. S6. However, the
pattern of cycle lengths and amplitudes in Fig. S6 is the same as without such variation and
indicates that our conclusions are robust to moderate environmental stochasticity.
6. Stochasticity in the date of snow melt
Stochastic variation in the earlier date of snow melt due to climate change is another form of
stochasticity that could potentially affect our results. To test this, we run yet another set of
simulations in which a random variate within 10 or 20 days was added to the date of snow
melt. The effect of such stochasticity was tested in scenario A, and applied independently in
each year (n=112) in each new simulation (n=1010; Table 2). Ranges of parameter values and
the number of simulations were as in Fig. 3 (panel A1 and A2) and Table 2 (Scenario A).
The results were mostly very similar to the ones without variation in the date of snow
melt (Fig 3). However, an apparent stabilizing effect emerged for large changes in overall
summer length and stochastic mortality, especially for the predicted cycle length (see the
reduced error bars for the yellow dots in the insert in Fig. S7). Also, the yellow dots appeared
to be more sorted in the very last panel, compared to the original simulation (Fig. 3 panel A2),
indicating that such stochastic variation in the date of snow melt would slightly reduce the
amplitude of cycles.
6
Figure S1.
Outline of the impact of climate change on lemming-predator interactions in northeast
Greenland. Under past climate, the rate of change in the numbers of the collared lemming
(∆N/∆t) is linked to predation, and the rates of change in predators (∆Predators/∆t) to lemming
densities (large grey outer arrows); these interactions produce the cyclic dynamics (Gilg et al.
2003; Gilg et al. 2006). Under climate change scenarios (small black arrows), the expected
extension of the snow-free period (+SFP) will allow predators to arrive and prey upon lemmings
earlier in the season (+ phenology). This will mechanistically reduce the average annual growth
rate of lemmings since their growth rate in winter (rw) is higher than the growth rate in summer
(rs). The altered quality of the snow (-SQ) by rain-on-snow and frost-melt events will induce
stochastic mortality of lemmings in the spring (+Sto) or alternatively reduce the growth rate of
the lemming population (-rw; Fig. S4 and S5). Altered snow quality might also cause an increase
in the alternate food (e.g. through higher muskox mortality), and in turn, improve the functional
(+phenology )
+SFP
-SQ
(+D +D 3 +a 3 -b )
∆N/ ∆t
∆Predators/∆ t
+SFP (-dN/dt )
-SQ (+Sto or -rw )
(+D, +D3) and/or numerical (-b, +a3) responses of the mammalian predators.
7
Figure S2. Proportion of lemming winter nest aggregations with juvenile carcasses (red
squares; left y-axis). Collared lemming density (individuals per ha) at snow melt is given for
15
1.40
13
1.20
11
1.00
9
0.80
7
0.60
5
0.40
3
0.20
1
0.00
-1
19
88
19
89
19
90
19
91
19
92
19
93
19
94
19
95
19
96
19
97
19
98
19
99
20
00
20
01
20
02
20
03
20
04
20
05
20
06
20
07
1.60
Lemming densities (ind./ha)
Lemming winter nest agregations
with juvenile carcasses (%)
comparison (black circles; right y-axis).
Juvenile mortality
Lemming densities
8
Figure S3. Altered lemming dynamics under the four climate change scenarios described in
the main text. The simulations in this figure assume reduced lemming growth rate in winter
(rw) in response to altered quality of the snow (instead of increasing stochastic mortality in the
spring as in Fig. 3).
9
Figure S4. Lemming dynamics at Karupelv (black symbols) and Zackenberg (white). Panels
a and b present the empirical data, c and d the dynamics predicted by the model (see Table 1
for parameter values), e and f the altered dynamics for a snow-free period of 130 days
(prolongation by one month) and the winter growth rate of the lemming set at 3.8 (5%
reduction or approximately the loss of half of the weaned young for one litter or month), and
g and h the altered dynamics with a snow-free period of 160 days and winter growth rate of
3.6 for the lemming (one full litter lost).
b
a
1988
12
-1
1996
2000
2004
2008
1988
12
10
10
8
8
6
6
4
4
2
2
0
0
c
Lemming density at snow melt (ind.ha )
1992
1992
1996
2000
2004
2008
'
d
12
12
10
10
8
8
6
6
4
4
2
2
0
0
e
f
12
12
10
10
8
8
6
6
4
4
2
2
0
0
g
h
12
12
10
10
8
8
6
6
4
4
2
2
0
0
10
Figure S5. Average annual breeding success of the four predator species at Karupelv (filled
symbols) and Zackenberg (open symbols). For each species, we present the empirical data,
the original model predictions for parameter values given in Table 1, the altered breeding
successes under scenario (A) for small changes in parameter values (as in Fig. S4ef; snowfree period of 130 days and lemming winter growth rate rw =3.8), and for moderate changes in
parameter values (as in Fig. 4gh; snow-free period of 160 days and lemming winter growth
rate rw =3.6). Values are given in fledglings (skua and owl), weaned young (fox) or young
produced (stoat) per km². Note that there are no direct density estimates available for the stoat
0,25
0,2
0,15
0,25
0,25
Scenario A
moderate changes
Scenario A
small changes
0,1
0,05
0,05
0
0
0,2
0,2
0,15
0,15
0,1
0,1
0,05
0,05
0
0
Long-tailed skua
Snowy owl
0,25
0,25
0,2
0,2
0,15
0,15
0,1
0,1
0,05
0,05
0
0
0,25
Baseline model
Empirical data
0,25
0,2
0,15
0,1
Predators' average annual breeding success
(young produced per km²)
Scenario A
moderate changes
Scenario A
small changes
Baseline model
Empirical data
in the empirical data. Other missing values represent local extinctions.
Arctic fox
0,25
0,2
0,2
0,15
0,15
0,1
0,1
0,05
0,05
0
0
Stoat
11
Figure S6. Altered lemming dynamics under the four climate change scenarios described in
the main text (in Fig. 3), but with 10 and 20% random variation in all the constant parameters
listed in Table 1. Ranges of parameter values and the number of simulations are the same as
in Fig. 3 and Table 2.
12
Figure S6. (continued)
13
Figure S7. Altered lemming dynamics under scenario A described in the main text (in Fig. 3), but
with random variation around the mean date of snow melt, up to 10 or 20 days. Ranges of
parameter values and the number of simulations are the same as in Fig. 3 and Table 2.
14
References
Christiansen, H.H., Sigsgaard, C., Humlum, O., Rash, M., & Hansen, B.U. (2008) Permafrost and Periglacial
geomorphology at Zackenberg. Advances in Ecological Research, 40, 151-174.
Forchhammer, M.C., Schmidt, N.M., Høye, T.T., Berg, T.B., Hendrichsen, D.K., & Post, E. (2008) Population
dynamical responses to climate change. Advances in Ecological Research, 40, 391-419.
Gilg, O., Hanski, I., & Sittler, B. (2003) Cyclic dynamics in a simple vertebrate predator-prey community.
Science, 302, 866-868.
Gilg, O., Sittler, B., Sabard, B., Hurstel, A., Sané, R., Delattre, P., & Hanski, I. (2006) Functional and numerical
responses of four lemming predators in high arctic Greenland. Oikos, 113, 196-213.
Hansen, B.U., Sigsgaard, C., Rasmussen, L., et al. (2008) Present-day climate at Zackenberg. Advances in
Ecological Research, 40, 111-149.
Hinkler, J., Hansen, B.U., Tamstorf, M.P., Sigsgaard, C., & Petersen, D. (2008) Snow and snow-cover in central
Northeast Greenland. Advances in Ecological Research, 40, 175-195.
Manniche, A.L.V. (1910) The terrestrial mammals and birds of northeast Greenland. Meddelelser om Grønland,
45, 1-199.
Pantenburg, V. (1952) Grandes chasses au Groenland Amiot Dumont, Paris.
Ricklefs, R.E. (1967) A graphical method of fitting equations to growth curves. Ecology, 48, 978-983.
Schmidt, N.M., Berg, T.B., Forchhammer, M., Hendrichsen, D.K., Kyhn, L.A., Meltofte, H., & Høye, T.T.
(2008) Vertebrate predator-prey interactions in a seasonal environment. Advances in Ecological
Research, 40, 345-370.
Stendel, M., Christensen, J.H., & Petersen, D. (2008) Arctic climate and climate change with a focus on
Greenland. Advances in Ecological Research, 40, 13-43.
15